Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval
Abstract
1. Introduction
- (i)
- Elicit long-lasting protective immune responses;
- (ii)
- Should be given to everyone regardless of comorbidity or age, immune status, pregnancy/breastfeeding status;
- (iii)
- Lack the potential to cause antibody dependent enhancement (ADE) or pulmonary immunopathology;
- (iv)
- Should be thermostable in order to enable transportation and storage in developing countries with poor refrigeration facilities;
- (v)
- Be highly immunogenic in the general population including a population with pre-existing anti-vector antibodies.
2. SARS-CoV-2 Candidate Vaccines with Approved EUA
2.1. The BNT162b2 Vaccine
- (i)
- We do not know whether NGMATs in children (12–17 year) and adults (18–65 years) are similar to those of older adults (>65 years old). It is also not clear whether the vaccine will be tested in children <12 years old.
- (ii)
- We do not know the NGMAT beyond one week after the second immunization; this is very important because in phase I trial, there was a slight reduction in NGMAT, two weeks after the second immunization [33].
- (iii)
- The proportion of participants in the whole trial that were used to assess the efficacy of >94% is not known.
- (iv)
- It is not clear whether the 162 cases of COVID-19 (and 9 severe cases) detected in the placebo group were within a certain age group, race/ethnicity, or had prior SARS-CoV-2 infection.
- (v)
- It is not clear whether the vaccine was tested in participants with comorbidities, which is a group in dire need of a COVID-19 vaccine.
2.2. The mRNA-1273 Vaccine
- (i)
- We do not know whether the participants in the vaccine group who had COVID-19 or severe cases of the disease had lower NGMATs compared to those that did not have the disease. Thus, data comparing NGMAT in these two groups are warranted. It would also be nice to know the percentage of adults, 18–55 years old, in the vaccine group that had COVID-19 compared to older adults, 56–70 years old. This is very important because in phase I trial, NGMAT in older adults (immunized with same dose of antigen/schedule as in phase II/III) seemed to be slightly higher (878) [36] compared to those in 18–55 years old (654.3) [35].
- (ii)
- We do not know what percentage of participants with comorbidities had COVID-19 (if any) in the vaccine group.
- (iii)
- Data on the percentage of individuals that were seropositive, for SARS-CoV-2, after the second dose are not known. This information is very important because it will shed light on how many participants within a group (population) will respond, immunogenically, to the vaccine.
- (iv)
- We do not know whether the NGMAT will change three weeks (and beyond) after the second immunization and/or whether the number of COVID-19 cases in the vaccine group will increase. So far, we have data only on the number of COVID-19 cases, two weeks after the second dose.
- (v)
- The proportion of participants in the whole trial that were used to assess the efficacy of 94.5% is not known. A larger proportion size will reflect reliable data and vice versa.
- (vi)
- Although the study included participants from diverse racial/ethnic backgrounds, the study was conducted only in one country (the U.S.) and did not include children and young adults < 18 years old. Data from other geographic regions around the world and from an age group < 18 years are needed.
3. SARS-CoV-2 Candidate Vaccines in Phase II/III Trials
3.1. Inactivated SARS-CoV-2 Vaccines
3.1.1. CoronaVac
- (i)
- Data showing NGMAT in individuals with comorbidities and from different ethnic/racial backgrounds; the phase II trial was conducted only on a healthy group of participants and the ongoing phase III trial (Table 1) excludes individuals with an immunodeficient immune system. Thus, it is not clear how individuals with comorbidities (in dire need of SARS-CoV-2 vaccine) or patients with a compromised immune system will respond to CoronaVac.
- (ii)
- Data showing NGMATs in different age groups. It is not clear whether the immunogenicity of CoronaVac decreases with age as has been observed with other SARS-CoV-2 candidate vaccines [31,33,35,44,45]. In other words, do older adults (50–59 years old) mount a lower response to the vaccine compared to younger adults (18–29 years old)?
- (iii)
- Data demonstrating the immunogenicity of the vaccine in older adults (>60 years, who are in dire need of a SARS-CoV-2 vaccine). We do not know how immunogenic the vaccine will be in people in this age group. If immune responses in the 50–59 years old group are lower than those in the 18–29 years old group, a dose of more than 6 μg/dose of CoronaVac may be required to elicit a strong immune response in adults who are >60 years old. Thus, in phase III trial, the first priority should evaluate 6 μg dose (instead of 3 μg as indicated in Zhang et al., [30]), at 28 days schedule, given the fact that this dose had minor adverse effects (e.g., pain at injection site), which were not different from the 3 μg dose.
- (iv)
- Data showing the longevity of neutralizing antibody titers beyond 28 days; phase II neutralizing antibody titers were conducted using sera collected 28 days (after the second dose). Thus, it is not clear how long the neutralizing antibody titers will last.
- (v)
- Assess the efficacy and safety of the vaccine in an age group, which is 9–17 years old. As mentioned above, CoronaVac is an inactivated vaccine formulated with the adjuvant, aluminum hydroxide; unfortunately, some inactivated viral vaccines against respiratory diseases (e.g., respiratory syncytia virus [46]) formulated with aluminum adjuvant have been associated, in a few cases, with vaccine-associated enhanced viral disease such as pulmonary immunopathology. Although pulmonary immunopathology, in non-human primates or human primates, has not been reported for CoronaVac or any SARS-CoV-2 vaccine, it has been reported in preclinical studies with SARS-CoV and MERS [47].
- (vi)
- Assess the potential for long-term adverse effect(s). Preclinical studies in non-human primates did not show that CoronaVac can promote ADE [48]. However, it is not known whether the same will be true once antibody titers wane.
- (vii)
- Determine whether the NGMATs (44.1 and 65.4) elicited by the vaccine will offer efficient protection. Preclinical studies of the vaccine with non-human primates showed that a lower NGMAT (of 24) offers complete protection from SARS-CoV-2 infection [48]; however, the titers in clinical trials (44.1 and 65.4) are lower than those in convalescent sera from patients who have recovered from COVID-19 (with NGMAT of at least 70) [33,49,50].
- (viii)
- Data showing the efficacy of the vaccine in a larger number of participants. Phase II trials were conducted only with 600 participants, which is a very small proportion of the general population.
3.1.2. Inactivated SARS-CoV-2 and BBIBP-CorV
3.2. Replication Deficient Vector Vaccines
3.2.1. Ad5 nCoV
3.2.2. Sputnik V (Gam-COVID-Vac)
- (i)
- Determine the efficacy of the vaccine in a group with high (>200) pre-existing anti-Ad26 and Ad5-neutralizing antibody titers. Phase I/II studies were conducted in participants with low pre-existing anti-Ad-neutralizing antibody titers (~25); 43–67% of people in some African countries, ~54% in Thailand, and 5.4–17.8% of people in other regions around the world have pre-existing anti-Ad26 antibodies [53]. It is worth noting that some of these people have neutralizing antibody titers of 200–1000.
- (ii)
- It is also recommended that a larger number of participants be included in future studies as well as a control or placebo group; phase I/II studies did not have a control group and the number of participants were very low.
- (iii)
- Assess the efficacy and safety of the vaccine in other age groups, <18 years and >60 years.
3.2.3. ChAdOx1 nCoV-19
- (i)
- Assess the efficacy of the vaccine in a population that has high levels (>200) of pre-existing anti-ChAdOx1-neutralizing antibody titers. In a phase I/II trial, only one participant had high levels (>200) of pre-existing anti-ChAdOx1-neutralizing antibody titers. Pre-existing antibodies against chimpanzee adenoviruses in the western world are very low and may not affect the efficacy of the vaccine. However, in countries (especially African countries) with natural habitats for chimpanzee, pre-existing antibodies against chimpanzee adenoviruses are high in the human population [56,57]. Thus, the efficacy of the vaccine in this group of individuals is warranted.
- (ii)
- Assess the efficacy of ChAdOx1 nCoV-19 vaccine in age groups, <18 years and >56 years.
3.2.4. Ad26.COV2.S (JNJ-78436735 or Ad26COVS1)
- (i)
- Assess the efficacy of the vaccine in a population that has high levels (>200) of pre-existing anti-Ad26-neutralizing antibody titers. Seropositivity/titers for Ad26 vector in participants were not reported and thus it is difficult to assess whether the vaccine will be immunogenic in a population with high titer anti-Ad26-neutralizing antibodies.
- (ii)
- It is recommended that a larger number of participants, especially individuals ≥65 years old, be included in future studies.
3.3. Recombinant Protein Vaccine
NVX-CoV2373
4. Outlook and Perspectives for the Future
5. Limitations in Efficacy Data between Vaccine Trials
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Lu, H.; Stratton, C.W.; Tang, Y.W. Outbreak of pneumonia of unknown etiology in Wuhan, China: The mystery and the miracle. J. Med. Virol. 2020, 92, 401–402. [Google Scholar] [CrossRef] [PubMed]
- Hui, D.S.; Azhar, E.I.; Madani, T.A.; Ntoumi, F.; Kock, R.; Dar, O.; Ippolito, G.; McHugh, T.D.; Memish, Z.A.; Drosten, C.; et al. The continuing 2019-nCoV epidemic threat of novel coronaviruses to global health—The latest 2019 novel coronavirus outbreak in Wuhan, China. Int. J. Infect. Dis. 2020, 91, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- JHU. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). Available online: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6 (accessed on 1 December 2020).
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef]
- Chen, N.; Zhou, M.; Dong, X.; Qu, J.; Gong, F.; Han, Y.; Qiu, Y.; Wang, J.; Liu, Y.; Wei, Y.; et al. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: A descriptive study. Lancet 2020, 395, 507–513. [Google Scholar] [CrossRef]
- Li, R.; Tian, J.; Yang, F.; Lv, L.; Yu, J.; Sun, G.; Ma, Y.; Yang, X.; Ding, J. Clinical characteristics of 225 patients with COVID-19 in a tertiary Hospital near Wuhan, China. J. Clin. Virol. 2020, 127, 104363. [Google Scholar] [CrossRef]
- Kaushik, S.; Aydin, S.I.; Derespina, K.R.; Bansal, P.B.; Kowalsky, S.; Trachtman, R.; Gillen, J.K.; Perez, M.M.; Soshnick, S.H.; Conway, E.E., Jr.; et al. Multisystem Inflammatory Syndrome in Children Associated with Severe Acute Respiratory Syndrome Coronavirus 2 Infection (MIS-C): A Multi-institutional Study from New York City. J. Pediatrics 2020, 224, 24–29. [Google Scholar] [CrossRef]
- Kaushik, S.; Ahluwalia, N.; Gangadharan, S.; Esperenza, M.; Murthy, R.; Ofori-Amanfo, G.; Aydin, S.I. ECMO support in SARS-CoV2 multisystem inflammatory syndrome in children in a child. Perfusion 2020, 267659120954386. [Google Scholar] [CrossRef]
- Flores, G.; Kumar, J.I.; Pressman, E.; Sack, J.; Alikhani, P. Spontaneous Brainstem Hemorrhagic Stroke in the Setting of Novel Coronavirus Disease 2019—A Case Report. Cureus 2020, 12, e10809. [Google Scholar] [CrossRef]
- Dakay, K.; Cooper, J.; Bloomfield, J.; Overby, P.; Mayer, S.A.; Nuoman, R.; Sahni, R.; Gulko, E.; Kaur, G.; Santarelli, J.; et al. Cerebral Venous Sinus Thrombosis in COVID-19 Infection: A Case Series and Review of The Literature. J. Stroke Cerebrovasc. Dis. 2020, 30, 105434. [Google Scholar] [CrossRef]
- Webb Hooper, M.; Napoles, A.M.; Perez-Stable, E.J. COVID-19 and Racial/Ethnic Disparities. JAMA 2020. [Google Scholar] [CrossRef]
- CDC. COVID-19 Hospitalization and Death by Race/Ethnicity. Available online: https://www.cdc.gov/coronavirus/2019-ncov/covid-data/investigations-discovery/hospitalization-death-by-race-ethnicity.html (accessed on 30 November 2020).
- Tian, X.; Li, C.; Huang, A.; Xia, S.; Lu, S.; Shi, Z.; Lu, L.; Jiang, S.; Yang, Z.; Wu, Y.; et al. Potent binding of 2019 novel coronavirus spike protein by a SARS coronavirus-specific human monoclonal antibody. Emerg. Microbes. Infect. 2020, 9, 382–385. [Google Scholar] [CrossRef]
- Yan, R.; Zhang, Y.; Li, Y.; Xia, L.; Guo, Y.; Zhou, Q. Structural basis for the recognition of SARS-CoV-2 by full-length human ACE2. Science 2020, 367, 1444–1448. [Google Scholar] [CrossRef]
- Lan, J.; Ge, J.; Yu, J.; Shan, S.; Zhou, H.; Fan, S.; Zhang, Q.; Shi, X.; Wang, Q.; Zhang, L.; et al. Structure of the SARS-CoV-2 spike receptor-binding domain bound to the ACE2 receptor. Nature 2020, 581, 215–220. [Google Scholar] [CrossRef]
- Shang, J.; Wan, Y.; Luo, C.; Ye, G.; Geng, Q.; Auerbach, A.; Li, F. Cell entry mechanisms of SARS-CoV-2. Proc. Natl. Acad. Sci. USA 2020, 117, 11727–11734. [Google Scholar] [CrossRef]
- WHO. Solidarity Therapeutics Trial Produces Conclusive Evidence on the Effectiveness of Repurposed Drugs for COVID-19 in Record Time. Available online: https://www.who.int/news/item/15-10-2020-solidarity-therapeutics-trial-produces-conclusive-evidence-on-the-effectiveness-of-repurposed-drugs-for-covid-19-in-record-time (accessed on 30 November 2020).
- FDA. FDA’s Approval of Veklury (Remdesivir) for the Treatment of COVID-19—The Science of Safety and Effectiveness. Available online: https://www.fda.gov/drugs/drug-safety-and-availability/fdas-approval-veklury-remdesivir-treatment-covid-19-science-safety-and-effectiveness (accessed on 30 November 2020).
- FDA. Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 30 November 2020).
- Sheridan, C. Convalescent serum lines up as first-choice treatment for coronavirus. Nat. Biotechnol. 2020. [Google Scholar] [CrossRef]
- Casadevall, A.; Pirofski, L.A. The convalescent sera option for containing COVID-19. J. Clin. Investig. 2020, 130, 1545–1548. [Google Scholar] [CrossRef]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef]
- FDA. Pfizer-BioNTech COVID-19 Vaccine. Available online: https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/pfizer-biontech-covid-19-vaccine (accessed on 24 December 2020).
- FDA. FDA Takes Additional Action in Fight against COVID-19 by Issuing Emergency Use Authorization for Second COVID-19 Vaccine. Available online: https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid (accessed on 24 December 2020).
- Pfizer. Pfizer and BioNTech Achieve First Authorization in the World for a Vaccine to Combat COVID-19. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-achieve-first-authorization-world (accessed on 24 December 2020).
- Pfizer. Pfizer and BioNTech Receive Authorization in the European Union for COVID-19 Vaccine. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-receive-authorization-european-union (accessed on 24 December 2020).
- PBS. EU Agency Moves Forward Meeting on Moderna COVID-19 Vaccine. Available online: https://www.pbs.org/newshour/health/eu-agency-moves-forward-meeting-on-moderna-covid-19-vaccine (accessed on 24 December 2020).
- Zhang, Y.; Zeng, G.; Pan, H.; Li, C.; Hu, Y.; Chu, K.; Han, W.; Chen, Z.; Tang, R.; Yin, W.; et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine in healthy adults aged 18-59 years: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Zhu, F.C.; Guan, X.H.; Li, Y.H.; Huang, J.Y.; Jiang, T.; Hou, L.H.; Li, J.X.; Yang, B.F.; Wang, L.; Wang, W.J.; et al. Immunogenicity and safety of a recombinant adenovirus type-5-vectored COVID-19 vaccine in healthy adults aged 18 years or older: A randomised, double-blind, placebo-controlled, phase 2 trial. Lancet 2020, 396, 479–488. [Google Scholar] [CrossRef]
- Logunov, D.Y.; Dolzhikova, I.V.; Zubkova, O.V.; Tukhvatullin, A.I.; Shcheblyakov, D.V.; Dzharullaeva, A.S.; Grousova, D.M.; Erokhova, A.S.; Kovyrshina, A.V.; Botikov, A.G.; et al. Safety and immunogenicity of an rAd26 and rAd5 vector-based heterologous prime-boost COVID-19 vaccine in two formulations: Two open, non-randomised phase 1/2 studies from Russia. Lancet 2020, 396, 887–897. [Google Scholar] [CrossRef]
- Walsh, E.E.; Frenck, R.W., Jr.; Falsey, A.R.; Kitchin, N.; Absalon, J.; Gurtman, A.; Lockhart, S.; Neuzil, K.; Mulligan, M.J.; Bailey, R.; et al. Safety and Immunogenicity of Two RNA-Based Covid-19 Vaccine Candidates. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Pfizer. Pfizer and BioNTech Conclude Phase 3 Study of COVID-19 Vaccine Candidate, Meeting All Primary Efficacy Endpoints. Available online: https://www.pfizer.com/news/press-release/press-release-detail/pfizer-and-biontech-conclude-phase-3-study-covid-19-vaccine (accessed on 25 November 2020).
- Jackson, L.A.; Anderson, E.J.; Rouphael, N.G.; Roberts, P.C.; Makhene, M.; Coler, R.N.; McCullough, M.P.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; et al. An mRNA Vaccine against SARS-CoV-2—Preliminary Report. N. Engl. J. Med. 2020, 383, 1920–1931. [Google Scholar] [CrossRef]
- Anderson, E.J.; Rouphael, N.G.; Widge, A.T.; Jackson, L.A.; Roberts, P.C.; Makhene, M.; Chappell, J.D.; Denison, M.R.; Stevens, L.J.; Pruijssers, A.J.; et al. Safety and Immunogenicity of SARS-CoV-2 mRNA-1273 Vaccine in Older Adults. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Moderna. Moderna’s COVID-19 Vaccine Candidate Meets Its Primary Efficacy Endpoint in the First Interim Analysis of the Phase 3 COVE Study. Available online: https://investors.modernatx.com/news-releases/news-release-details/modernas-covid-19-vaccine-candidate-meets-its-primary-efficacy (accessed on 27 November 2020).
- ClinicalTrials.gov. COVID-19 Vaccine, SARS COV-2 Vaccine. Available online: https://clinicaltrials.gov/ct2/results?cond=Covid19+vaccine&age_v=&gndr=&type=&rslt=&Search=Apply (accessed on 18 November 2020).
- WHO. Draft Landscape of COVID-19 Candidate Vaccines. Available online: https://www.who.int/publications/m/item/draft-landscape-of-covid-19-candidate-vaccines (accessed on 18 November 2020).
- Le, T.T.; Cramer, J.P.; Chen, R.; Mayhew, S. Evolution of the COVID-19 vaccine development landscape. Nat. Rev. Drug Discov. 2020. [Google Scholar] [CrossRef]
- Krammer, F. SARS-CoV-2 vaccines in development. Nature 2020, 586, 516–527. [Google Scholar] [CrossRef]
- Poland, G.A.; Ovsyannikova, I.G.; Kennedy, R.B. SARS-CoV-2 immunity: Review and applications to phase 3 vaccine candidates. Lancet 2020, 396, 1595–1606. [Google Scholar] [CrossRef]
- Prasad, A.N.; Borisevich, V.; Woolsey, C.; Agans, K.N.; Deer, D.J.; Dobias, N.S.; Geisbert, J.B.; Fenton, K.A.; Geisbert, T.W. Use of Convalescent Serum Reduces Severity of COVID-19 in Nonhuman Primates. Available online: https://www.biorxiv.org/content/10.1101/2020.10.14.340091v1 (accessed on 30 November 2020).
- Xia, S.; Zhang, Y.; Wang, Y.; Wang, H.; Yang, Y.; Gao, G.F.; Tan, W.; Wu, G.; Xu, M.; Lou, Z.; et al. Safety and immunogenicity of an inactivated SARS-CoV-2 vaccine, BBIBP-CorV: A randomised, double-blind, placebo-controlled, phase 1/2 trial. Lancet. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Sadoff, J.; Le Gars, M.; Shukarev, G.; Heerwegh, D.; Truyers, C.; de Groot, A.M.; Stoop, J.; Tete, S.; Van Damme, W.; Leroux-Roels, I.; et al. Safety and Immunogenicity of the Ad26.COV2.S COVID-19 Vaccine Candidate: Interim Results of a Phase 1/2a, Double-blind, Randomized, Placebo-Controlled Trial. Available online: https://www.medrxiv.org/content/10.1101/2020.09.23.20199604v1 (accessed on 1 December 2020).
- Fulginiti, V.A.; Eller, J.J.; Sieber, O.F.; Joyner, J.W.; Minamitani, M.; Meiklejohn, G. Respiratory virus immunization. I. A field trial of two inactivated respiratory virus vaccines; an aqueous trivalent parainfluenza virus vaccine and an alum-precipitated respiratory syncytial virus vaccine. Am. J. Epidemiol. 1969, 89, 435–448. [Google Scholar] [CrossRef]
- Tseng, C.T.; Sbrana, E.; Iwata-Yoshikawa, N.; Newman, P.C.; Garron, T.; Atmar, R.L.; Peters, C.J.; Couch, R.B. Immunization with SARS coronavirus vaccines leads to pulmonary immunopathology on challenge with the SARS virus. PLoS ONE 2012, 7, e35421. [Google Scholar] [CrossRef]
- Gao, Q.; Bao, L.; Mao, H.; Wang, L.; Xu, K.; Yang, M.; Li, Y.; Zhu, L.; Wang, N.; Lv, Z.; et al. Development of an inactivated vaccine candidate for SARS-CoV-2. Science 2020, 369, 77–81. [Google Scholar] [CrossRef]
- Wang, X.; Guo, X.; Xin, Q.; Pan, Y.; Hu, Y.; Li, J.; Chu, Y.; Feng, Y.; Wang, Q. Neutralizing Antibodies Responses to SARS-CoV-2 in COVID-19 Inpatients and Convalescent Patients. Clin. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Keech, C.; Albert, G.; Cho, I.; Robertson, A.; Reed, P.; Neal, S.; Plested, J.S.; Zhu, M.; Cloney-Clark, S.; Zhou, H.; et al. Phase 1-2 Trial of a SARS-CoV-2 Recombinant Spike Protein Nanoparticle Vaccine. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Xia, S.; Duan, K.; Zhang, Y.; Zhao, D.; Zhang, H.; Xie, Z.; Li, X.; Peng, C.; Zhang, Y.; Zhang, W.; et al. Effect of an Inactivated Vaccine Against SARS-CoV-2 on Safety and Immunogenicity Outcomes: Interim Analysis of 2 Randomized Clinical Trials. JAMA 2020, 324, 951–960. [Google Scholar] [CrossRef]
- Fausther-Bovendo, H.; Kobinger, G.P. Pre-existing immunity against Ad vectors: Humoral, cellular, and innate response, what’s important? Hum. Vaccin. Immunother. 2014, 10, 2875–2884. [Google Scholar] [CrossRef]
- Barouch, D.H.; Kik, S.V.; Weverling, G.J.; Dilan, R.; King, S.L.; Maxfield, L.F.; Clark, S.; Ng’ang’a, D.; Brandariz, K.L.; Abbink, P.; et al. International seroepidemiology of adenovirus serotypes 5, 26, 35, and 48 in pediatric and adult populations. Vaccine 2011, 29, 5203–5209. [Google Scholar] [CrossRef]
- Pilankatta, R.; Chawla, T.; Khanna, N.; Swaminathan, S. The prevalence of antibodies to adenovirus serotype 5 in an adult Indian population and implications for adenovirus vector vaccines. J. Med. Virol. 2010, 82, 407–414. [Google Scholar] [CrossRef]
- Folegatti, P.M.; Ewer, K.J.; Aley, P.K.; Angus, B.; Becker, S.; Belij-Rammerstorfer, S.; Bellamy, D.; Bibi, S.; Bittaye, M.; Clutterbuck, E.A.; et al. Safety and immunogenicity of the ChAdOx1 nCoV-19 vaccine against SARS-CoV-2: A preliminary report of a phase 1/2, single-blind, randomised controlled trial. Lancet 2020, 396, 467–478. [Google Scholar] [CrossRef]
- Xiang, Z.; Li, Y.; Cun, A.; Yang, W.; Ellenberg, S.; Switzer, W.M.; Kalish, M.L.; Ertl, H.C. Chimpanzee adenovirus antibodies in humans, sub-Saharan Africa. Emerg. Infect. Dis. 2006, 12, 1596–1599. [Google Scholar] [CrossRef]
- Dicks, M.D.; Spencer, A.J.; Edwards, N.J.; Wadell, G.; Bojang, K.; Gilbert, S.C.; Hill, A.V.; Cottingham, M.G. A novel chimpanzee adenovirus vector with low human seroprevalence: Improved systems for vector derivation and comparative immunogenicity. PLoS ONE 2012, 7, e40385. [Google Scholar] [CrossRef]
- CDC. Interim Clinical Considerations for Use of mRNA COVID-19 Vaccines Currently Authorized in the United States. Available online: https://www.cdc.gov/vaccines/covid-19/info-by-product/clinical-considerations.html (accessed on 25 December 2020).
- Matthias, D.M.; Robertson, J.; Garrison, M.M.; Newland, S.; Nelson, C. Freezing temperatures in the vaccine cold chain: A systematic literature review. Vaccine 2007, 25, 3980–3986. [Google Scholar] [CrossRef]
- Edstam, J.S.; Dulmaa, N.; Tsendjav, O.; Dambasuren, B.; Densmaa, B. Exposure of hepatitis B vaccine to freezing temperatures during transport to rural health centers in Mongolia. Prev. Med. 2004, 39, 384–388. [Google Scholar] [CrossRef]
- Seelenfreund, E.; Robinson, W.A.; Amato, C.M.; Tan, A.C.; Kim, J.; Robinson, S.E. Long term storage of dry versus frozen RNA for next generation molecular studies. PLoS ONE 2014, 9, e111827. [Google Scholar] [CrossRef]
- Peabody, J.; Muttil, P.; Chackerian, B.; Tumban, E. Characterization of a spray-dried candidate HPV L2-VLP vaccine stored for multiple years at room temperature. Papillomavirus Res. 2017, 3, 116–120. [Google Scholar] [CrossRef]
- Tumban, E.; Muttil, P.; Escobar, C.A.; Peabody, J.; Wafula, D.; Peabody, D.S.; Chackerian, B. Preclinical refinements of a broadly protective VLP-based HPV vaccine targeting the minor capsid protein, L2. Vaccine 2015, 33, 3346–3353. [Google Scholar] [CrossRef]
- Zhai, L.; Yadav, R.; Kunda, N.K.; Anderson, D.; Bruckner, E.; Miller, E.K.; Basu, R.; Muttil, P.; Tumban, E. Oral immunization with bacteriophage MS2-L2 VLPs protects against oral and genital infection with multiple HPV types associated with head & neck cancers and cervical cancer. Antivir. Res. 2019, 166, 56–65. [Google Scholar] [CrossRef]
- Wu, F.; Liu, M.; Wang, A.; Lu, L.; Wang, Q.; Gu, C.; Chen, J.; Wu, Y.; Xia, S.; Ling, Y.; et al. Evaluating the Association of Clinical Characteristics With Neutralizing Antibody Levels in Patients Who Have Recovered From Mild COVID-19 in Shanghai, China. JAMA Intern. Med. 2020, 180, 1356–1362. [Google Scholar] [CrossRef]
- Long, Q.X.; Tang, X.J.; Shi, Q.L.; Li, Q.; Deng, H.J.; Yuan, J.; Hu, J.L.; Xu, W.; Zhang, Y.; Lv, F.J.; et al. Clinical and immunological assessment of asymptomatic SARS-CoV-2 infections. Nat. Med. 2020, 26, 1200–1204. [Google Scholar] [CrossRef]
- NPR. Poll: Americans Are Growing Less Reluctant To Take COVID-19 Vaccine. Available online: https://www.npr.org/sections/coronavirus-live-updates/2020/12/15/946761737/poll-americans-are-growing-less-reluctant-to-take-covid-19-vaccine (accessed on 26 December 2020).
- Bar-On, Y.M.; Flamholz, A.; Phillips, R.; Milo, R. SARS-CoV-2 (COVID-19) by the numbers. Elife 2020, 9. [Google Scholar] [CrossRef]
- Nobusawa, E.; Sato, K. Comparison of the mutation rates of human influenza A and B viruses. J. Virol. 2006, 80, 3675–3678. [Google Scholar] [CrossRef]
- CoV-Glue. Amino Acid Replacements. Available online: http://cov-glue.cvr.gla.ac.uk/#/replacement (accessed on 5 December 2020).
- Korber, B.; Fischer, W.M.; Gnanakaran, S.; Yoon, H.; Theiler, J.; Abfalterer, W.; Hengartner, N.; Giorgi, E.E.; Bhattacharya, T.; Foley, B.; et al. Tracking Changes in SARS-CoV-2 Spike: Evidence that D614G Increases Infectivity of the COVID-19 Virus. Cell 2020, 182, 812–827.e19. [Google Scholar] [CrossRef]
- Rogers, T.F.; Zhao, F.; Huang, D.; Beutler, N.; Burns, A.; He, W.T.; Limbo, O.; Smith, C.; Song, G.; Woehl, J.; et al. Isolation of potent SARS-CoV-2 neutralizing antibodies and protection from disease in a small animal model. Science 2020, 369, 956–963. [Google Scholar] [CrossRef]
- ECDC. Rapid Increase of a SARS-CoV-2 Variant with Multiple Spike Protein Mutations Observed in the United Kingdom. Available online: https://www.ecdc.europa.eu/sites/default/files/documents/SARS-CoV-2-variant-multiple-spike-protein-mutations-United-Kingdom.pdf (accessed on 25 December 2020).
- Szebeni, J. Complement activation-related pseudoallergy: A new class of drug-induced acute immune toxicity. Toxicology 2005, 216, 106–121. [Google Scholar] [CrossRef]
- Mohamed, M.; Abu Lila, A.S.; Shimizu, T.; Alaaeldin, E.; Hussein, A.; Sarhan, H.A.; Szebeni, J.; Ishida, T. PEGylated liposomes: Immunological responses. Sci. Technol. Adv. Mater. 2019, 20, 710–724. [Google Scholar] [CrossRef]
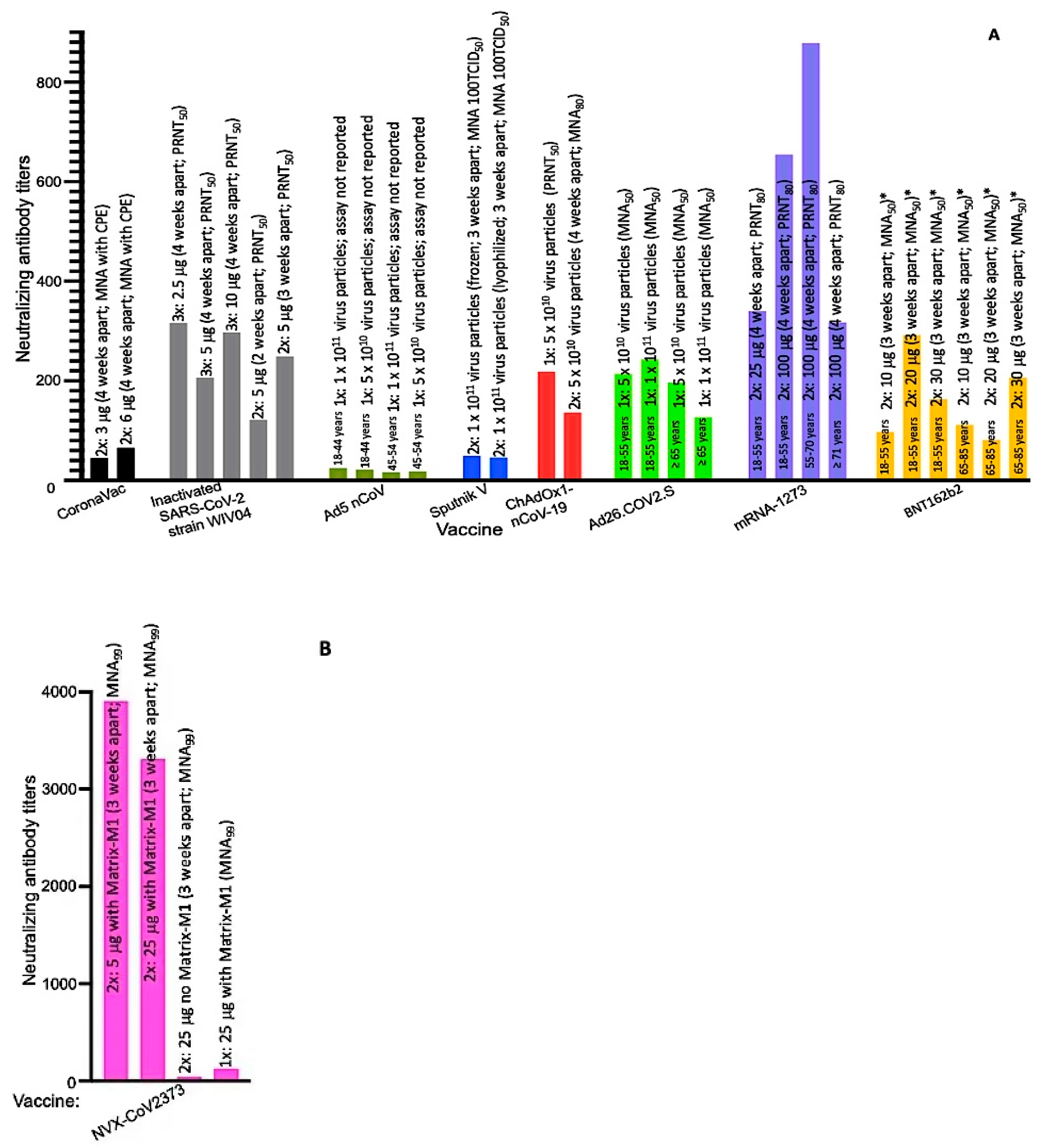
| Developer | Vaccine Name (Component) | Storage Temp. ϕ | Required Doses | Weeks Apart | Age Group (Years) | Exclusion Criteria from Studies | Clinical Trial Registration Numbers |
|---|---|---|---|---|---|---|---|
| Inactivated Vaccines | |||||||
| Sinovac | CoronaVac (inactivated SARS-CoV-2 in aluminum hydroxide adjuvant) | 2–8 °C | 2x: 600 SU/dose * | 2 and 4 | 18–59 | -History of COVID-19 or presence of SARS-CoV-2 antibodies -Pregnant/breastfeeding -Used immunosuppressive agents within 6 months -Immunodeficient (HIV) | NCT04582344 NCT04508075 |
| Sinopharm | Inactivated SARS-CoV-2 (inactivated SARS-CoV-2, strain WIV04, in aluminum hydroxide adjuvant) | 2–8 °C | 2x (dosage not provided) | 3 | ≥18–85 | -History of SARS-CoV-2, SARS-CoV or MERS-CoV infections -Pregnant -Received other investigational CoV vaccines (SARS-CoV and MERS-CoV) -Immunodeficient (HIV) -Receiving anti-TB therapy | NCT04510207 NCT04560881 NCT04510207 NCT04612972 |
| BBIBP-CorV (inactivated SARS-CoV-2, strain HB02, in aluminum hydroxide adjuvant) | |||||||
| Bharat Biotech | BBV152 (inactivated SARS-CoV-2 in aluminum hydroxide gel—imidazoquinoline adjuvant) | 2–8 °C | 2x: 6 μg/dose | 4 | ≥18 | -History of SARS-CoV infection -History of COVID-19 investigational or licensed vaccination -Pregnant/breastfeeding -Immunodeficient (HIV) -Hepatitis B or C infection | NCT04641481 |
| Replication Deficient Vector Vaccines | |||||||
| CanSino | Ad5 nCoV % (replication-deficient Ad type 5 vector expressing full-length spike protein) | 2–8 °C | 1x (VP not provided) ** | N/A | ≥18 | -History of COVID-19 or presence of SARS-CoV-2 antibodies -Pregnant/breastfeeding -Using immunosuppressive agents or immunodeficient -Adenovirus vectored vaccines | NCT04526990 NCT04540419 |
| Gamaleya National Center of Epidemiology and Microbiology | Sputnik V €,# or Gam-COVID-Vac (combined replication-deficient Ad types 5 and 26 vectors each expressing full-length spike protein) | Frozen version (−18 °C) and lyophilized version (2–8 °C) | 2x (prime with rAd26-S and boost with rAd5-S): VP dosage not provided *** | 3 | ≥18 | -History of COVID-19 or presence of SARS-CoV-2 antibodies -Pregnant/breastfeeding -Immunosuppressive agents within 3 months -Immunodeficient (HIV) -Tuberculosis | NCT04564716 NCT04530396 |
| AstraZeneca | ChAdOx1 nCoV-19 (replication-deficient Ad type 5 vector expressing full-length spike protein) | 2–8 °C | 1x: 5 × 1010 VP 2x: 5 × 1010 VP and 3.5–6.5 × 1010 VP | 4-12 | 18–55 56–69 ≥70 | -History of COVID-19 or presence of SARS-CoV-2 antibodies -Pregnant/breastfeeding | NCT04536051 NCT04516746 NCT04540393 NCT04400838 |
| Johnson & Johnson/Janssen Pharma | Ad26.COV2.S or JNJ-78436735 (replication-deficient Ad-type 26 vector expressing full-length spike protein) | 2–8 °C | 1x: 5 × 1010 VP | N/A | ≥18 | -Previous vaccination with CoV vaccine -Received investigational adenoviral-vectored vaccines within 6 months | NCT04505722 |
| mRNA Vaccines | |||||||
| Moderna | mRNA-1273 ∑ (mRNA of full-length spike protein in a lipid nanoparticle) | Frozen between −25 and −15 °C | 2x: 100 μg each | 4 | ≥18 | -History of SARS-CoV-2 infection -Pregnant/breastfeeding -Received other investigational CoV vaccines (SARS-CoV and MERS-CoV) -Received systemic Immunosuppressants for >14 days within 6 months | NCT04470427 |
| Pfizer and BioNTech | BNT162b2 ∑ (mRNA of full-length spike protein in a lipid nanoparticle) | Frozen at −70 °C ± 10 °C | 2x: 30 μg each | 3 | ≥12 | -Symptoms/diagnosis of COVID 19 by NAAT $ -Pregnant/breastfeeding -Immunocompromised -Previous vaccination with CoV vaccine -Immunosuppressive therapy Note: Positive serological test for SARS-CoV-2 was not excluded | NCT04368728 Protocol C4591001 |
| Recombinant Protein Vaccine | |||||||
| Novavax | NVX-CoV2373 (a “nanoparticle” of trimeric full-length recombinant spike protein formulated in Matrix-M1 adjuvant) | 2–8 °C | 2x: 5 µg SARS-CoV-2 rS + 50 µg Matrix-M1 adjuvant/dose | 3 | 18–84 | -History of COVID-19 or presence of SARS-CoV-2 antibodies -Pregnant/breastfeeding -Immunosuppressive or immunodeficient status | NCT04583995 NCT04611802 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tumban, E. Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval. Viruses 2021, 13, 54. https://doi.org/10.3390/v13010054
Tumban E. Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval. Viruses. 2021; 13(1):54. https://doi.org/10.3390/v13010054
Chicago/Turabian StyleTumban, Ebenezer. 2021. "Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval" Viruses 13, no. 1: 54. https://doi.org/10.3390/v13010054
APA StyleTumban, E. (2021). Lead SARS-CoV-2 Candidate Vaccines: Expectations from Phase III Trials and Recommendations Post-Vaccine Approval. Viruses, 13(1), 54. https://doi.org/10.3390/v13010054





