Semipersistently Transmitted, Phloem Limited Plant Viruses Are Inoculated during the First Subphase of Intracellular Stylet Penetrations in Phloem Cells
Abstract
1. Introduction
2. Materials and Methods
2.1. Plants, Aphid and Virus Maintenance
2.2. EPG Setup and Transmission Tests
2.3. Determining Phloem-pd Occurrence
2.4. Duration of Aphid Stylet Phloem Intrusion
2.5. Statistical Analysis
3. Results
3.1. Aphid Stylet Activities and BYV Transmission
3.2. Duration of Phloem-pd Subphases
3.3. BYV Transmission in Association with the Duration of Subphase II-1
3.4. Identification of the Phloem-pd
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fereres, A. Aphid behavior and the transmission of noncirculative viruses. In Vector-Mediated Transmission of Plant Pathogens; Brown, J.K., Ed.; APS: St Paul, MN, USA, 2016; pp. 31–45. [Google Scholar] [CrossRef]
- Mauck, K.E.; Chesnais, Q.; Shapiro, L.R. Evolutionary determinants of host and vector manipulation by plant viruses. Adv. Virus Res. 2018, 101, 189–250. [Google Scholar] [CrossRef]
- Scheller, H.V.; Shukle, R.H. Feeding behavior and transmission of Barley yellow dwarf virus by Sitobion avenae on oats. Entomol. Exp. Appl. 1986, 40, 189–195. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electronic recording of penetration behavior by aphids. Entomol. Exp. Appl. 1978, 24, 521–530. [Google Scholar] [CrossRef]
- Prado, E.; Tjallingii, W.F. Aphid activities during sieve elements punctures. Entomol. Exp. Appl. 1994, 72, 157–165. [Google Scholar] [CrossRef]
- Martín, B.; Collar, J.L.; Tjallingii, W.F.; Fereres, A. Intracellular ingestion and salivation by aphids may cause the acquisition and inoculation of non-persistently transmitted plant viruses. J. Gen. Virol. 1997, 78, 2701–2705. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Membrane potentials as an indicator for plant-cell penetration by aphid stylets. Entomol. Exp. Appl. 1985, 38, 187–193. [Google Scholar] [CrossRef]
- Moreno, A.; Tjallingii, W.F.; Fernández-Mata, G.; Fereres, A. Differences in the mechanism of inoculation between a semi-persistent and non-persistent aphid-transmitted plant virus. J. Gen. Virol. 2012, 93, 662–667. [Google Scholar] [CrossRef]
- Powell, G. Intracellular salivation is the aphid activity associated with inoculation of non-persistently transmitted viruses. J. Gen. Virol. 2005, 86, 469–472. [Google Scholar] [CrossRef]
- Powell, G.; Pirone, T.; Hardie, J. Aphid stylet activities during potyvirus acquisition from plants and an in vitro system that correlate with subsequent transmission. Eur. J. Plant Pathol. 1995, 101, 411–420. [Google Scholar] [CrossRef]
- Agranovsky, A.A.; Koonin, E.V.; Boyko, V.P.; Maiss, E.; Frötschl, R.; Lunina, N.A.; Atabekov, J.G. Beet yellows closterovirus: Complete genome structure and identification of a leader papain-like thiol protease. Virology 1994, 198, 311–324. [Google Scholar] [CrossRef]
- Dolja, V. Beet yellows virus: The importance of being different. Mol. Plant Pathol. 2003, 4, 91–98. [Google Scholar] [CrossRef]
- Nault, L.R. Arthropod transmission of plant viruses: A new synthesis. Ann. Entomol. Soc. Am. 1997, 90, 522–541. [Google Scholar] [CrossRef]
- Pirone, T.P.; Blanc, S. Helper-dependent vector transmission of plant viruses. Annu. Rev. Phytopathol. 1996, 34, 227–247. [Google Scholar] [CrossRef]
- Ng, J.C.K.; Zhou, J.S. Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: Current perspectives and future challenges. Curr. Opin. Virol. 2015, 15, 48–55. [Google Scholar] [CrossRef]
- Chen, A.Y.S.; Walker, G.P.; Carter, D.; Ng, J.C.K. A virus capsid Protein component mediates virion and transmission by its insect vector. Proc. Natl. Acad. Sci. USA 2011, 108, 16777–16782. [Google Scholar] [CrossRef]
- Killiny, N.; Harper, S.J.; Alfaress, S.; El Mohtar, C.; Dawson, W.O. Minor coat and heat-shock proteins are involved in binding of Citrus tristeza virus to the foregut of its aphid vector, Toxoptera citricida. Appl. Environ. Microbiol. 2016, 82, 6294–6302. [Google Scholar] [CrossRef]
- Alzhanova, D.V.; Prokhnevsky, A.I.; Peremyslov, V.V.; Dolja, V.V. Virions tails of Beet yellows virus: Coordinated assembly by three structural proteins. Virology 2007, 359, 220–226. [Google Scholar] [CrossRef]
- Jiménez, J.; Tjallingii, W.F.; Moreno, A.; Fereres, A. Newly distinguished cell punctures associated with transmission of the semipersistent phloem-limited Beet yellows virus. J. Virol. 2018, 92, e01076-18. [Google Scholar] [CrossRef]
- Jiménez, J.; Garzo, E.; Alba-Tercedor, J.; Moreno, A.; Fereres, A.; Walker, G.P. The phloem-pd: A distinctive brief sieve element stylet puncture prior to sieve element phase of aphid feeding behavior. Arthropod Plant Interact. 2020, 14, 67–78. [Google Scholar] [CrossRef]
- Jiménez, J.; Arias-Martín, M.; Moreno, A.; Garzo, E.; Fereres, A. Barley yellow dwarf virus can be inoculated during brief intracellular punctures in phloem cells before the sieve element continuous salivation phase. Phytopathology 2020, 110, 85–93. [Google Scholar] [CrossRef]
- Clark, M.F.; Adams, A.N. Characteristics of the microplate method of enzyme-linked immunosorbent assay for the detection of plant viruses. J. Gen. Virol. 1977, 34, 475–483. [Google Scholar] [CrossRef]
- Tjallingii, W.F.; Garzo, E.; Fereres, A. New structure in cell puncture activities by aphid stylets: A Dual-Mode EPG study. Entomol. Exp. Appl. 2010, 135, 193–207. [Google Scholar] [CrossRef]
- Harris, K.F. An ingestion-egestion hypothesis of noncirculative virus transmission. In Aphids as Virus Vectors; Harris, K.F., Maramorosch, K., Eds.; Academic Press: New York, NY, USA, 1977; pp. 165–220. [Google Scholar] [CrossRef]
- Harris, K.F.; Harris, L.J. Ingestion-egestion theory of cuticula-borne virus transmission. In Virus-Insect-Plant Interactions; Harris, K.F., Smith, O.P., Duffus, J.E., Eds.; Academic Press: New York, NY, USA, 2001; pp. 111–132. [Google Scholar] [CrossRef]
- IBM Corp. IBM SPSS Statistics for Macintosh; Version 25.0; IBM Corp: Armonk, NY, USA, 2017; Available online: https://www.ibm.com/support/pages/downloading-ibm-spss-statistics-25 (accessed on 2 August 2020).
- Uzest, M.; Gargani, D.; Dombrovsky, A.; Cazevieille, C.; Cot, D.; Blanc, S. The “acrostyle”: A newly described anatomical structure in aphid stylets. Arthropod Struct. Dev. 2010, 39, 221–229. [Google Scholar] [CrossRef]
- Tjallingii, W.F. Electrical recording of stylet penetration activities. In Aphids: Their Biology. Nature Enemies and Control; Minks, A.K., Harrewijn, P.J., Eds.; Elsevier: Amsterdam, The Netherlands, 1988; Volume 2B, pp. 95–108. Available online: https://research.wur.nl/en/publications/electrical-recording-of-stylet-penetration-activities (accessed on 2 October 2020).
- Bradley, R.H.E.; Sylvester, E.S. Do aphids carry transmissible sugar Beet yellows virus via their stylets? Evidence from ultraviolet irradiation. Virology 1962, 17, 599–601. [Google Scholar] [CrossRef]
- Murant, A.F.; Roberts, I.M.; Elnagar, S. Association of virus-like particles with the foregut of the aphid Cavariella aegopodii transmitting the semi-persistent viruses Anthriscus yellows and Parsnip yellow fleck. J. Gen. Virol. 1976, 31, 47–57. [Google Scholar] [CrossRef]
- Stevens, M.; Lacomme, C. Transmission of plant viruses. In Aphids as Crop Pests, 2nd ed.; Van Emden, H.F., Harrington, R., Eds.; CAB International: Oxfordshire, UK, 2018; pp. 323–361. [Google Scholar] [CrossRef]
- Peng, H.C.; Walker, G.P. Sieve element occlusion provides resistance against Aphis gossypii in TGR-1551 melons. Insect Sci. 2018, 1, 33–48. [Google Scholar] [CrossRef]
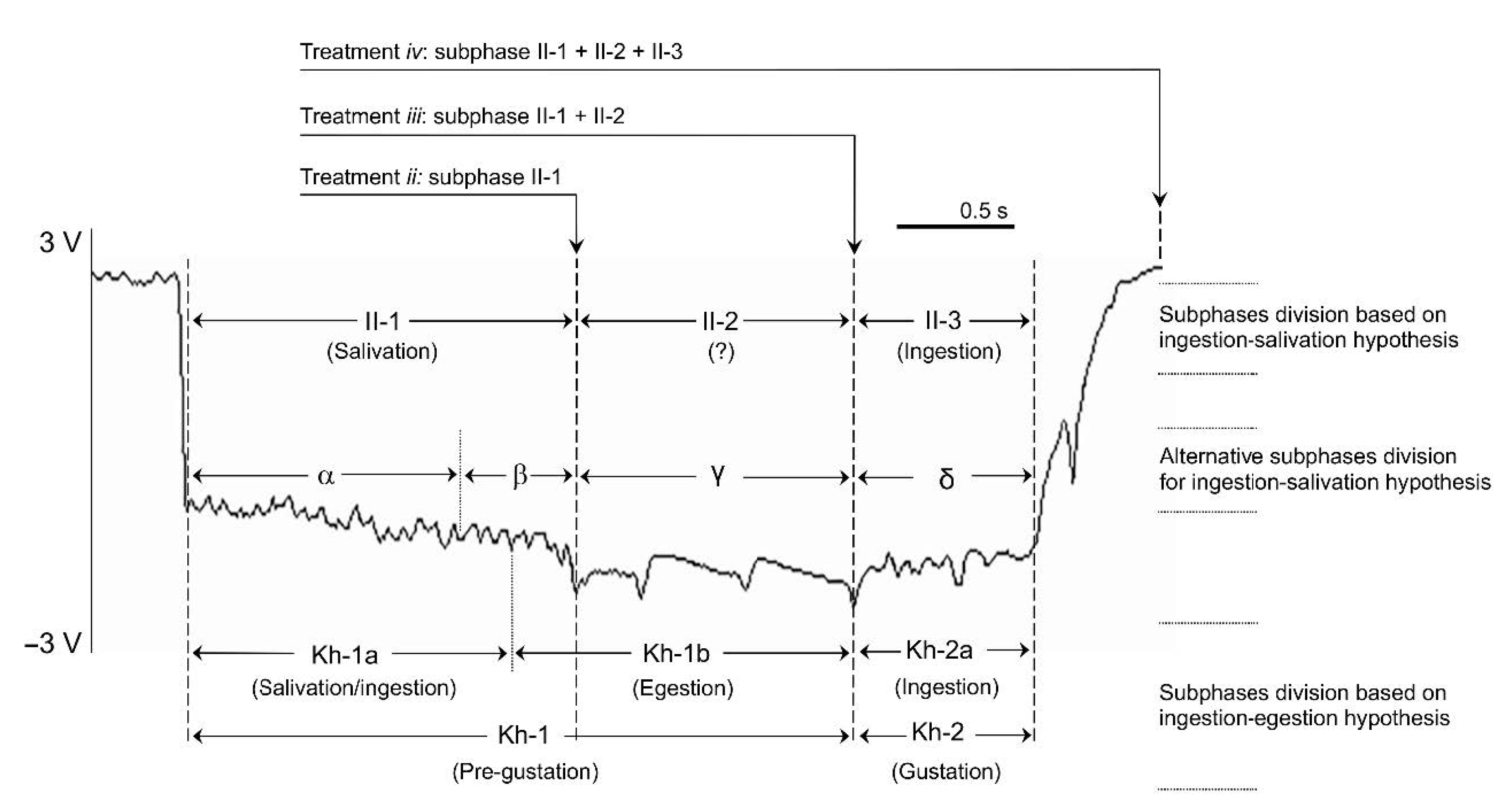


| Aphid Stylet Activities (EPG Waveforms Observed) a | Total EPG Recordings | Discarded Data b | Infected Plants | Total | % BYV Transmission Efficiency d | |
|---|---|---|---|---|---|---|
| Plant A+ (A+ B+ or A+ B−) c | Only Plant B+ | |||||
| (i) A single complete standard-pd | 47 | 29 | 0 | 18 | 0/18 | 0% a |
| (ii) Subphase II-1 of the phloem-pd | 78 | 62 | 9 | 7 | 9/16 | 56% b |
| (iii) Subphase II-2 of the phloem-pd (subphases II-1 + II-2) | 48 | 34 | 10 | 4 | 10/14 | 71% b |
| (iv) A single complete phloem-pd (subphases II-1 + II-2 + II-3) | 50 | 34 | 11 | 5 | 11/16 | 69% b |
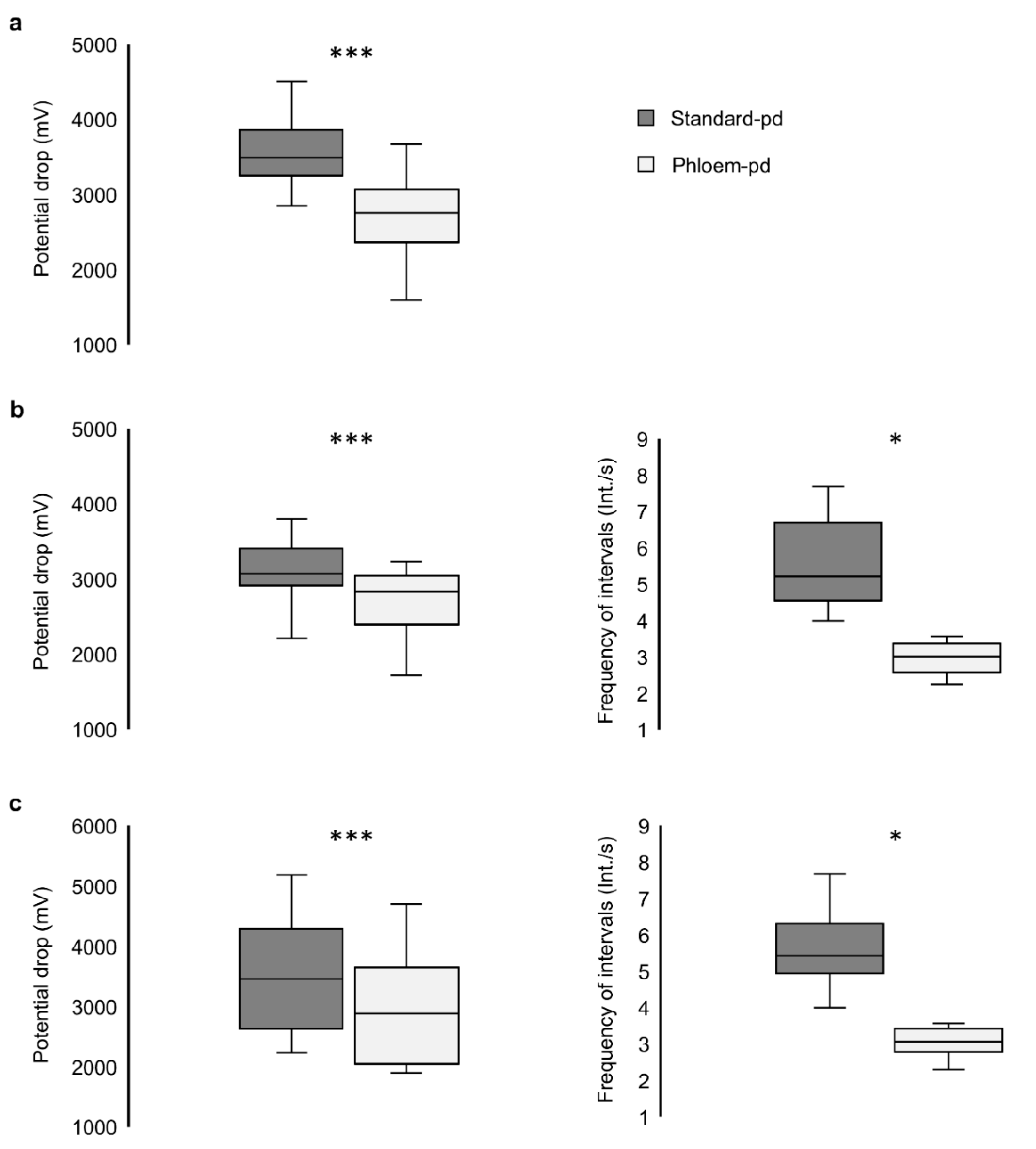
| EPG Recording | Infected Plants | No. of Complete Standard Potential Drops | Lower Subphase II-2 Frequency (Int./s) | % ΔV Phloem-pd (mV) | Duration Subphase II-1 of the Phloem-pd (s) |
|---|---|---|---|---|---|
| 1 | Only A | 37 | 4.17 | 69.24 | 1.34 |
| 2 | Only A | 47 | 5.0 | 84.19 | 0.98 |
| 3 | A + B | 29 | 5.15 | 67.82 | 1.12 |
| 4 | A + B | 34 | 4.34 | 73.93 | 1.36 |
| 5 | A + B | 26 | 5.26 | 67.74 | 1.45 |
| 6 | A + B | 46 | 4.0 | 72.33 | 1.31 |
| 7 | A + B | 28 | 4.29 | 82.17 | 0.76 |
| 8 | A + B | 44 | 4.35 | 81.98 | 0.93 |
| 9 | A + B | 58 | 4.55 | 81.47 | 1.57 |
| 10 | Only B | 73 | 4.17 | 79.33 | 0.78 |
| 11 | Only B | 42 | 4.35 | 83.53 | 1.27 |
| 12 | Only B | 29 | 5.88 | 74.86 | 1.19 |
| 13 | Only B | 42 | 4.55 | 77.54 | 0.87 |
| 14 | Only B | 26 | 4.55 | 83.43 | 1.2 |
| 15 | Only B | 51 | 4.42 | 84.25 | 1.09 |
| 16 | Only B | 24 | 5.41 | 83.04 | 1.28 |
| Mean | - | 39.75 | 4.65 | 77.93 | 1.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiménez, J.; Moreno, A.; Fereres, A. Semipersistently Transmitted, Phloem Limited Plant Viruses Are Inoculated during the First Subphase of Intracellular Stylet Penetrations in Phloem Cells. Viruses 2021, 13, 137. https://doi.org/10.3390/v13010137
Jiménez J, Moreno A, Fereres A. Semipersistently Transmitted, Phloem Limited Plant Viruses Are Inoculated during the First Subphase of Intracellular Stylet Penetrations in Phloem Cells. Viruses. 2021; 13(1):137. https://doi.org/10.3390/v13010137
Chicago/Turabian StyleJiménez, Jaime, Aránzazu Moreno, and Alberto Fereres. 2021. "Semipersistently Transmitted, Phloem Limited Plant Viruses Are Inoculated during the First Subphase of Intracellular Stylet Penetrations in Phloem Cells" Viruses 13, no. 1: 137. https://doi.org/10.3390/v13010137
APA StyleJiménez, J., Moreno, A., & Fereres, A. (2021). Semipersistently Transmitted, Phloem Limited Plant Viruses Are Inoculated during the First Subphase of Intracellular Stylet Penetrations in Phloem Cells. Viruses, 13(1), 137. https://doi.org/10.3390/v13010137







