MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species
Abstract
1. Introduction
2. Materials and Methods
2.1. Genome Sequences
2.2. Potential miR Expression and Link Analysis
2.3. Protein–Protein Network Interaction Analysis for miR Target Proteins
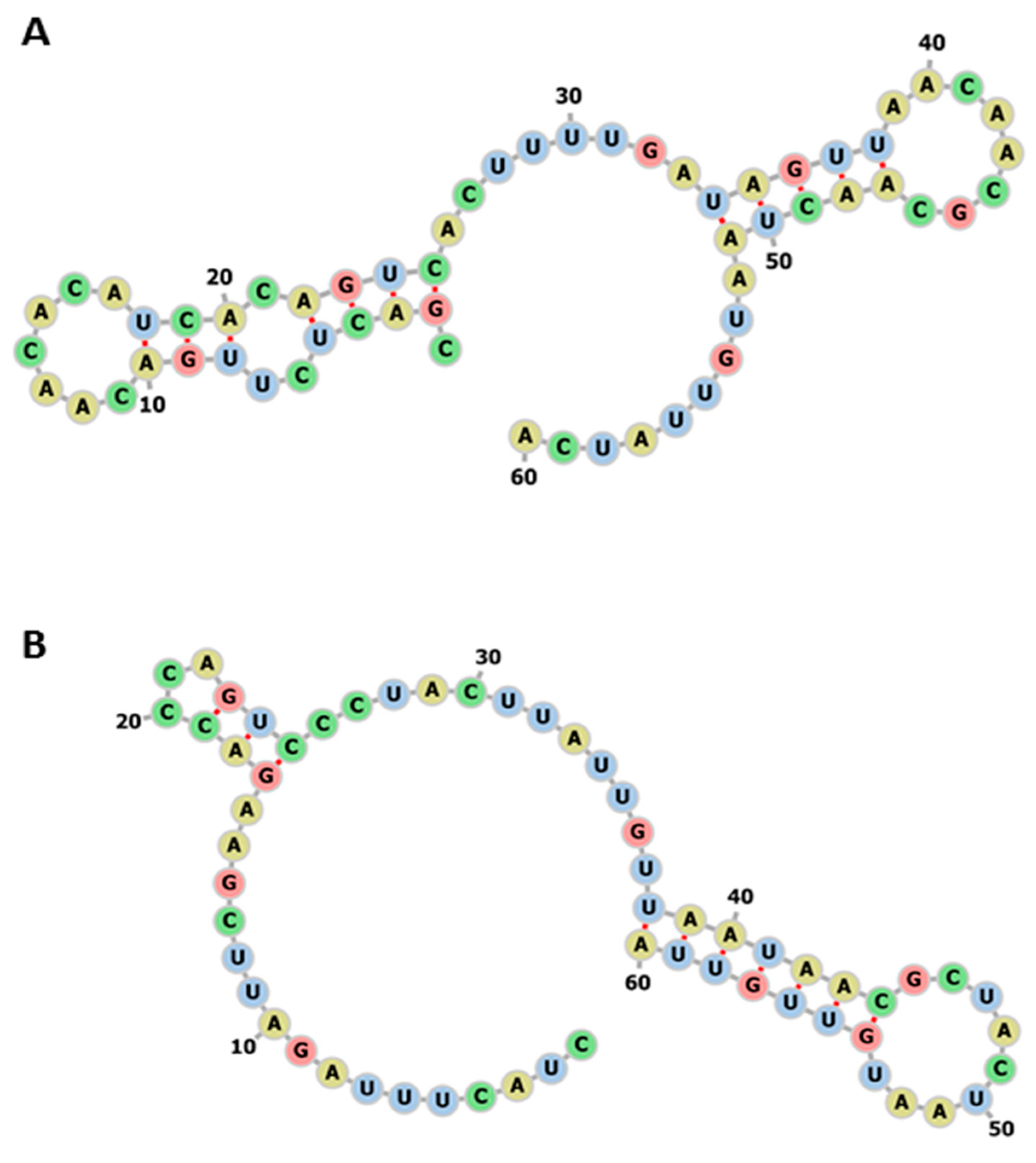
3. Results
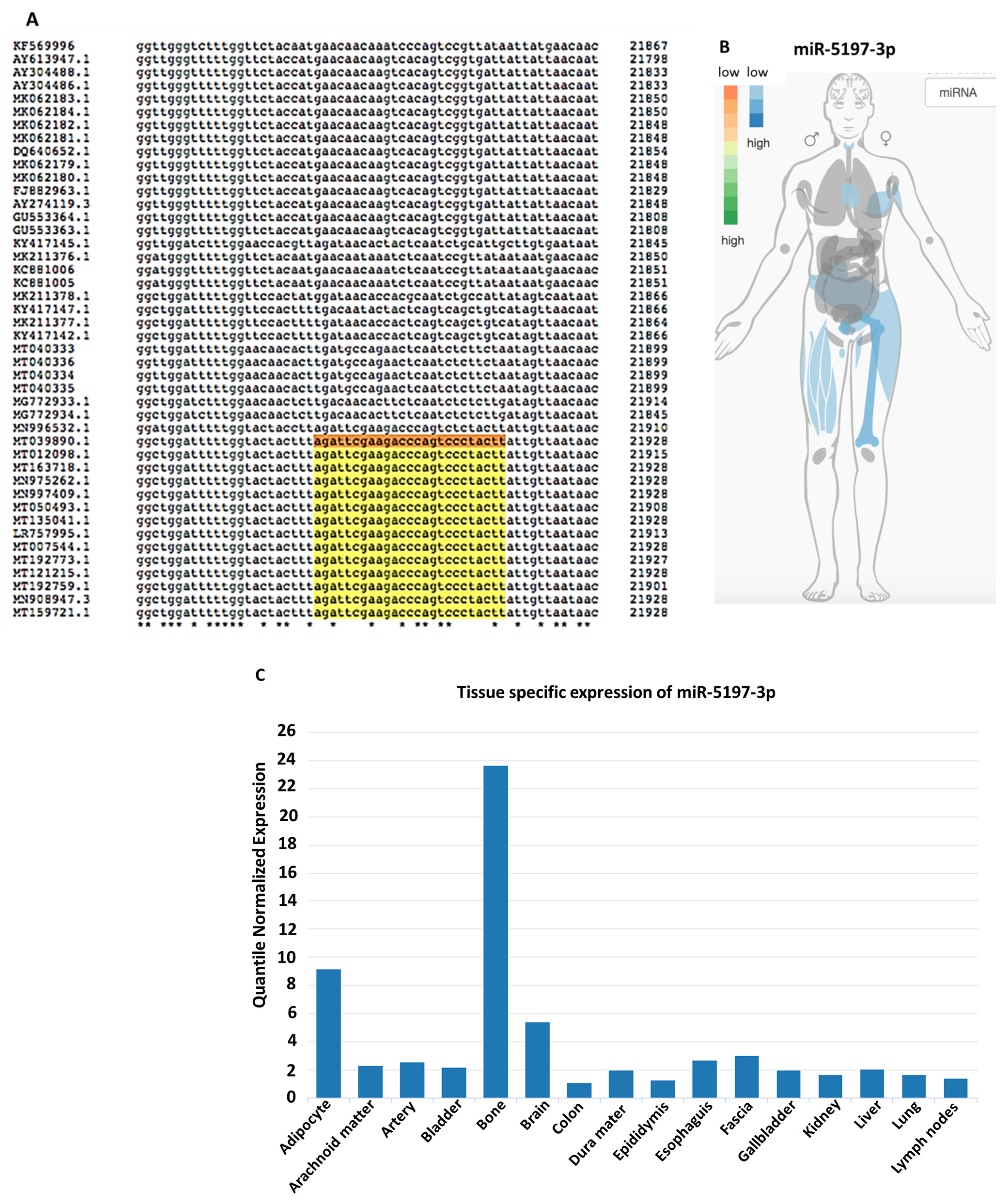
3.1. Conservation of Seven SARS-CoV-2 miRs Across Zoonotic Species and Human
3.2. Genomic Sequence Analysis
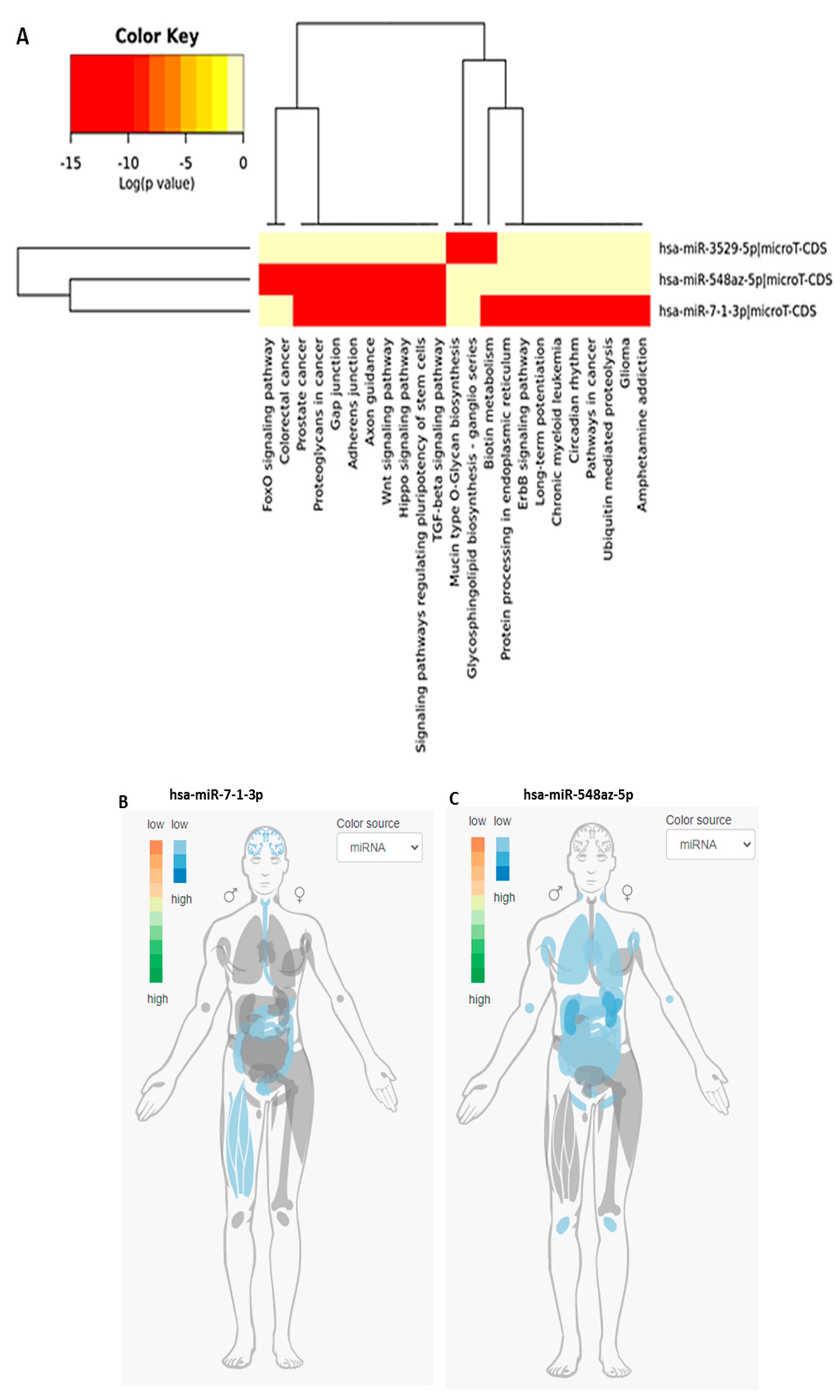
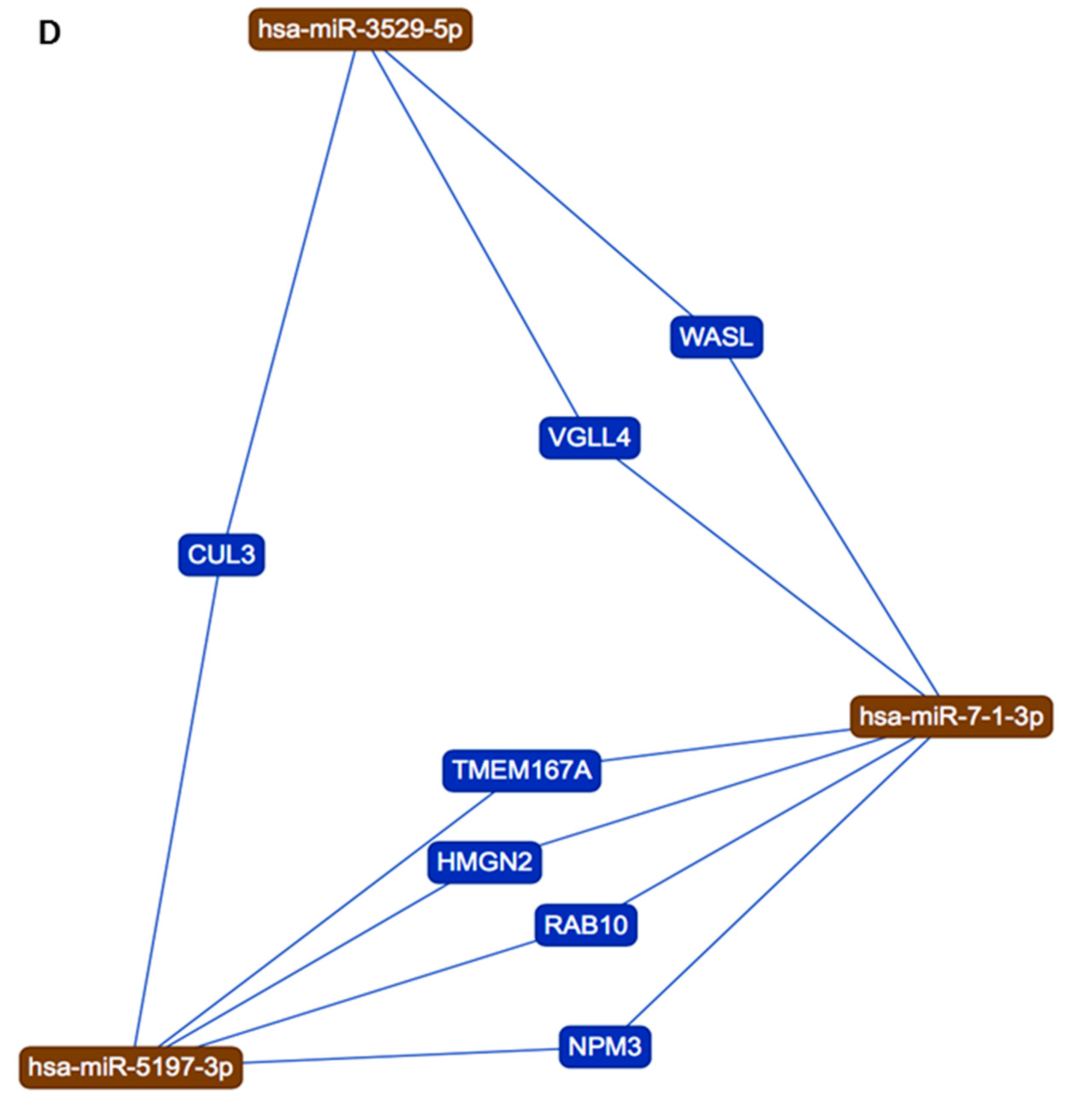
3.3. KEGG Pathway Analysis for the Three New miRs (miR-3529-5p, miR-7-1-3p, and miR-548az-5p) Generated by Changes in miR-5197
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Recalcati, S. Cutaneous manifestations in COVID-19: A first perspective. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e212–e213. [Google Scholar] [CrossRef] [PubMed]
- Driggin, E.; Madhavan, M.V.; Bikdeli, B.; Chuich, T.; Laracy, J.; Biondi-Zoccai, G.; Brown, T.S.; Der Nigoghossian, C.; Zidar, D.A.; Haythe, J.; et al. Cardiovascular Considerations for Patients, Health Care Workers, and Health Systems During the Coronavirus Disease 2019 (COVID-19) Pandemic. J. Am. Coll. Cardiol. 2020, 75, 2352–2371. [Google Scholar] [CrossRef] [PubMed]
- Mousa, A.Y.; Broce, M.; Lucas, B.D., Jr. Cardiovascular Disease Novel Coronavirus and the Search for Investigational Therapies. J. Vasc. Surg. 2020. [Google Scholar] [CrossRef]
- Mahmud, E.; Dauerman, H.L.; Welt, F.G.; Messenger, J.C.; Rao, S.V.; Grines, C.; Mattu, A.; Kirtane, A.J.; Jauhar, R.; Meraj, P.; et al. Management of Acute Myocardial Infarction During the COVID-19 Pandemic. J. Am. Coll. Cardiol. 2020. [CrossRef]
- Hirsch, J.S.; Ng, J.H.; Ross, D.W.; Sharma, P.; Shah, H.H.; Barnett, R.L.; Hazzan, A.D.; Fishbane, S.; Jhaveri, K.D. Northwell COVID-19 Research Consortium, & Northwell Nephrology COVID-19 Research Consortium, 2020. Acute kidney injury in patients hospitalized with COVID-19. Kidney Int. 2020, 98, 209–218. [Google Scholar] [PubMed]
- Ronco, C.; Reis, T.; Husain-Syed, F. Management of acute kidney injury in patients with COVID-19. Lancet Respir. Med. 2020, 8, 738–742. [Google Scholar] [CrossRef]
- Gu, J.; Han, B.; Wang, J. COVID-19: Gastrointestinal Manifestations and Potential Fecal-Oral Transmission. Gastroenterology 2020, 158, 1518–1519. [Google Scholar] [CrossRef]
- Avula, A.; Nalleballe, K.; Narula, N.; Sapozhnikov, S.; Dandu, V.; Toom, S.; Glaser, A.; Elsayeg, D. COVID-19 presenting as stroke. Brain Behav. Immun. 2020, 87, 115–119. [Google Scholar] [CrossRef]
- Ellul, M.A.; Benjamin, L.; Singh, B.; Lant, S.; Michael, B.D.; Easton, A.; Kneen, R.; Defres, S.; Sejvar, J.; Solomon, T. Neurological associations of COVID-19. Lancet Neurol. 2020. [Google Scholar] [CrossRef]
- Varatharaj, A.; Thomas, N.; Ellul, M.A.; Davies, N.; Pollak, T.A.; Tenorio, E.L.; Sultan, M.; Easton, A.; Breen, G.; Zandi, M.; et al. Neurological and neuropsychiatric complications of COVID-19 in 153 patients: A UK-wide surveillance study. Lancet Psychiatry 2020. [Google Scholar] [CrossRef]
- Luan, J.; Lu, Y.; Jin, X.; Zhang, L. Spike protein recognition of mammalian ACE2 predicts the host range and an optimized ACE2 for SARS-CoV-2 infection. Biochem. Biophys. Res. Commun. 2020, 526, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.Y.; Liu, C.C.; Chen, R.H. Cul3-KLHL20 ubiquitin ligase: Physiological functions, stress responses, and disease implications. Cell Div. 2016, 11, 5. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.A.; Thiel, V.; Dobbe, J.C.; van der Meer, Y.; Snijder, E.J.; Ziebuhr, J. Multiple enzymatic activities associated with severe acute respiratory syndrome coronavirus helicase. J. Virol. 2004, 78, 5619–5632. [Google Scholar] [CrossRef] [PubMed]
- Feder, M.; Pas, J.; Wyrwicz, L.S.; Bujnicki, J.M. Molecular phylogenetics of the RrmJ/fibrillarin superfamily of ribose 2′-O-methyltransferases. Gene 2003, 302, 129–138. [Google Scholar] [CrossRef]
- Damas, N.D.; Fossat, N.; Scheel, T.K.H. Functional Interplay between RNA Viruses and Non-Coding RNA in Mammals. Noncoding RNA. Noncoding RNA 2019, 5, 7. [Google Scholar]
- Janssen, H.L.; Reesink, H.W.; Lawitz, E.J.; Zeuzem, S.; Rodriguez-Torres, M.; Patel, K.; van der Meer, A.J.; Patick, A.K.; Chen, A.; Zhou, Y.; et al. Treatment of HCV infection by targeting microRNA. N. Engl. J. Med. 2013, 368, 1685–1694. [Google Scholar] [CrossRef]
- Nassar, D.; Blanpain, C. Cancer Stem Cells: Basic Concepts and Therapeutic Implications. Annu. Rev. Pathol. Mech. Dis. 2016, 11, 47–76. [Google Scholar] [CrossRef]
- Barbu, M.G.; Condrat, C.E.; Thompson, D.C.; Bugnar, O.L.; Cretoiu, D.; Toader, N.; Suciu, O.D.; Voinea, S.C. MicroRNA Involvement in Signaling Pathways During Viral Infection. Front. Cell. Dev. Biol. 2020, 8, 143. [Google Scholar] [CrossRef]
- Arisan, E.D.; Dart, A.; Grant, G.H.; Arisan, S.; Cuhadaroglu, S.; Lange, S.; Uysal-Onganer, P. The Prediction of miRNAs in SARS-CoV-2 Genomes: Hsa-miR Databases Identify 7 Key miRs Linked to Host Responses and Virus Pathogenicity-Related KEGG Pathways Significant for Comorbidities. Viruses 2020, 12, 614. [Google Scholar] [CrossRef]
- Li, X.; Giorgi, E.E.; Marichann, M.H.; Foley, B.; Xiao, C.; Kong, X.P.; Chen, Y.; Korber, B.; Gao, F. Emergence of SARS-CoV-2 through Recombination and Strong Purifying Selection. bioRxiv 2020. [Google Scholar] [CrossRef]
- Voskarides, K.; Christaki, E.; Nikolopoulos, G.K. Influenza Virus-Host Co-evolution. A Predator-Prey Relationship? Front. Immunol. 2018, 9, 2017. [Google Scholar] [CrossRef] [PubMed]
- Kaján, G.L.; Doszpoly, A.; Tarján, Z.L.; Vidovszky, M.Z.; Papp, T. Virus-Host Coevolution with a Focus on Animal and Human DNA Viruses. J. Mol. Evol. 2020, 88, 41–56. [Google Scholar] [CrossRef] [PubMed]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Söding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, V.; Backes, C.; Ludwig, N.; Fehlmann, T.; Kern, F.; Meese, E.; Keller, A. IMOTA: An interactive multi-omics tissue atlas for the analysis of human miRNA–target interactions. Nucleic Acids Res. 2018, 46, D770–D775. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, N.; Leidinger, P.; Becker, K.; Backes, C.; Fehlmann, T.; Pallasch, C.; Rheinheimer, S.; Meder, B.; Stähler, C.; Meese, E.; et al. Distribution of miRNA expression across human tissues. Nucleic Acids Res. 2016, 44, 3865–3877. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.; Tsafou, K.; Stolte, C.; Pletscher-Frankild, S.; O’Donoghue, S.I.; Jensen, L.J. Comprehensive comparison of large-scale tissue expression datasets. PeerJ 2015, 3, e1054. [Google Scholar] [CrossRef]
- Hamberg, M.; Backes, C.; Fehlmann, T.; Hart, M.; Meder, B.; Meese, E.; Keller, A. miRTargetLink-miRNAs, Genes and Interaction Networks. Int. J. Mol. Sci. 2016, 17, 564. [Google Scholar] [CrossRef]
- Bernhart, S.H.; Hofacker, I.L.; Will, S.; Gruber, A.R.; Stadler, P.F. RNAalifold: Improved consensus structure prediction for RNA alignments. BMC Bioinf. 2008, 9, 474. [Google Scholar] [CrossRef]
- Zuker, M.; Stiegler, P. Optimal computer folding of large RNA sequences using thermodynamics and auxiliary information. Nucleic Acids Res. 1981, 9, 133–148. [Google Scholar] [CrossRef]
- Ding, Y.; Chan, C.Y.; Lawrence, C.E. RNA secondary structure prediction by centroids in a Boltzmann weighted ensemble. RNA 2005, 11, 1157–1166. [Google Scholar] [CrossRef] [PubMed]
- Parlikar, A.; Kalia, K.; Sinha, S.; Patnaik, S.; Sharma, N.; Vemuri, S.G.; Sharma, G. Understanding genomic diversity, pan-genome, and evolution of SARS-CoV-2. PeerJ 2020, 8, e9576. [Google Scholar] [CrossRef] [PubMed]
- Kadri, S.; Hinman, V.; Benos, P.V. HHMMiR: Efficient de novo prediction of microRNAs using hierarchical hidden Markov models. BMC Bioinform. 2009, 10 (Suppl. 1), S35. [Google Scholar] [CrossRef]
- Wu, Y.; Wei, B.; Liu, H.; Li, T.; Rayner, S. MiRPara: A SVM-based software tool for prediction of most probable microRNA coding regions in genome scale sequences. BMC Bioinform. 2011, 12, 107. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S. miRBase: The microRNA sequence database. Methods Mol. Biol. 2006, 342, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Griffiths-Jones, S.; Saini, H.K.; van Dongen, S.; Enright, A.J. miRBase: Tools for microRNA genomics. Nucleic Acids Res. 2008, 36, D154–D158. [Google Scholar] [CrossRef] [PubMed]
- Choo, S.W.; Rayko, M.; Tan, T.K.; Hari, R.; Komissarov, A.; Wee, W.Y.; Yurchenko, A.A.; Kliver, S.; Tamazian, G.; Antunes, A.; et al. Pangolin genomes and the evolution of mammalian scales and immunity. Genome Res. 2016, 26, 1312–1322. [Google Scholar] [CrossRef]
- Luo, X.Y.; Yuan, J.L.; Liu, J.; Luo, C.N.; Yang, M.H.; Wei, Q.; Yang, M.; Chen, Y.; Liu, Y.; Yuan, G.H. Increased Macroautophagy in Interferon-Gamma-Producing T Cells from Patients with Newly Diagnosed Systemic Lupus Erythematosus. Chin. Med. J. 2018, 131, 1527–1532. [Google Scholar] [CrossRef]
- Wang, B.X.; Fish, E.N. Global virus outbreaks: Interferons as 1st responders. Semin. Immunol. 2019, 43, 101300. [Google Scholar] [CrossRef]
- Green, R.; Ireton, R.C.; Gale, M., Jr. Interferon-stimulated genes: New platforms and computational approaches. Mamm. Genome 2018, 29, 593–602. [Google Scholar] [CrossRef]
- Fischer, H.; Tschachler, E.; Eckhart, L. Pangolins Lack IFIH1/MDA5, a Cytoplasmic RNA Sensor That Initiates Innate Immune Defense Upon Coronavirus Infection. Front. Immunol. 2020, 11, 939. [Google Scholar] [CrossRef] [PubMed]
- Fischer, H.; Tschachler, E.; Eckhart, L. Cytosolic DNA sensing through cGAS and STING is inactivated by gene mutations in pangolins. Apoptosis 2020, 25, 474–480. [Google Scholar] [CrossRef] [PubMed]
- Sok, D.; Le, K.M.; Vadnais, M.; Saye-Francisco, K.L.; Jardine, J.G.; Torres, J.L.; Berndsen, Z.T.; Kong, L.; Stanfield, R.; Ruiz, J.; et al. Rapid elicitation of broadly neutralizing antibodies to HIV by immunization in cows. Nature 2017, 548, 108–111. [Google Scholar] [CrossRef]
- Amer, H.M. Bovine-like coronaviruses in domestic and wild ruminants. Anim. Health Res. Rev. 2018, 19, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Saif, L.J.; Jung, K. Comparative Pathogenesis of Bovine and Porcine Respiratory Coronaviruses in the Animal Host Species and SARS-CoV-2 in Humans. J. Clin. Microbiol. 2020, 58, e01355-20. [Google Scholar] [CrossRef]
- Mazurov, D.; Ilinskaya, A.; Heidecker, G.; Filatov, A. Role of O-glycosylation and expression of CD43 and CD45 on the surfaces of effector T cells in human T cell leukemia virus type 1 cell-to-cell infection. J. Virol. 2012, 86, 2447–2458. [Google Scholar] [CrossRef]
- Simon, E.J.; Linstedt, A.D. Site-specific glycosylation of Ebola virus glycoprotein by human polypeptide GalNAc-transferase 1 induces cell adhesion defects. J. Biol. Chem. 2018, 293, 19866–19873. [Google Scholar] [CrossRef]
- Lantéri, M.; Giordanengo, V.; Hiraoka, N.; Fuzibet, J.G.; Auberger, P.; Fukuda, M.; Baum, L.G.; Lefebvre, J.C. Altered T cell surface glycosylation in HIV-1 infection results in increased susceptibility to galectin-1-induced cell death. Glycobiology 2003, 13, 909–918. [Google Scholar] [CrossRef]
- Gaunitz, S.; Liu, J.; Nilsson, A.; Karlsson, N.; Holgersson, J. Avian influenza H5 hemagglutinin binds with high avidity to sialic acid on different O-linked core structures on mucin-type fusion proteins. Glycoconj. J. 2014, 31, 145–159. [Google Scholar] [CrossRef]
- Nordén, R.; Nyström, K.; Adamiak, B.; Halim, A.; Nilsson, J.; Larson, G.; Trybala, E.; Olofsson, S. Involvement of viral glycoprotein gC-1 in expression of the selectin ligand sialyl-Lewis X induced after infection with herpes simplex virus type 1. APMIS 2013, 121, 280–289. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, L.; Qiu, L.; Pan, R.; Bai, H.; Jiang, Y.; Wang, Z.; Bi, Y.; Chen, G.; Chang, G. Expression patterns of novel circular RNAs in chicken cells after avian leukosis virus subgroup J infection. Gene 2019, 15, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Jiang, J.; Liang, B.; Wei, F.; Huang, J.; Pan, P.; Su, J.; Zhou, B.; Zang, N.; Ye, L.; et al. Opiate use inhibits TLR9 signaling pathway in vivo: Possible role in pathogenesis of HIV-1 infection. Sci. Rep. 2017, 7, 13071. [Google Scholar] [CrossRef] [PubMed]
- Nyland, S.B.; Cao, C.; Bai, Y.; Loughran, T.P.; Ugen, K.E. Modulation of infection and type 1 cytokine expression parameters by morphine during in vitro coinfection with human T-cell leukemia virus type I and HIV-1. J. Acquir. Immune Defic. Syndr. 2003, 32, 406–416. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, T.; Douglas, S.D.; Lai, J.P.; Xiao, W.D.; Pleasure, D.E.; Ho, W.Z. Morphine enhances hepatitis C virus (HCV) replicon expression. Am. J. Pathol. 2003, 163, 1167–1175. [Google Scholar] [CrossRef]
- Li, Y.; Ye, L.; Peng, J.S.; Wang, C.Q.; Luo, G.X.; Zhang, T.; Wan, Q.; Ho, W.Z. Morphine inhibits intrahepatic interferon- alpha expression and enhances complete hepatitis C virus replication. J. Infect. Dis. 2007, 196, 719–730. [Google Scholar] [CrossRef]
- Chuang, R.Y.; Suzuki, S.; Chuang, T.K.; Miyagi, T.; Chuang, L.F.; Doi, R.H. Opioids and the progression of simian AIDS. Front. Biosci. 2005, 10, 1666–1677. [Google Scholar] [CrossRef][Green Version]
- Coussons-Read, M.E.; Daniels, M.; Gilmour, M.I. Morphine alters the immune response to influenza virus infection in Lewis rats. Adv. Exp. Med. Biol. 1998, 437, 73–82. [Google Scholar]
- Chinnapaiyan, S.; Dutta, R.K.; Nair, M.; Chand, H.S.; Rahman, I.; Unwalla, H.J. TGF-β1 increases viral burden and promotes HIV-1 latency in primary differentiated human bronchial epithelial cells. Sci. Rep. 2019, 9, 12552. [Google Scholar] [CrossRef]
- Alejandre-Alcázar, M.A.; Michiels-Corsten, M.; Vicencio, A.G.; Reiss, I.; Ryu, J.; de Krijger, R.R.; Haddad, G.G.; Tibboel, D.; Seeger, W.; Eickelberg, O.; et al. TGF-beta signaling is dynamically regulated during the alveolarization of rodents and human lungs. Dev. Dyn. 2008, 237, 259–269. [Google Scholar] [CrossRef]
- Gordon, K.J.; Blobe, G.C. Role of transforming growth factor-beta superfamily signaling pathways in human disease. Biochim. Biophys. Acta 2008, 1782, 197–228. [Google Scholar] [CrossRef]
- Morty, R.E.; Königshoff, M.; Eickelberg, O. Transforming growth factor-beta signaling across ages: From distorted lung development to chronic obstructive pulmonary disease. Proc. Am. Thorac. Soc. 2009, 6, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.; Negi, S.; Szebeni, A.; Olson, M.O. Protein NPM3 interacts with the multifunctional nucleolar protein B23/nucleophosmin and inhibits ribosome biogenesis. J. Biol. Chem. 2005, 280, 5496–5502. [Google Scholar] [CrossRef] [PubMed]
- Okuwaki, M.; Sumi, A.; Hisaoka, M.; Saotome-Nakamura, A.; Akashi, S.; Nishimura, Y.; Nagata, K. Function of homo- and hetero-oligomers of human nucleoplasmin/nucleophosmin family proteins NPM1, NPM2 and NPM3 during sperm chromatin remodeling. Nucleic Acids Res. 2012, 40, 4861–4878. [Google Scholar] [CrossRef] [PubMed]
- Ciribilli, Y.; Singh, P.; Inga, A.; Borlak, J. c-Myc targeted regulators of cell metabolism in a transgenic mouse model of papillary lung adenocarcinoma. Oncotarget 2016, 7, 65514–65539. [Google Scholar] [CrossRef][Green Version]
- Bao, S.; Zhu, J.; Garvey, W.T. Cloning of Rab GTPases expressed in human skeletal muscle: Studies in insulin-resistant subjects. Horm. Metab. Res. 1998, 30, 656–662. [Google Scholar] [CrossRef]
- Jeng, E.E.; Bhadkamkar, V.; Ibe, N.U.; Gause, H.; Jiang, L.; Chan, J.; Jian, R.; Jimenez-Morales, D.; Stevenson, E.; Krogan, N.J.; et al. Systematic Identification of Host Cell Regulators of Legionella pneumophila Pathogenesis Using a Genome-wide CRISPR Screen. Cell Host Microbe 2019, 26, 551–563.e6. [Google Scholar] [CrossRef]
- Feng, Y.; Huang, N.; Wu, Q.; Wang, B. HMGN2: A novel antimicrobial effector molecule of human mononuclear leukocytes? J. Leukoc. Biol. 2005, 78, 1136–1141. [Google Scholar] [CrossRef]
- Zheng, S.; Ren, L.; Li, H.; Shen, X.; Yang, X.; Li, N.; Wang, X.; Guo, X.; Wang, X.; Huang, N. High-mobility group nucleosome-binding domain 2 protein inhibits the invasion of Klebsiella pneumoniae into mouse lungs in vivo. Mol. Med. Rep. 2015, 12, 1279–1285. [Google Scholar] [CrossRef]
- Portela, M.; Segura-Collar, B.; Argudo, I.; Sáiz, A.; Gargini, R.; Sánchez-Gómez, P.; Casas-Tintó, S. Oncogenic dependence of glioma cells on kish/TMEM167A regulation of vesicular trafficking. Glia 2019, 67, 404–417. [Google Scholar] [CrossRef]
- Segura-Collar, B.; Gargini, R.; Tovar-Ambel, E.; Hernández-SanMiguel, E.; Epifano, C.; Pérez de Castro, I.; Hernández-Laín, A.; Casas-Tintó, S.; Sánchez-Gómez, P. The EGFR-TMEM167A-p53 Axis Defines the Aggressiveness of Gliomas. Cancers 2020, 12, 208. [Google Scholar] [CrossRef]
- Sakaue, T.; Maekawa, M.; Nakayama, H.; Higashiyama, S. Prospect of divergent roles for the CUL3 system in vascular endothelial cell function and angiogenesis. J. Biochem. 2017, 162, 237–245. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, A.; Wu, Q.; Deng, Y.; Liu, Y.; Lu, J.; Liu, L.; Li, X.; Liao, C.; Zhao, B.; Song, H. Loss of VGLL4 suppresses tumor PD-L1 expression and immune evasion. EMBO J. 2019, 38, e99506. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Yoo, Y.; Okuhama, N.N.; Tucker, P.W.; Liu, G.; Guan, J.L. Regulation of RNA-polymerase-II-dependent transcription by N-WASP and its nuclear-binding partners. Nat. Cell Biol. 2006, 8, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Miki, H.; Sasaki, T.; Takai, Y.; Takenawa, T. Induction of filopodium formation by a WASP-related actin-depolymerizing protein N-WASP. Nature 1998, 391, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, E.M.; Verma, S.; Ali, R.G.; Ratheesh, A.; Hamilton, N.A.; Akhmanova, A.; Yap, A.S. N-WASP regulates the epithelial junctional actin cytoskeleton through a non-canonical post-nucleation pathway. Nat. Cell Biol. 2011, 13, 934–943. [Google Scholar] [CrossRef] [PubMed]
- Al Absi, A.; Wurzer, H.; Guerin, C.; Hoffmann, C.; Moreau, F.; Mao, X.; Brown-Clay, J.; Petrolli, R.; Casellas, C.P.; Dieterle, M.; et al. Actin Cytoskeleton Remodeling Drives Breast Cancer Cell Escape from Natural Killer-Mediated Cytotoxicity. Cancer Res. 2018, 78, 5631–5643. [Google Scholar] [CrossRef]
- Jiang, H.; Leung, C.; Tahan, S.; Wang, D. Entry by multiple picornaviruses is dependent on a pathway that includes TNK2, WASL, and NCK1. eLife 2019, 8, e50276. [Google Scholar] [CrossRef]
- Uenishi, E.; Shibasaki, T.; Takahashi, H.; Seki, C.; Hamaguchi, H.; Yasuda, T.; Tatebe, M.; Oiso, Y.; Takenawa, T.; Seino, S. Actin dynamics regulated by the balance of neuronal Wiskott-Aldrich syndrome protein (N-WASP) and cofilin activities determines the biphasic response of glucose-induced insulin secretion. J. Biol. Chem. 2013, 288, 25851–25864. [Google Scholar] [CrossRef]
- Guenin-Macé, L.; Veyron-Churlet, R.; Thoulouze, M.I.; Romet-Lemonne, G.; Hong, H.; Leadlay, P.F.; Danckaert, A.; Ruf, M.T.; Mostowy, S.; Zurzolo, C.; et al. Mycolactone activation of Wiskott-Aldrich syndrome proteins underpins Buruli ulcer formation. J. Clin. Investig. 2013, 123, 1501–1512. [Google Scholar] [CrossRef]
- Wang, X. Composition of seed sequence is a major determinant of microRNA targeting patterns. Bioinformatics 2014, 30, 1377–1383. [Google Scholar] [CrossRef]
- O’Shea, T.J.; Cryan, P.M.; Cunningham, A.A.; Fooks, A.R.; Hayman, D.T.; Luis, A.D.; Peel, A.J.; Plowright, R.K.; Wood, J.L. Bat flight and zoonotic viruses. Emerg. Infect. Dis. 2014, 20, 741–745. [Google Scholar] [CrossRef] [PubMed]
- Carmel, I.; Shomron, N.; Heifetz, Y. Does base-pairing strength play a role in microRNA repression? RNA (N. Y.) 2012, 18, 1947–1956. [Google Scholar] [CrossRef] [PubMed]
- Rolle, K.; Piwecka, M.; Belter, A.; Wawrzyniak, D.; Jeleniewicz, J.; Barciszewska, M.Z.; Barciszewski, J. The sequence and structure determine the function of mature human miRNAs. PLoS ONE 2016, 11, e0151246. [Google Scholar] [CrossRef] [PubMed]
- Roche, B.; Guégan, J.F. Ecosystem dynamics, biological diversity and emerging infectious diseases. C. R. Biol. 2011, 334, 385–392. [Google Scholar] [CrossRef]
- Johnson, C.K.; Hitchens, P.L.; Pandit, P.S.; Rushmore, J.; Evans, T.S.; Young, C.; Doyle, M.M. Global shifts in mammalian population trends reveal key predictors of virus spillover risk. Proc. Biol. Sci. 2020, 287, 20192736. [Google Scholar] [CrossRef]
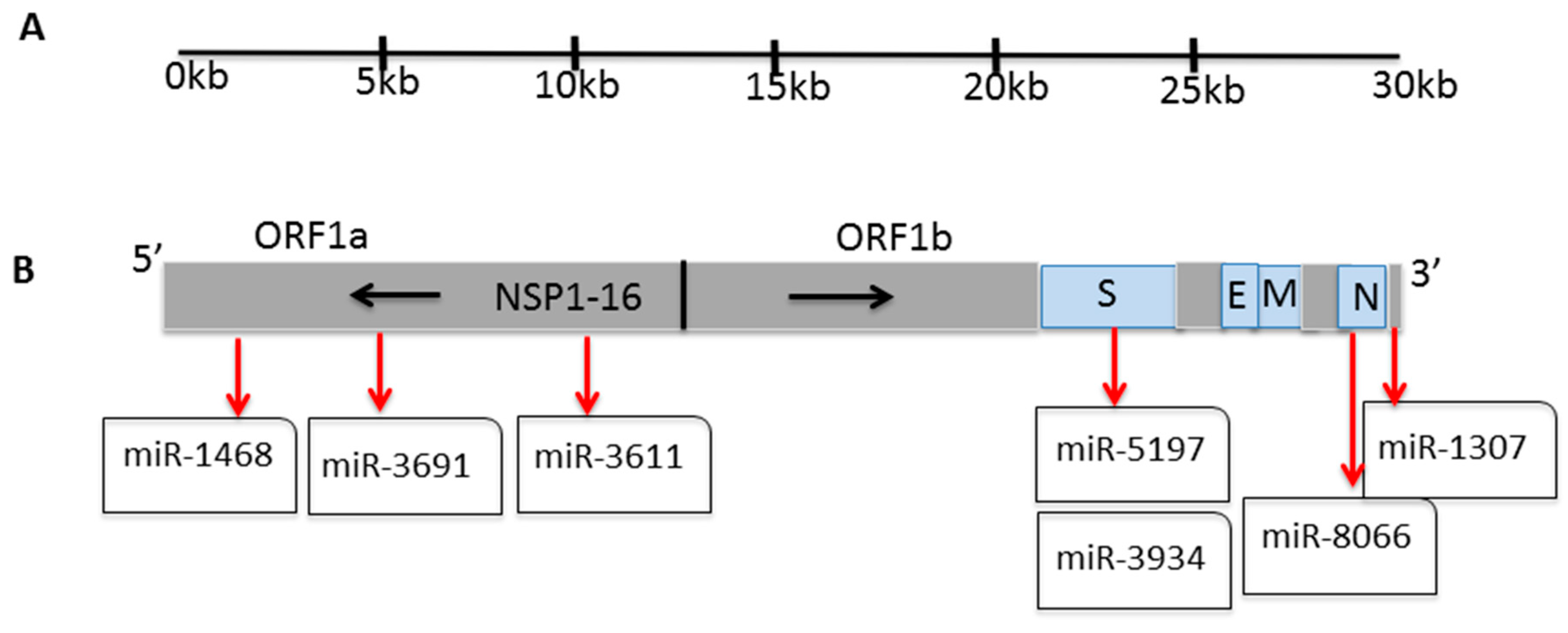
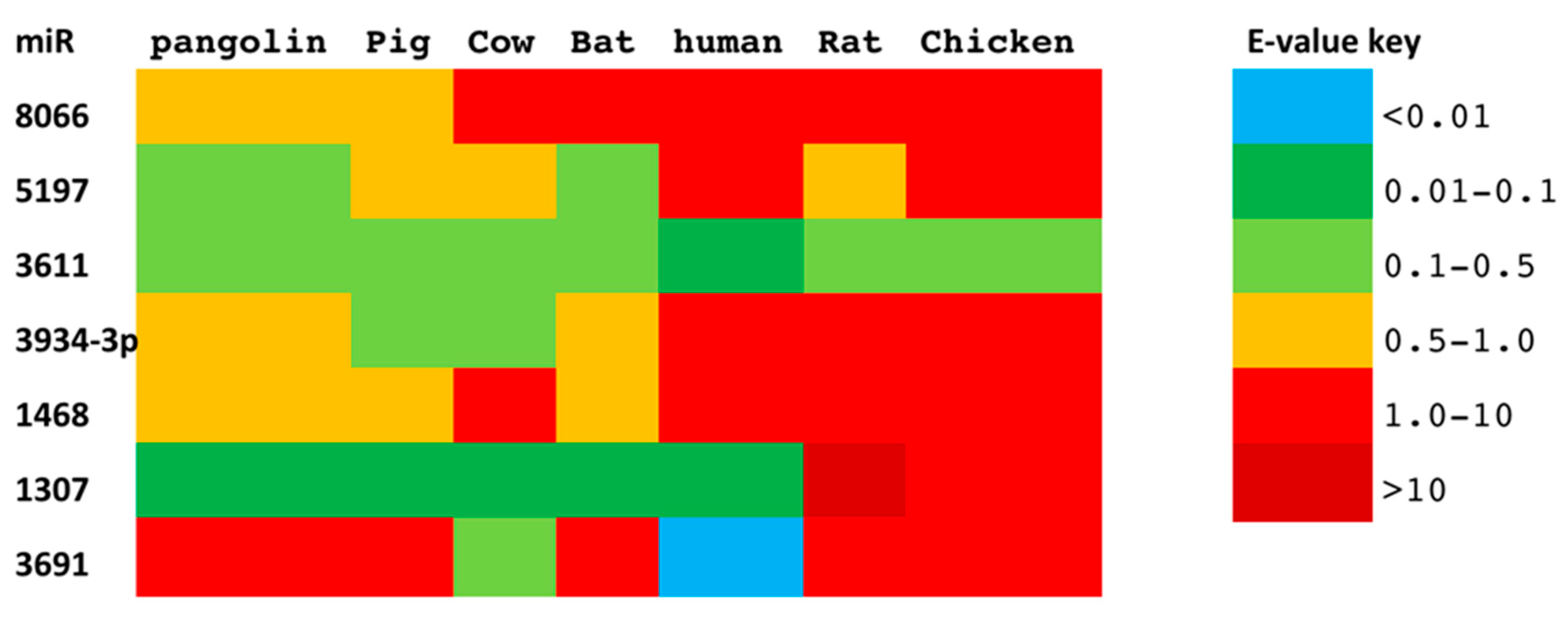

| miR | miR Sequence Alignment | GC Content(%) | Body Temperature (°C) | ||
|---|---|---|---|---|---|
| 8066 | ATATGGGTTGCAAATGAGGGAGCCTTGAATACACCTAAAGATCACATTGGCACCCGAAA | 28677 | Pangolin | 31 | 32 |
| ATATGGGTTGCAACTGAGGGAGCCTTGAATACACCAAAAGATCACATTGGCACCCGCAAT | 28723 | Human | 37.5 | 37 | |
| ATATGGGTTGCAACTGAGGGAGCCTTGAATACACCAAAAGATCACATTGGCACCCGCAAT | 28689 | Bat | 37.5 | 39–42 | |
| 5197 | CGACTCTTGACAACACATCACAGTCACTTTTGATAGTTAACAACGCAACTAATGTTATCA | 21922 | Pangolin | 40 | 32 |
| CTACTTTAGATTCGAAGACCCAGTCCCTACTTATTGTTAATAACGCTACTAATGTTGTTA | 21944 | Human | 48 | 37 | |
| CTACCTTAGATTCGAAGACCCAGTCTCTACTTATTGTTAATAACGCTACTAATGTTGTTA | 21926 | Bat | 43 | 39–42 | |
| 3611 | CAAGAGCGCTTTTTACATACTACCATCCATTGTCTCTAATGAGAAAGAAGAAATTCTTGG | 4350 | Pangolin | 25 | 32 |
| TAAAAGTGCCTTTTACATTCTACCATCTATTATCTCTAATGAGAAGCAAGAAATTCTTGG | 4371 | Human | 31 | 37 | |
| TAAAAGTGCCTTTTACATTCTACCATCTATTATCTCTAATGAGAAGCAAGAAATTCTTGG | 4353 | Bat | 31 | 39–42 | |
| 3934 | GACCCCATGCCTAATAAT---------GGCTGGACAGTCTTTTCAGCTGCTTATTACGTG | 22329 | Pangolin | 32 | |
| TATTTGACTCCTGGTGATTCTTCTTCAGGTTGGACAGCTGGTGCTGCAGCTTATTATGTG | 22363 | Human | 42 | 37 | |
| TATTTGACTCCTGGTGATTCTTCTTCAGGTTGGACAGCTGGTGCTGCAGCTTATTATGTG | 22345 | Bat | 42 | 39–42 | |
| 1468 | ACACGTCCAACTCAGTTTGCCTGTTTTACAGGTTCGCGACGTGCTCGTACGTGGCTTTGG | 360 | Pangolin | 42 | 32 |
| ACACGTCCAACTCAGTTTGCCTGTTTTACAGGTTCGCGACGTGCTCGTACGTGGCTTTGG | 360 | Human | 42 | 37 | |
| ACACGTCCAACTCAGTTTGCCTGTCTTACAGGTTCGCGACGTGCTCGTACGTGGCTTTGG | 345 | Bat | 50 | 39–42 | |
| 1307 | TGTGTAACATTAGGGAGGACTTGAAAGAGCCACCACATTTTCACCGAGGCCACGCGGAGT | 29702 | Pangolin | 76 | 32 |
| TGTGTAACATTAGGGAGGACTTGAAAGAGCCACCACATTTTCACCGAGGCCACGCGGAGT | 29748 | Human | 76 | 37 | |
| TGTGTAACATTAGGGAGGACTTGAAAGAGCCACCACATTTTCACCGAGGCCACGCGGAGT | 29714 | Bat | 76 | 39–42 | |
| 3691 | GAGATGTTGATACAGACTTTGTGAATGAGTTTTATGCATATTTGCGTAAACACTTCTCAA | 15682 | Pangolin | 36 | 32 |
| GAGATGTTGACACAGACTTTGTGAATGAGTTTTACGCATATTTGCGTAAACATTTCTCAA | 15703 | Human | 41 | 37 | |
| GAGATGTTGACACAGACTTTGTGAATGAGTTTTACGCATATTTGCGTAAACATTTCTCAA | 15685 | Bat | 41 | 39–42 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, S.; Arisan, E.D.; Grant, G.H.; Uysal-Onganer, P. MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species. Viruses 2021, 13, 117. https://doi.org/10.3390/v13010117
Lange S, Arisan ED, Grant GH, Uysal-Onganer P. MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species. Viruses. 2021; 13(1):117. https://doi.org/10.3390/v13010117
Chicago/Turabian StyleLange, Sigrun, Elif Damla Arisan, Guy H. Grant, and Pinar Uysal-Onganer. 2021. "MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species" Viruses 13, no. 1: 117. https://doi.org/10.3390/v13010117
APA StyleLange, S., Arisan, E. D., Grant, G. H., & Uysal-Onganer, P. (2021). MicroRNAs for Virus Pathogenicity and Host Responses, Identified in SARS-CoV-2 Genomes, May Play Roles in Viral-Host Co-Evolution in Putative Zoonotic Host Species. Viruses, 13(1), 117. https://doi.org/10.3390/v13010117








