Interaction between NS1 and Cellular MAVS Contributes to NS1 Mitochondria Targeting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plasmids
2.2. Cells and Virus
2.3. Western Blotting (WB)
2.4. Transfection and Immunoprecipitation (IP)
2.5. Immunofluorescent Assay (IFA)
2.6. Reporter Assay for IFN-β Promoter Activity
2.7. ELISA for Measuring IFN-β Concentrations
3. Results
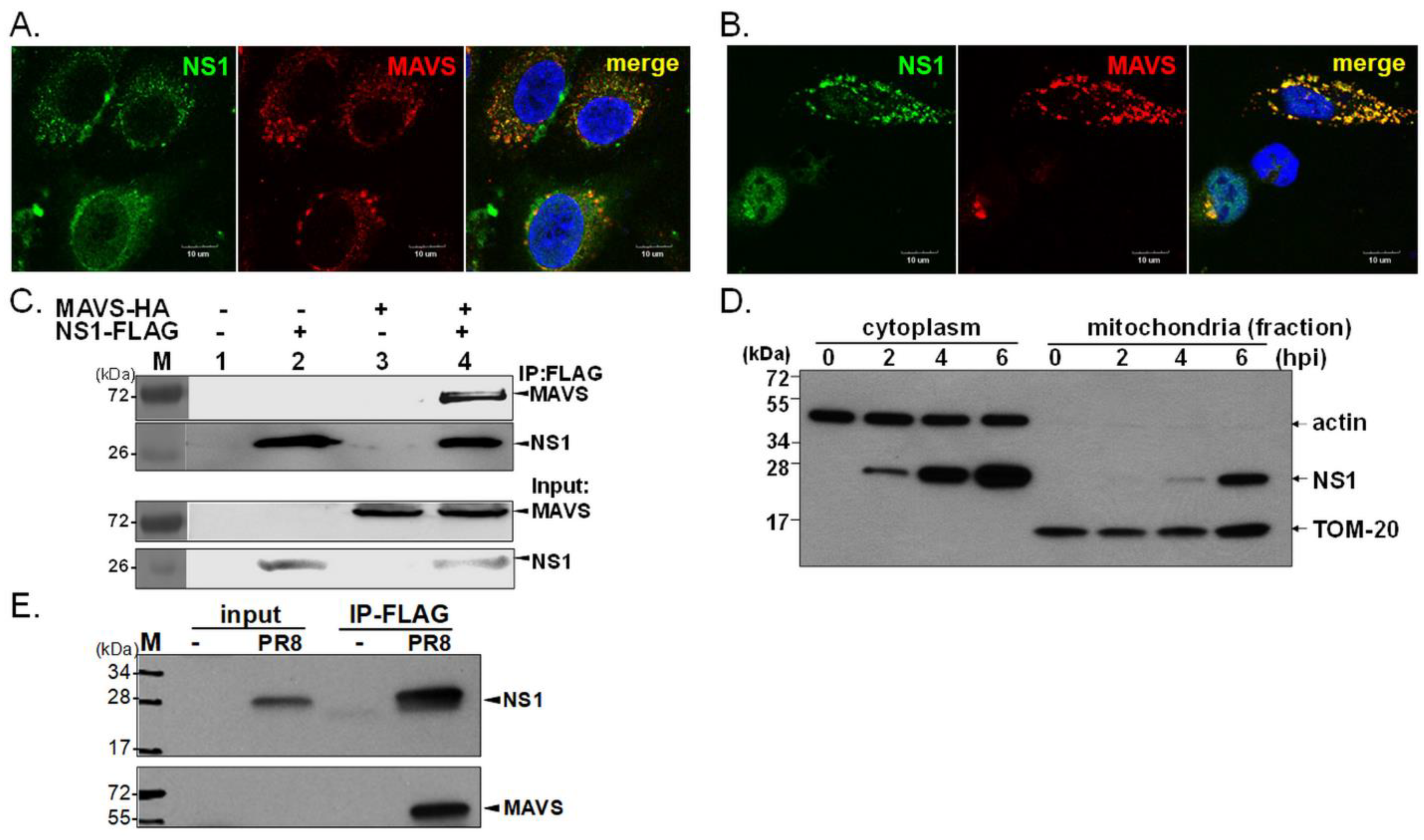
3.1. Mitochondrial Localization of NS1 and Its Interaction with MAVS
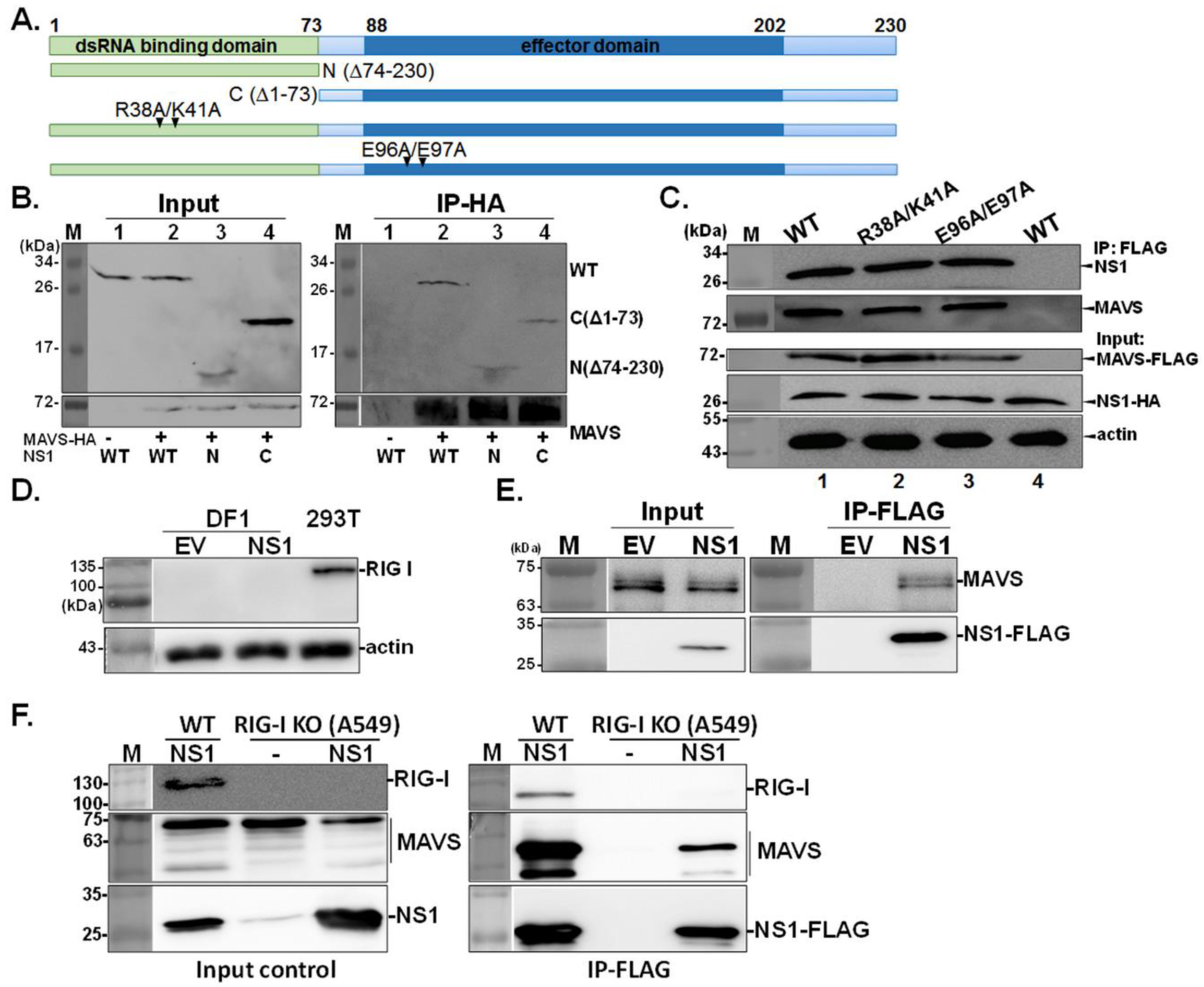
3.2. Identification of Domains of NS1 Required for the NS1-MAVS Interaction
3.3. MAVS Enhances the Mitochondrial Targeting of NS1, and the TM Tegion of MAVS Is Essential for the NS1-MAVS Interaction
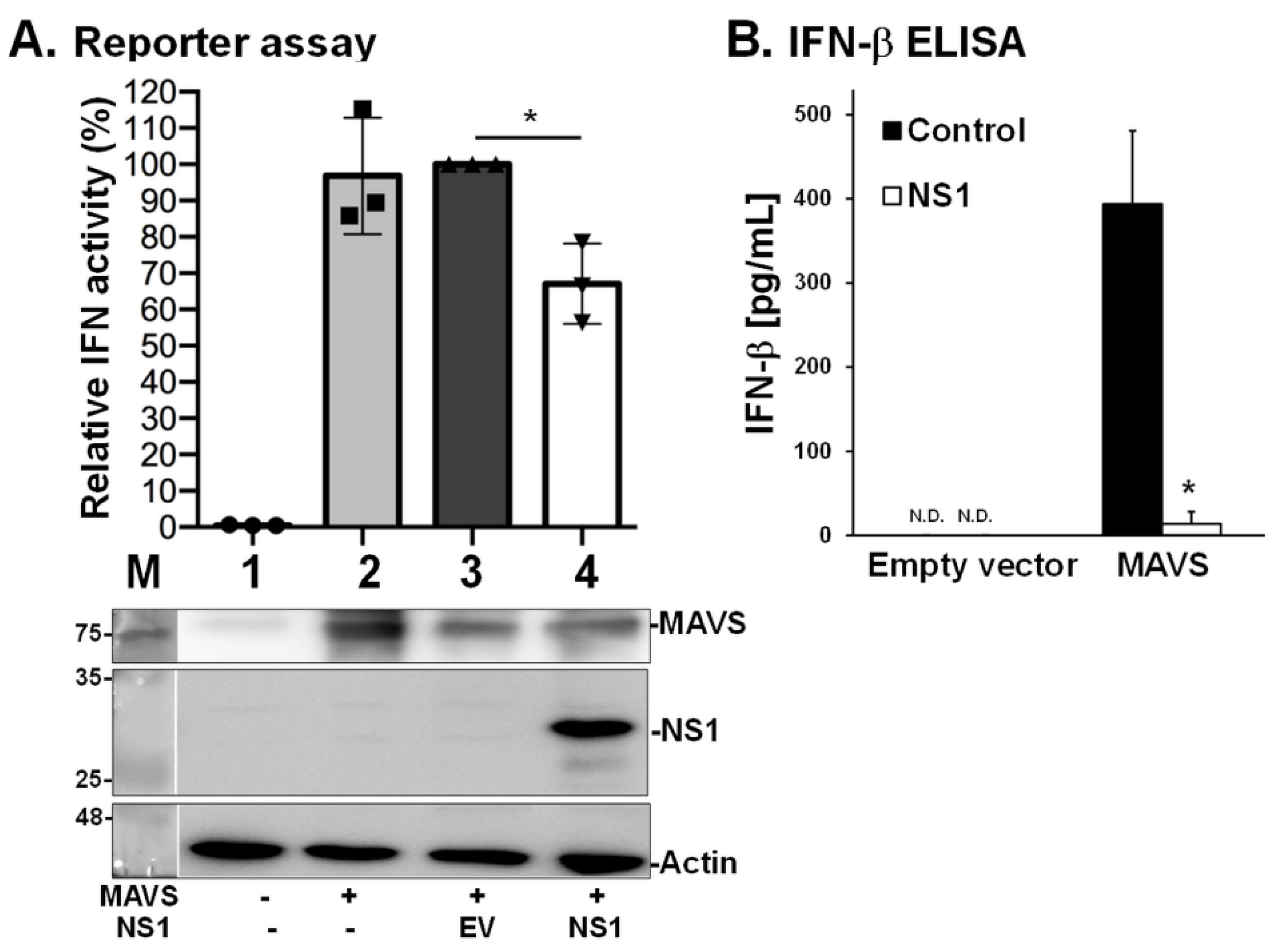
3.4. NS1 Decreases MAVS-Induced IFN Expression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Krug, R.M.; Etkind, P.R. Cytoplasmic and nuclear virus-specific proteins in influenza virus-infected MDCK cells. Virology 1973, 56, 334–348. [Google Scholar] [CrossRef]
- Suarez, D.L.; Perdue, M.L. Multiple alignment comparison of the non-structural genes of influenza A viruses. Virus Res. 1998, 54, 59–69. [Google Scholar] [CrossRef]
- Hale, B.G.; Randall, R.E.; Ortin, J.; Jackson, D. The multifunctional ns1 protein of influenza A viruses. J. Gen. Virol. 2008, 89, 2359–2376. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.Y.; Chien, C.Y.; Lu, Y.; Montelione, G.T.; Krug, R.M. An amino-terminal polypeptide fragment of the influenza virus NS1 protein possesses specific RNA-binding activity and largely helical backbone structure. RNA 1995, 1, 948–956. [Google Scholar] [PubMed]
- Wang, X.; Basler, C.F.; Williams, B.R.; Silverman, R.H.; Palese, P.; Garcia-Sastre, A. Functional replacement of the carboxy-terminal two-thirds of the influenza A virus NS1 protein with short heterologous dimerization domains. J. Virol. 2002, 76, 12951–12962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenspan, D.; Palese, P.; Krystal, M. Two nuclear location signals in the influenza virus NS1 nonstructural protein. J. Virol. 1988, 62, 3020–3026. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Yamakita, Y.; Krug, R.M. Regulation of a nuclear export signal by an adjacent inhibitory sequence: The effector domain of the influenza virus NS1 protein. Proc. Natl. Acad. Sci. USA 1998, 95, 4864–4869. [Google Scholar] [CrossRef] [Green Version]
- Murayama, R.; Harada, Y.; Shibata, T.; Kuroda, K.; Hayakawa, S.; Shimizu, K.; Tanaka, T. Influenza A virus non-structural protein 1 (NS1) interacts with cellular multifunctional protein nucleolin during infection. Biochem. Biophys. Res. Commun. 2007, 362, 880–885. [Google Scholar] [CrossRef]
- Han, H.; Cui, Z.-Q.; Wang, W.; Zhang, Z.-P.; Wei, H.-P.; Zhou, Y.-F.; Zhang, X.-E. New regulatory mechanisms for the intracellular localization and trafficking of influenza A virus NS1 protein revealed by comparative analysis of A/PR/8/34 and A/Sydney/5/97. J. Gen. Virol. 2010, 91, 2907–2917. [Google Scholar] [CrossRef]
- Melen, K.; Kinnunen, L.; Fagerlund, R.; Ikonen, N.; Twu, K.Y.; Krug, R.M.; Julkunen, I. Nuclear and nucleolar targeting of influenza A virus NS1 protein: Striking differences between different virus subtypes. J. Virol. 2007, 81, 5995–6006. [Google Scholar] [CrossRef] [Green Version]
- Volmer, R.; Mazel-Sanchez, B.; Volmer, C.; Soubies, S.M.; Guerin, J.-L. Nucleolar localization of influenza a NS1: Striking differences between mammalian and avian cells. Virol. J. 2010, 7, 63. [Google Scholar] [CrossRef] [PubMed]
- Garaigorta, U.; Falcon, A.M.; Ortin, J. Genetic analysis of influenza virus NS1 gene: A temperature-sensitive mutant shows defective formation of virus particles. J. Virol. 2005, 79, 15246–15257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, Y.; Yoshioka, K.; Suzuki, C.; Awashima, S.; Hosaka, Y.; Yewdell, J.; Kuroda, K. Localization of influenza virus proteins to nuclear dot 10 structures in influenza virus-infected cells. Virology 2003, 310, 29–40. [Google Scholar] [CrossRef] [Green Version]
- Raman, S.N.T.; Zhou, Y. Networks of host factors that interact with NS1 protein of influenza A virus. Front. Microbiol. 2016, 7, 654. [Google Scholar]
- Garcia-Sastre, A.; Egorov, A.; Matassov, D.; Brandt, S.; Levy, D.E.; Durbin, J.E.; Palese, P.; Muster, T. Influenza A virus lacking the NS1 gene replicates in interferon-deficient systems. Virology 1998, 252, 324–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Talon, J.; Horvath, C.M.; Polley, R.; Basler, C.F.; Muster, T.; Palese, P.; Garcia-Sastre, A. Activation of interferon regulatory factor 3 is inhibited by the influenza A virus NS1 protein. J. Virol. 2000, 74, 7989–7996. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, M.; Zheng, H.; Muster, T.; Palese, P.; Beg, A.A.; Garcia-Sastre, A. Influenza A virus NS1 protein prevents activation of nf-kappab and induction of alpha/beta interferon. J. Virol. 2000, 74, 11566–11573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nemeroff, M.E.; Barabino, S.M.; Li, Y.; Keller, W.; Krug, R.M. Influenza virus NS1 protein interacts with the cellular 30 kDa subunit of cpsf and inhibits 3’end formation of cellular pre-mRNAs. Mol. Cell 1998, 1, 991–1000. [Google Scholar] [CrossRef]
- Fortes, P.; Beloso, A.; Ortin, J. Influenza virus NS1 protein inhibits pre-mRNA splicing and blocks mRNA nucleocytoplasmic transport. EMBO J. 1994, 13, 704–712. [Google Scholar] [CrossRef]
- Lu, Y.; Qian, X.Y.; Krug, R.M. The influenza virus NS1 protein: A novel inhibitor of pre-mRNA splicing. Genes Dev. 1994, 8, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Min, J.-Y.; Krug, R.M. The primary function of RNA binding by the influenza A virus NS1 protein in infected cells: Inhibiting the 2’-5’ oligo (A) synthetase/RNase l pathway. Proc. Natl. Acad. Sci. USA 2006, 103, 7100–7105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Min, J.Y.; Li, S.; Sen, G.C.; Krug, R.M. A site on the influenza A virus NS1 protein mediates both inhibition of PKR activation and temporal regulation of viral RNA synthesis. Virology 2007, 363, 236–243. [Google Scholar] [CrossRef] [Green Version]
- Rajsbaum, R.; Albrecht, R.A.; Wang, M.K.; Maharaj, N.P.; Versteeg, G.A.; Nistal-Villan, E.; Garcia-Sastre, A.; Gack, M.U. Species-specific inhibition of RIG-I ubiquitination and ifn induction by the influenza A virus NS1 protein. PLoS Pathog. 2012, 8, e1003059. [Google Scholar] [CrossRef] [PubMed]
- Mibayashi, M.; Martinez-Sobrido, L.; Loo, Y.-M.; Cardenas, W.B.; Gale, M., Jr.; Garcia-Sastre, A. Inhibition of retinoic acid-inducible gene I-mediated induction of beta interferon by the NS1 protein of influenza A virus. J. Virol. 2007, 81, 514–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gack, M.U.; Albrecht, R.A.; Urano, T.; Inn, K.-S.; Huang, I.-C.; Carnero, E.; Farzan, M.; Inoue, S.; Jung, J.U.; Garcia-Sastre, A. Influenza A virus NS1 targets the ubiquitin ligase TRIM25 to evade recognition by the host viral RNA sensor RIG-I. Cell Host Microbe 2009, 5, 439–449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schuberth-Wagner, C.; Ludwig, J.; Bruder, A.K.; Herzner, A.-M.; Zillinger, T.; Goldeck, M.; Schmidt, T.; Schmid-Burgk, J.L.; Kerber, R.; Wolter, S.; et al. A conserved histidine in the RNA sensor RIG-I controls immune tolerance to N1-2’o-methylated self RNA. Immunity 2015, 43, 41–51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heaton, N.S.; Moshkina, N.; Fenouil, R.; Gardner, T.J.; Aguirre, S.; Shah, P.S.; Zhao, N.; Manganaro, L.; Hultquist, J.F.; Noel, J.; et al. Targeting viral proteostasis limits influenza virus, hiv, and dengue virus infection. Immunity 2016, 44, 46–58. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.B.; Park, Y.H.; Chungu, K.; Woo, S.J.; Han, S.T.; Choi, H.J.; Rengaraj, D.; Han, J.Y. Targeted knockout of mda5 and tlr3 in the df-1 chicken fibroblast cell line impairs innate immune response against RNA ligands. Front. Immunol. 2020, 11, 678. [Google Scholar] [CrossRef]
- Varga, Z.T.; Ramos, I.; Hai, R.; Schmolke, M.; Garcia-Sastre, A.; Fernandez-Sesma, A.; Palese, P. The influenza virus protein pb1-f2 inhibits the induction of type i interferon at the level of the mavs adaptor protein. PLoS Pathog. 2011, 7, e1002067. [Google Scholar] [CrossRef]
- Tawaratsumida, K.; Phan, V.; Hrincius, E.R.; High, A.A.; Webby, R.; Redecke, V.; Hacker, H. Quantitative proteomic analysis of the influenza A virus nonstructural proteins NS1 and NS2 during natural cell infection identifies pact as an NS1 target protein and antiviral host factor. J. Virol. 2014, 88, 9038–9048. [Google Scholar] [CrossRef] [Green Version]
- Ayllon, J.; Garcia-Sastre, A. The NS1 protein: A multitasking virulence factor. Curr. Top. Microbiol. Immunol. 2015, 386, 73–107. [Google Scholar] [PubMed]
- Nogales, A.; Rodriguez, L.; DeDiego, M.L.; Topham, D.J.; Martinez-Sobrido, L. Interplay of PA-X and NS1 proteins in replication and pathogenesis of a temperature-sensitive 2009 pandemic H1N1 influenza A virus. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twu, K.Y.; Noah, D.L.; Rao, P.; Kuo, R.-L.; Krug, R.M. The cpsf30 binding site on the NS1A protein of influenza A virus is a potential antiviral target. J. Virol. 2006, 80, 3957–3965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramos, I.; Carnero, E.; Bernal-Rubio, D.; Seibert, C.W.; Westera, L.; Garcia-Sastre, A.; Fernandez-Sesma, A. Contribution of double-stranded RNA and CPSF30 binding domains of influenza virus NS1 to the inhibition of type I interferon production and activation of human dendritic cells. J. Virol. 2013, 87, 2430–2440. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, L.; Nogales, A.; Iqbal, M.; Perez, D.R.; Martinez-Sobrido, L. Identification of amino acid residues responsible for inhibition of host gene expression by influenza A H9N2 NS1 targeting of CPSF30. Front. Microbiol. 2018, 9, 2546. [Google Scholar] [CrossRef]
- Keiner, B.; Maenz, B.; Wagner, R.; Cattoli, G.; Capua, I.; Klenk, H.D. Intracellular distribution of NS1 correlates with the infectivity and interferon antagonism of an avian influenza virus (H7N1). J. Virol. 2010, 84, 11858–11865. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Lu, X.; Li, J.; Berube, N.; Giest, K.-L.; Liu, Q.; Anderson, D.H.; Zhou, Y. Genetically engineered, biarsenically labeled influenza virus allows visualization of viral NS1 protein in living cells. J. Virol. 2010, 84, 7204–7213. [Google Scholar] [CrossRef] [Green Version]
- Mok, B.-W.; Liu, H.; Chen, P.; Liu, S.; Lau, S.-Y.; Huang, X.; Liu, Y.-C.; Wang, P.; Yuen, K.-Y.; Chen, H. The role of nuclear NS1 protein in highly pathogenic H5N1 influenza viruses. Microbes Infect. 2017, 19, 587–596. [Google Scholar] [CrossRef]
- Tsai, C.F.; Lin, H.Y.; Hsu, W.L.; Tsai, C.H. The novel mitochondria localization of influenza A virus NS1 visualized by flash labeling. FEBS Open Bio. 2017, 7, 1960–1971. [Google Scholar] [CrossRef] [Green Version]
- Sanchez-Aparicio, M.T.; Ayllon, J.; Leo-Macias, A.; Wolff, T.; Garcia-Sastre, A. Subcellular localizations of RIG-I, TRIM25, and MAVS complexes. J. Virol. 2017, 91, e01155-16. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Dong, X.; He, Z.; Wu, Y.; Zhang, S.; Lin, J.; Yang, Y.; Chen, J.; An, S.; Yin, Y.; et al. Zika virus antagonizes interferon response in patients and disrupts RIG-I-mavs interaction through its CARD-TM domains. Cell Biosci. 2019, 9, 46. [Google Scholar] [CrossRef] [PubMed]
- Pollpeter, D.; Parsons, M.; Sobala, A.E.; Coxhead, S.; Lang, R.D.; Bruns, A.M.; Papaioannou, S.; McDonnell, J.M.; Apolonia, L.; Chowdhury, J.A.; et al. Deep sequencing of HIV-1 reverse transcripts reveals the multifaceted antiviral functions of APOBEC3G. Nat. Microbiol. 2018, 3, 220–233. [Google Scholar] [CrossRef] [PubMed]
- Koshiba, T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta 2013, 1833, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seth, R.B.; Sun, L.; Ea, C.K.; Chen, Z.J. Identification and characterization of MAVS, a mitochondrial antiviral signaling protein that activates NF-KB and IRF 3. Cell 2005, 122, 669–682. [Google Scholar] [CrossRef] [Green Version]
- Shi, Y.; Yuan, B.; Qi, N.; Zhu, W.; Su, J.; Li, X.; Qi, P.; Zhang, D.; Hou, F. An autoinhibitory mechanism modulates MAVS activity in antiviral innate immune response. Nat. Commun. 2015, 6, 7811. [Google Scholar] [CrossRef] [Green Version]
- Tang, E.D.; Wang, C.-Y. Mavs self-association mediates antiviral innate immune signaling. J. Virol. 2009, 83, 3420–3428. [Google Scholar] [CrossRef] [Green Version]
- Yoshinaka, T.; Kosako, H.; Yoshizumi, T.; Furukawa, R.; Hirano, Y.; Kuge, O.; Tamada, T.; Koshiba, T. Structural basis of mitochondrial scaffolds by prohibitin complexes: Insight into a role of the Coiled-Coil region. iScience 2019, 19, 1065–1078. [Google Scholar] [CrossRef]
- Yoshizumi, T.; Ichinohe, T.; Sasaki, O.; Otera, H.; Kawabata, S.-I.; Mihara, K.; Koshiba, T. Influenza A virus protein PB1-F2 translocates into mitochondria via TOM40 channels and impairs innate immunity. Nat. Commun. 2014, 5, 4713. [Google Scholar] [CrossRef]
- Varga, Z.T.; Grant, A.; Manicassamy, B.; Palese, P. Influenza virus protein PB1-F2 INHIBITS the induction of type I interferon by binding to MAVS and decreasing mitochondrial membrane potential. J. Virol. 2012, 86, 8359–8366. [Google Scholar] [CrossRef] [Green Version]
- Boyapalle, S.; Wong, T.; Garay, J.; Teng, M.; Juan-Vergara, H.S.; Mohapatra, S.; Mohapatra, S. Respiratory syncytial virus NS1 protein colocalizes with mitochondrial antiviral signaling protein mavs following infection. PLoS ONE 2012, 7, e29386. [Google Scholar] [CrossRef]
- Nandi, S.; Chanda, S.; Bagchi, P.; Nayak, M.K.; Bhowmick, R.; Chawla-Sarkar, M. Mavs protein is attenuated by rotavirus nonstructural protein 1. PLoS ONE 2014, 9, e92126. [Google Scholar]
- Ma, J.; Ketkar, H.; Geng, T.; Lo, E.; Wang, L.; Xi, J.; Sun, Q.; Zhu, Z.; Cui, Y.; Yang, L.; et al. Zika virus non-structural protein 4A blocks the RLR-MAVS signaling. Front. Microbiol. 2018, 9, 1350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Z.; Zhu, X.; Wen, W.; Yuan, J.; Hu, Y.; Chen, J.; An, S.; Dong, X.; Lin, C.; Yu, J.; et al. Dengue virus subverts host innate immunity by targeting adaptor protein MAVS. J. Virol. 2016, 90, 7219–7230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jensen, S.; Thomsen, A.R. Sensing of RNA viruses: A review of innate immune receptors involved in recognizing RNA virus invasion. J. Virol. 2012, 86, 2900–2910. [Google Scholar] [CrossRef] [Green Version]
- Chow, K.T.; Gale, M., Jr.; Loo, Y.-M. RIG-I and other RNA sensors in antiviral immunity. Annu. Rev. Immunol. 2018, 36, 667–694. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tseng, Y.-Y.; Kuan, C.-Y.; Mibayashi, M.; Chen, C.-J.; Palese, P.; Albrecht, R.A.; Hsu, W.-L. Interaction between NS1 and Cellular MAVS Contributes to NS1 Mitochondria Targeting. Viruses 2021, 13, 1909. https://doi.org/10.3390/v13101909
Tseng Y-Y, Kuan C-Y, Mibayashi M, Chen C-J, Palese P, Albrecht RA, Hsu W-L. Interaction between NS1 and Cellular MAVS Contributes to NS1 Mitochondria Targeting. Viruses. 2021; 13(10):1909. https://doi.org/10.3390/v13101909
Chicago/Turabian StyleTseng, Yeu-Yang, Chih-Ying Kuan, Masaki Mibayashi, Chi-Jene Chen, Peter Palese, Randy A. Albrecht, and Wei-Li Hsu. 2021. "Interaction between NS1 and Cellular MAVS Contributes to NS1 Mitochondria Targeting" Viruses 13, no. 10: 1909. https://doi.org/10.3390/v13101909
APA StyleTseng, Y.-Y., Kuan, C.-Y., Mibayashi, M., Chen, C.-J., Palese, P., Albrecht, R. A., & Hsu, W.-L. (2021). Interaction between NS1 and Cellular MAVS Contributes to NS1 Mitochondria Targeting. Viruses, 13(10), 1909. https://doi.org/10.3390/v13101909





