HIV-1 Envelope Glycoprotein Cell Surface Localization Is Associated with Antibody-Induced Internalization
Abstract
:1. Introduction
2. Material and Methods
2.1. Ethics Statement
2.2. Cell Lines and Primary Cells
2.3. Plasmids and Proviral Constructs
2.4. Viral Production, Infections, and Ex Vivo Amplification
2.5. Antibodies and Reagents
2.6. Antibody-Induced Internalization Assay by Flow Cytometry
2.7. Antibody-Induced Internalization Assay by Confocal Microscopy
2.8. Biochemical Isolation of Lipid Microdomains and Western Blot Analysis
2.9. Statistical Analyses
3. Results and Discussion
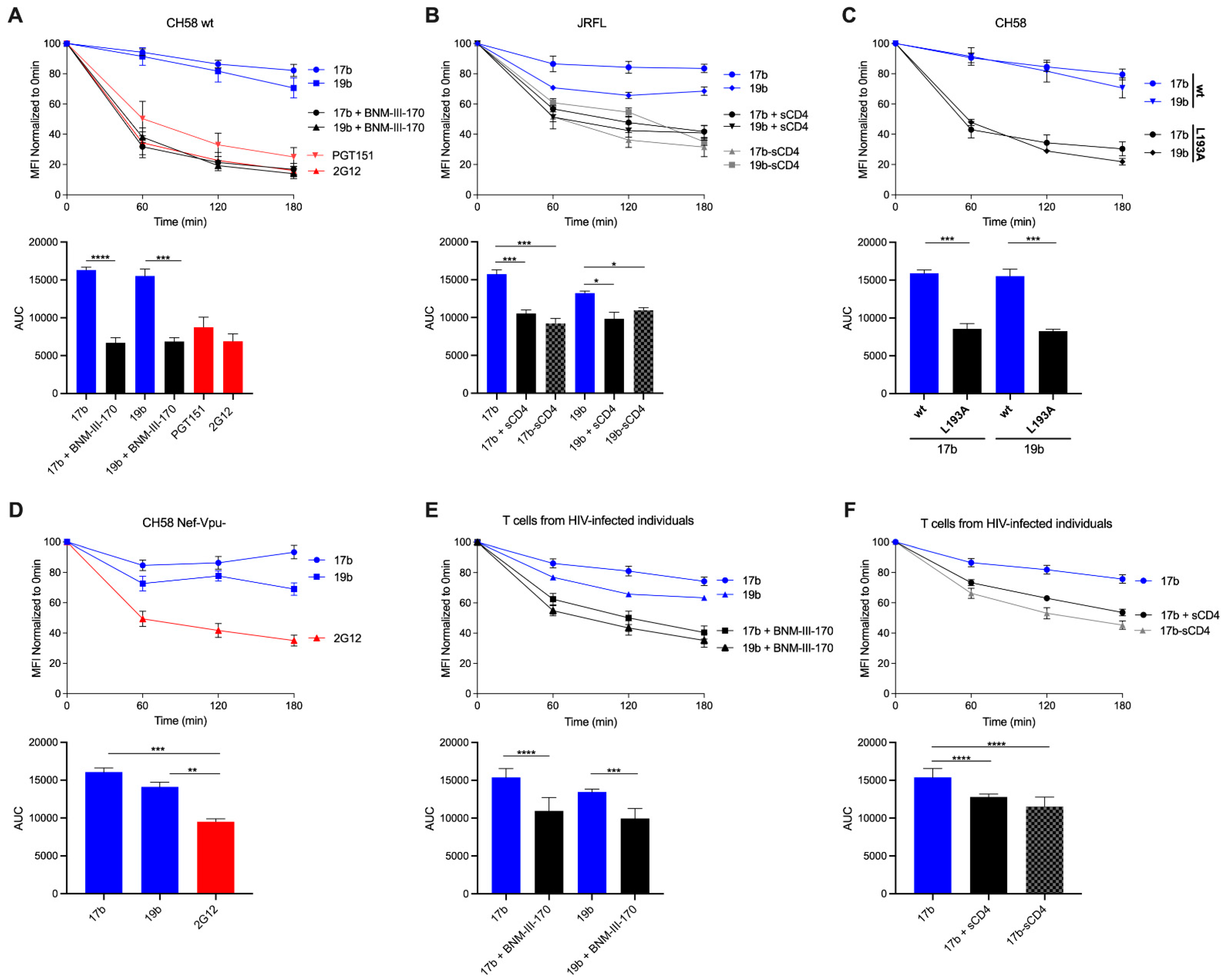
3.1. HIV-1 Env ‘Opening’ Accelerates Its Antibody-Mediated Internalization from the Cell Surface
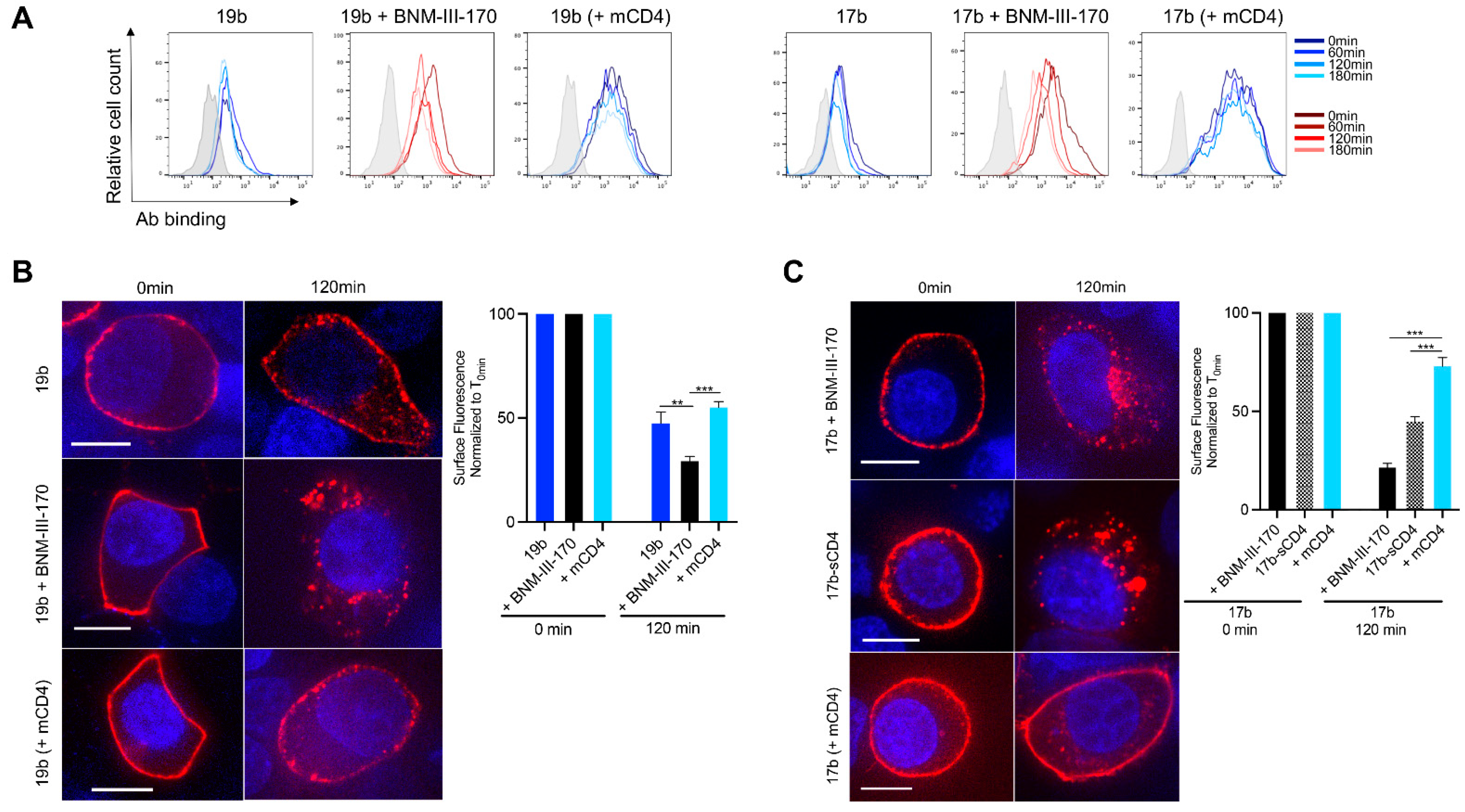
3.2. Visualizing nNAb-Mediated Env Internalization from the Cell Surface
3.3. Different Membrane Microdomains Could Determine Antibody-Mediated Env Internalization
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Boge, M.; Wyss, S.; Bonifacino, J.S.; Thali, M. A Membrane-proximal Tyrosine-based Signal Mediates Internalization of the HIV-1 Envelope Glycoprotein via Interaction with the AP-2 Clathrin Adaptor. J. Biol. Chem. 1998, 273, 15773–15778. [Google Scholar] [CrossRef] [Green Version]
- Byland, R.; Vance, P.J.; Hoxie, J.A.; Marsh, M. A Conserved Dileucine Motif Mediates Clathrin and AP-2–dependent Endocytosis of the HIV-1 Envelope Protein. Mol. Biol. Cell 2007, 18, 414–425. [Google Scholar] [CrossRef] [Green Version]
- von Bredow, B.; Arias, J.F.; Heyer, L.N.; Gardner, M.R.; Farzan, M.; Rakasz, E.G.; Evans, D.T. Envelope Glycoprotein Internalization Protects Human and Simian Immunodeficiency Virus-Infected Cells from Antibody-Dependent Cell-Mediated Cytotoxicity. J. Virol. 2015, 89, 10648–10655. [Google Scholar] [CrossRef] [Green Version]
- Anand, S.P.; Grover, J.R.; Tolbert, W.D.; Prévost, J.; Richard, J.; Ding, S.; Baril, S.; Medjahed, H.; Evans, D.T.; Pazgier, M.; et al. Antibody-Induced Internalization of HIV-1 Env Proteins Limits Surface Expression of the Closed Conformation of Env. J. Virol. 2019, 93, e00293-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panova, V.; Attig, J.; Young, G.R.; Stoye, J.P.; Kassiotis, G. Antibody-induced internalisation of retroviral envelope glycoproteins is a signal initiation event. PLoS Pathog. 2020, 16, e1008605. [Google Scholar] [CrossRef] [PubMed]
- Chew, H.Y.; de Lima, P.O.; Cruz, J.L.G.; Banushi, B.; Echejoh, G.; Hu, L.; Joseph, S.R.; Lum, B.; Rae, J.; O’Donnell, J.S.; et al. Endocytosis Inhibition in Humans to Improve Responses to ADCC-Mediating Antibodies. Cell 2020, 180, 895–914. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Nguyen, H.T.; Ding, H.; Wang, J.; Zou, S.; Liu, L.; Guha, D.; Gabuzda, D.; Ho, D.D.; Kappes, J.C.; et al. Dual Pathways of Human Immunodeficiency Virus Type 1 Envelope Glycoprotein Trafficking Modulate the Selective Exclusion of Uncleaved Oligomers from Virions. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Zou, S.; Zhang, S.; Gaffney, A.; Ding, H.; Lu, M.; Grover, J.R.; Farrell, M.; Nguyen, H.T.; Zhao, C.; Anang, S.; et al. Long-Acting BMS-378806 Analogues Stabilize the State-1 Conformation of the Human Immunodeficiency Virus Type 1 Envelope Glycoproteins. J. Virol. 2020, 94. [Google Scholar] [CrossRef]
- Salimi, H.; Johnson, J.; Flores, M.G.; Zhang, M.S.; O’Malley, Y.; Houtman, J.C.; Schlievert, P.M.; Haim, H. The lipid membrane of HIV-1 stabilizes the viral envelope glycoproteins and modulates their sensitivity to antibody neutralization. J. Biol. Chem. 2020, 295, 348–362. [Google Scholar] [CrossRef]
- Fontaine, J.; Chagnon-Choquet, J.; Valcke, H.S.; Poudrier, J.; Roger, M.; the Montreal Primary HIV Infection and Long-Term Non-Progressor Study Groups. High expression levels of B lymphocyte stimulator (BLyS) by dendritic cells correlate with HIV-related B-cell disease progression in humans. Blood 2011, 117, 145–155. [Google Scholar] [CrossRef]
- Fontaine, J.; Coutlée, F.; Tremblay, C.; Routy, J.-P.; Poudrier, J.; Roger, M. Montreal Primary HIV Infection and Long-Term Nonprogressor Study Groups HIV Infection Affects Blood Myeloid Dendritic Cells after Successful Therapy and despite Nonprogressing Clinical Disease. J. Infect. Dis. 2009, 199, 1007–1018. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.; Veillette, M.; Brassard, N.; Iyer, S.S.; Roger, M.; Martin, L.; Pazgier, M.; Schön, A.; Freire, E.; Routy, J.-P.; et al. CD4 mimetics sensitize HIV-1-infected cells to ADCC. Proc. Natl. Acad. Sci. USA 2015, 112, E2687–E2694. [Google Scholar] [CrossRef] [Green Version]
- Veillette, M.; Désormeaux, A.; Medjahed, H.; Gharsallah, N.-E.; Coutu, M.; Baalwa, J.; Guan, Y.; Lewis, G.; Ferrari, G.; Hahn, B.; et al. Interaction with Cellular CD4 Exposes HIV-1 Envelope Epitopes Targeted by Antibody-Dependent Cell-Mediated Cytotoxicity. J. Virol. 2014, 88, 2633–2644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodge, R.; LaLonde, J.; Lemay, G.; Cohen, É.A. The membrane-proximal intracytoplasmic tyrosine residue of HIV-1 envelope glycoprotein is critical for basolateral targeting of viral budding in MDCK cells. EMBO J. 1997, 16, 695–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Gonzalez, J.F.; Salazar, M.G.; Keele, B.F.; Learn, G.; Giorgi, E.E.; Li, H.; Decker, J.M.; Wang, S.; Baalwa, J.; Kraus, M.H.; et al. Genetic identity, biological phenotype, and evolutionary pathways of transmitted/founder viruses in acute and early HIV-1 infection. J. Exp. Med. 2009, 206, 1273–1289. [Google Scholar] [CrossRef] [PubMed]
- Ochsenbauer, C.; Edmonds, T.G.; Ding, H.; Keele, B.F.; Decker, J.; Salazar, M.G.; Salazar-Gonzalez, J.F.; Shattock, R.; Haynes, B.F.; Shaw, G.M.; et al. Generation of Transmitted/Founder HIV-1 Infectious Molecular Clones and Characterization of Their Replication Capacity in CD4 T Lymphocytes and Monocyte-Derived Macrophages. J. Virol. 2011, 86, 2715–2728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heigele, A.; Kmiec, D.; Regensburger, K.; Langer, S.; Peiffer, L.; Stürzel, C.M.; Sauter, D.; Peeters, M.; Pizzato, M.; Learn, G.H.; et al. The Potency of Nef-Mediated SERINC5 Antagonism Correlates with the Prevalence of Primate Lentiviruses in the Wild. Cell Host Microbe 2016, 20, 381–391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prévost, J.; Richard, J.; Ding, S.; Pacheco, B.; Charlebois, R.; Hahn, B.H.; Kaufmann, D.E.; Finzi, A. Envelope glycoproteins sampling states 2/3 are susceptible to ADCC by sera from HIV-1-infected individuals. Virology 2018, 515, 38–45. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, W.A.; Koyanagi, Y.; Namazie, A.; Zhao, J.-Q.; Diagne, A.; Ldler, K.; Zack, J.A.; Chen, I.S.Y. HIV-1 tropism for mononuclear phagocytes can be determined by regions of gp120 outside the CD4-binding domain. Nature 1990, 348, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Wang, L.; Gu, C.; Herschhorn, A.; Xiang, S.-H.; Haim, H.; Yang, X.; Sodroski, J. Subunit organization of the membrane-bound HIV-1 envelope glycoprotein trimer. Nat. Struct. Mol. Biol. 2012, 19, 893–899. [Google Scholar] [CrossRef] [Green Version]
- Bour, S.; Schubert, U.; Strebel, K. The human immunodeficiency virus type 1 Vpu protein specifically binds to the cytoplasmic domain of CD4: Implications for the mechanism of degradation. J. Virol. 1995, 69, 1510–1520. [Google Scholar] [CrossRef] [Green Version]
- Richard, J.; Nguyen, D.N.; Tolbert, W.D.; Gasser, R.; Ding, S.; Vézina, D.; Gong, S.Y.; Prévost, J.; Gendron-Lepage, G.; Medjahed, H.; et al. Across Functional Boundaries: Making Nonneutralizing Antibodies to Neutralize HIV-1 and Mediate Fc-Mediated Effector Killing of Infected Cells. mBio 2021, e01405-01421. [Google Scholar] [CrossRef]
- Finzi, A.; Xiang, S.-H.; Pacheco, B.; Wang, L.; Haight, J.; Kassa, A.; Danek, B.; Pancera, M.; Kwong, P.D.; Sodroski, J. Topological Layers in the HIV-1 gp120 Inner Domain Regulate gp41 Interaction and CD4-Triggered Conformational Transitions. Mol. Cell 2010, 37, 656–667. [Google Scholar] [CrossRef] [Green Version]
- Melillo, B.; Liang, S.; Park, J.; Schön, A.; Courter, J.R.; LaLonde, J.M.; Wendler, D.J.; Princiotto, A.M.; Seaman, M.S.; Freire, E.; et al. Small-Molecule CD4-Mimics: Structure-Based Optimization of HIV-1 Entry Inhibition. ACS Med. Chem. Lett. 2016, 7, 330–334. [Google Scholar] [CrossRef] [Green Version]
- Chen, J.; Park, J.; Kirk, S.M.; Chen, H.-C.; Li, X.; Lippincott, D.J.; Melillo, B.; Smith, A.B., III. Development of an Effective Scalable Enantioselective Synthesis of the HIV-1 Entry Inhibitor BNM-III-170 as the Bis-trifluoroacetate Salt. Org. Process. Res. Dev. 2019, 23, 2464–2469. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH Image to ImageJ: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]
- Laumaea, A.; Smith III, A.B.; Sodroski, J.; Finzi, A. Opening the HIV envelope: Potential of CD4 mimics as multifunctional HIV entry inhibitors. Curr. Opin. HIV AIDS 2020, 15, 300–308. [Google Scholar] [CrossRef]
- Richard, J.; Veillette, M.; Ding, S.; Zoubchenok, D.; Alsahafi, N.; Coutu, M.; Brassard, N.; Park, J.; Courter, J.R.; Melillo, B.; et al. Small CD4 Mimetics Prevent HIV-1 Uninfected Bystander CD4 + T Cell Killing Mediated by Antibody-dependent Cell-mediated Cytotoxicity. EBioMedicine 2016, 3, 122–134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anand, S.P.; Prévost, J.; Baril, S.; Richard, J.; Medjahed, H.; Chapleau, J.-P.; Tolbert, W.D.; Kirk, S.; Smith, A.B., III; Wines, B.D.; et al. Two Families of Env Antibodies Efficiently Engage Fc-Gamma Receptors and Eliminate HIV-1-Infected Cells. J. Virol. 2019, 93, e01823-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herschhorn, A.; Ma, X.; Gu, C.; Ventura, J.D.; Castillo-Menendez, L.; Melillo, B.; Terry, D.S.; Smith, A.B., III; Blanchard, S.C.; Munro, J.B.; et al. Release of gp120 Restraints Leads to an Entry-Competent Intermediate State of the HIV-1 Envelope Glycoproteins. mBio 2016, 7, e01598-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haim, H.; Salas, I.; Sodroski, J. Proteolytic Processing of the Human Immunodeficiency Virus Envelope Glycoprotein Precursor Decreases Conformational Flexibility. J. Virol. 2013, 87, 1884–1889. [Google Scholar] [CrossRef] [Green Version]
- Alsahafi, N.; Bakouche, N.; Kazemi, M.; Richard, J.; Ding, S.; Bhattacharyya, S.; Das, D.; Anand, S.P.; Prévost, J.; Tolbert, W.D.; et al. An Asymmetric Opening of HIV-1 Envelope Mediates Antibody-Dependent Cellular Cytotoxicity. Cell Host Microbe 2019, 25, 578–587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castillo-Menendez, L.R.; Witt, K.; Espy, N.; Princiotto, A.; Madani, N.; Pacheco, B.; Finzi, A.; Sodroski, J. Comparison of Uncleaved and Mature Human Immunodeficiency Virus Membrane Envelope Glycoprotein Trimers. J. Virol. 2018, 92, e00277-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stieh, D.J.; King, D.F.; Klein, K.; Aldon, Y.; McKay, P.F.; Shattock, R.J. Discrete partitioning of HIV-1 Env forms revealed by viral capture. Retrovirology 2015, 12, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campbell, S.; Crowe, S.; Mak, J. Lipid rafts and HIV-1: From viral entry to assembly of progeny virions. J. Clin. Virol. 2001, 22, 217–227. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Hildreth, J.E.K. Evidence for Budding of Human Immunodeficiency Virus Type 1 Selectively from Glycolipid-Enriched Membrane Lipid Rafts. J. Virol. 2000, 74, 3264–3272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedbury, P.R.; Freed, E.O. The role of matrix in HIV-1 envelope glycoprotein incorporation. Trends Microbiol. 2014, 22, 372–378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Waheed, A.A.; Freed, E.O. The Role of Lipids in Retrovirus Replication. Viruses 2010, 2, 1146–1180. [Google Scholar] [CrossRef] [Green Version]
- Laude, A.; Prior, I.A. Plasma membrane microdomains: Organization, function and trafficking (Review). Mol. Membr. Biol. 2004, 21, 193–205. [Google Scholar] [CrossRef]
- Popik, W.; Alce, T.M.; Au, W.-C. Human Immunodeficiency Virus Type 1 Uses Lipid Raft-Colocalized CD4 and Chemokine Receptors for Productive Entry into CD4+ T Cells. J. Virol. 2002, 76, 4709–4722. [Google Scholar] [CrossRef] [Green Version]
- Chojnacki, J.; Eggeling, C. Super-resolution fluorescence microscopy studies of human immunodeficiency virus. Retrovirology 2018, 15, 41. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anand, S.P.; Prévost, J.; Descôteaux-Dinelle, J.; Richard, J.; Nguyen, D.N.; Medjahed, H.; Chen, H.-C.; Smith, A.B., III; Pazgier, M.; Finzi, A. HIV-1 Envelope Glycoprotein Cell Surface Localization Is Associated with Antibody-Induced Internalization. Viruses 2021, 13, 1953. https://doi.org/10.3390/v13101953
Anand SP, Prévost J, Descôteaux-Dinelle J, Richard J, Nguyen DN, Medjahed H, Chen H-C, Smith AB III, Pazgier M, Finzi A. HIV-1 Envelope Glycoprotein Cell Surface Localization Is Associated with Antibody-Induced Internalization. Viruses. 2021; 13(10):1953. https://doi.org/10.3390/v13101953
Chicago/Turabian StyleAnand, Sai Priya, Jérémie Prévost, Jade Descôteaux-Dinelle, Jonathan Richard, Dung N. Nguyen, Halima Medjahed, Hung-Ching Chen, Amos B. Smith, III, Marzena Pazgier, and Andrés Finzi. 2021. "HIV-1 Envelope Glycoprotein Cell Surface Localization Is Associated with Antibody-Induced Internalization" Viruses 13, no. 10: 1953. https://doi.org/10.3390/v13101953
APA StyleAnand, S. P., Prévost, J., Descôteaux-Dinelle, J., Richard, J., Nguyen, D. N., Medjahed, H., Chen, H.-C., Smith, A. B., III, Pazgier, M., & Finzi, A. (2021). HIV-1 Envelope Glycoprotein Cell Surface Localization Is Associated with Antibody-Induced Internalization. Viruses, 13(10), 1953. https://doi.org/10.3390/v13101953





