Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Clinical Samples
2.3. Antibody Production and Selection
2.4. ELISA for the Detection and Quantification of CHIKV E1/E2
2.5. Lateral Immune Detection Methods for the Quantification of CHIKV E1/E2
2.6. Image Analysis
2.7. Limit of Detection (LoD) Analysis
2.8. RNA Extraction and Quantitative RT-PCR
2.9. Receiver Operator Characteristic (ROC) Analysis
3. Results
3.1. Antibody Selection for CHIKV ELISA and Lateral Flow Assays
3.2. Limits of Detection
3.3. Performance of CHIKV E1/E2 ELISA
3.4. Performance of CHIKV E1/E2 Lateral Flow
4. Discussion
Supplementary Materials
Author Contributions:
Funding
Acknowledgments
Conflicts of Interest
References
- Pialoux, G.; Gaüzère, B.-A.; Jauréguiberry, S.; Strobel, M. Chikungunya, an epidemic arbovirosis. Lancet Infect. Dis. 2007, 7, 319–327. [Google Scholar] [CrossRef]
- Ganesan, V.K.; Duan, B.; Reid, S.P. Chikungunya Virus: Pathophysiology, Mechanism, and Modeling Viruses 1 December 2017. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5744143/ (accessed on 14 January 2020).
- Vairo, F.; Haider, N.; Kock, R.; Ntoumi, F.; Ippolito, G.; Zumla, A. Chikungunya: Epidemiology, Pathogenesis, Clinical Features, Management, and Prevention. Infect. Dis. Clin. N. Am. 2019, 33, 1003–1025. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, S.; Mukherjee, S.; Haldar, S.K.; Bhattacharya, N.; Tripathi, A. Re-emergence of Chikungunya virus infection in Eastern India. Braz. J. Microbiol. 2020, 88, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Rueda, J.C.; Santos, A.M.; Angarita, J.-I.; Giraldo, R.B.; Saldarriaga, E.-L.; Muñoz, J.G.B.; Forero, E.; Valencia, H.; Somoza, F.; Martin-Arsanios, D.; et al. Demographic and clinical characteristics of chikungunya patients from six Colombian cities, 2014-2015. Emerg. Microbes Infect. 2019, 8, 1490–1500. [Google Scholar] [CrossRef] [PubMed]
- Silva, M.M.O.; Tauro, L.B.; Kikuti, M.; Anjos, R.O.; Santos, V.C.; Gonçalves, T.S.F.; Paploski, I.A.D.; Moreira, P.S.S.; Nascimento, L.C.J.; Campos, G.S.; et al. Concomitant Transmission of Dengue, Chikungunya, and Zika Viruses in Brazil: Clinical and Epidemiological Findings from Surveillance for Acute Febrile Illness. Clin. Infect. Dis. 2018, 69, 1353–1359. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Gresh, L.; Vargas, M.J.; Ballesteros, G.; Tellez, Y.; Soda, K.J.; Sahoo, M.K.; Nuñez, A.; Balmaseda, A.; Harris, E.; et al. Viremia and Clinical Presentation in Nicaraguan Patients Infected with Zika Virus, Chikungunya Virus, and Dengue Virus. Clin. Infect. Dis. 2016, 63, 1584–1590. [Google Scholar] [CrossRef]
- Proesmans, S.; Katshongo, F.; Milambu, J.; Fungula, B.; Mavoko, H.M.; Ahuka-Mundeke, S.; Da Luz, R.I.; Van Esbroeck, M.; Ariën, K.K.; Cnops, L.; et al. Dengue and chikungunya among outpatients with acute undifferentiated fever in Kinshasa, Democratic Republic of Congo: A cross-sectional study. PLoS Negl. Trop. Dis. 2019, 13, e0007047. [Google Scholar] [CrossRef]
- Carvalho, F.R.; Medeiros, T.; Vianna, R.A.D.O.; Douglass-Jaimes, G.; Nunes, P.C.G.; Quintans, M.D.S.; Souza, C.; Cavalcanti, S.M.B.; Dos Santos, F.B.; De Oliveira, S.A.; et al. Simultaneous circulation of arboviruses and other congenital infections in pregnant women in Rio de Janeiro, Brazil. Acta Trop. 2019, 192, 49–54. [Google Scholar] [CrossRef]
- Bagno, F.; Figueiredo, M.M.; Villarreal, J.; Pereira, G.C.; Godoi, L.C.; Da Fonseca, F.G. Undetected Chikungunya virus co-infections in a Brazilian region presenting hyper-endemic circulation of Dengue and Zika. J. Clin. Virol. 2019, 113, 27–30. [Google Scholar] [CrossRef]
- Mardekian, S.K.; Roberts, A.L. Diagnostic Options and Challenges for Dengue and Chikungunya Viruses. BioMed Res. Int. 2015, 2015, 834371. [Google Scholar] [CrossRef]
- Petitdemange, C.; Wauquier, N.; Vieillard, V. Control of immunopathology during chikungunya virus infection. J. Allergy Clin. Immunol. 2015, 135, 846–855. [Google Scholar] [CrossRef] [PubMed]
- Ng, L.-C.; Tan, L.-K.; Tan, C.-H.; Tan, S.S.; Hapuarachchi, H.C.; Pok, K.-Y.; Lai, Y.-L.; Lam-Phua, S.-G.; Bucht, G.; Lin, R.T.; et al. Entomologic and Virologic Investigation of Chikungunya, Singapore. Emerg. Infect. Dis. 2009, 15, 1243–1249. [Google Scholar] [CrossRef] [PubMed]
- Carletti, F.; Di Caro, A.; Capobianchi, M.R.; Chiappini, R.; Castilletti, C.; Ippolito, G.; Sciarrone, M.R.; Bordi, L. Rapid detection and quantification of Chikungunya virus by a one-step reverse transcription polymerase chain reaction real-time assay. Am. J. Trop. Med. Hyg. 2007, 77, 521–524. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Hasebe, F.; Parquet, M.C.; Pandey, B.D.; Mathenge, E.; Morita, K.; Balasubramaniam, V.; Saat, Z.; Yusop, A.; Sinniah, M.; Natkunam, S.; et al. Combined detection and genotyping ofChikungunya virus by a specific reverse transcription-polymerase chain reaction. J. Med. Virol. 2002, 67, 370–374. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.Y.; Babu, V.S.; Dev, S.S.; Gopalakrishnapai, J.; Harish, M.; Rajesh, M.D.; Anisha, S.; Mohankumar, C. Rapid detection and characterization of Chikungunya virus by RT-PCR in febrile patients from Kerala, India. Indian J. Exp. Biol. 2008, 46, 573–578. [Google Scholar] [PubMed]
- Laurent, P.; Le Roux, K.; Grivard, P.; Bertil, G.; Naze, F.; Picard, M.; Staikowsky, F.; Barau, G.; Schuffenecker, I.; Michault, A. Development of a Sensitive Real-Time Reverse Transcriptase PCR Assay with an Internal Control to Detect and Quantify Chikungunya Virus. Clin. Chem. 2007, 53, 1408–1414. [Google Scholar] [CrossRef]
- Panning, M.; Charrel, R.; Mantke, O.D.; Landt, O.; Niedrig, M.; Drosten, C. Coordinated Implementation of Chikungunya Virus Reverse Transcription–PCR. Emerg. Infect. Dis. 2009, 15, 469–471. [Google Scholar] [CrossRef]
- Yap, G.; Pok, K.-Y.; Lai, Y.-L.; Hapuarachchi, H.C.; Chow, A.; Leo, Y.-S.; Tan, L.-K.; Ng, L.C. Evaluation of Chikungunya Diagnostic Assays: Differences in Sensitivity of Serology Assays in Two Independent Outbreaks. PLoS Negl. Trop. Dis. 2010, 4, e753. [Google Scholar] [CrossRef]
- Nguyen, H.C.; Park, H.; Shin, H.-J.; Nguyen, N.M.; Nguyen, A.T.V.; Trinh, T.-T.T.; Duong, T.H.Y.; Tuong, H.T.; Hoang, V.T.; Seo, G.-E.; et al. Fluorescent Immunosorbent Assay for Chikungunya Virus Detection. Intervirology 2019, 62, 145–155. [Google Scholar] [CrossRef]
- Bagno, F.; Godoi, L.C.; Salazar, N.; Pereira, G.D.C.; Figueiredo, M.M.; Da FonSeca, F.G. Development of an enzyme-linked immunosorbent assay using recombinant protein antigen for the diagnosis of Chikungunya virus. Data Brief 2019, 25, 104015. [Google Scholar] [CrossRef]
- Kikuti, M.; Tauro, L.B.; Moreira, P.S.; Nascimento, L.C.J.; Portilho, M.M.; Soares, G.C.; Weaver, S.C.; Reis, M.G.; Kitron, U.; Ribeiro, G.S. Evaluation of two commercially available chikungunya virus IgM enzyme-linked immunoassays (ELISA) in a setting of concomitant transmission of chikungunya, dengue and Zika viruses. Int. J. Infect. Dis. 2019, 91, 38–43. [Google Scholar] [CrossRef] [PubMed]
- Pal, P.; Dowd, K.A.; Brien, J.D.; Edeling, M.A.; Gorlatov, S.; Johnson, S.; Lee, I.; Akahata, W.; Nabel, G.J.; Richter, M.K.S.; et al. Development of a Highly Protective Combination Monoclonal Antibody Therapy against Chikungunya Virus. PLoS Pathog. 2013, 9, e1003312. [Google Scholar] [CrossRef] [PubMed]
- Bosch, I.; De Puig, H.; Hiley, M.; Carré-Camps, M.; Perdomo-Celis, F.; Narváez, C.; Salgado, D.M.; Senthoor, D.; O’Grady, M.; Phillips, E.; et al. Rapid antigen tests for dengue virus serotypes and Zika virus in patient serum. Sci. Transl. Med. 2017, 9, eaan1589. [Google Scholar] [CrossRef] [PubMed]
- Blacksell, S.D.; Tanganuchitcharnchai, A.; Jarman, R.G.; Gibbons, R.V.; Paris, D.H.; Bailey, M.S.; Day, N.P.J.; Premaratna, R.; Lalloo, D.G.; de Silva, H.G. Poor Diagnostic Accuracy of Commercial Antibody-Based Assays for the Diagnosis of Acute Chikungunya Infection. Clin. Vaccine Immunol. 2011, 18, 1773–1775. [Google Scholar] [CrossRef]
- Rianthavorn, P.; Wuttirattanakowit, N.; Prianantathavorn, K.; Limpaphayom, N.; Theamboonlers, A.; Poovorawan, Y. Evaluation of a rapid assay for detection of IgM antibodies to chikungunya. Southeast Asian J. Trop. Med. Public Health 2010, 41, 92. [Google Scholar]
- Chopra, A.; Anuradha, V.; Lagoo-Joshi, V.; Kunjir, V.; Salvi, S.; Saluja, M. Chikungunya virus aches and pains: An emerging challenge. Arthritis Rheum. 2008, 58, 2921–2922. [Google Scholar] [CrossRef]
- Hoarau, J.-J.; Bandjee, M.-C.J.; Krejbich-Trotot, P.; Das, T.; Li-Pat-Yuen, G.; Dassa, B.; Denizot, M.; Guichard, E.; Ribera, A.; Henni, T.; et al. Persistent Chronic Inflammation and Infection by Chikungunya Arthritogenic Alphavirus in Spite of a Robust Host Immune Response. J. Immunol. 2010, 184, 5914–5927. [Google Scholar] [CrossRef]
- Grivard, P.; Le Roux, K.; Laurent, P.; Fianu, A.; Perrau, J.; Gigan, J.; Hoarau, G.; Grondin, N.; Staikowsky, F.; Favier, F.; et al. Molecular and serological diagnosis of Chikungunya virus infection. Pathol. Biol. 2007, 55, 490–494. [Google Scholar] [CrossRef]
- Shukla, J.; Khan, M.; Tiwari, M.; Sannarangaiah, S.; Sharma, S.; Rao, P.V.L.; Parida, M. Development and evaluation of antigen capture ELISA for early clinical diagnosis of chikungunya. Diagn. Microbiol. Infect. Dis. 2009, 65, 142–149. [Google Scholar] [CrossRef]
- Okabayashi, T.; Sasaki, T.; Masrinoul, P.; Chantawat, N.; Yoksan, S.; Nitatpattana, N.; Chusri, S.; Vargas, R.E.M.; Grandadam, M.; Brey, P.T.; et al. Detection of Chikungunya Virus Antigen by a Novel Rapid Immunochromatographic Test. J. Clin. Microbiol. 2014, 53, 382–388. [Google Scholar] [CrossRef]
- Jain, J.; Okabayashi, T.; Kaur, N.; Nakayama, E.; Shioda, T.; Gaind, R.; Kurosu, T.; Sunil, S. Evaluation of an immunochromatography rapid diagnosis kit for detection of chikungunya virus antigen in India, a dengue-endemic country. Virol. J. 2018, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Huits, R.; Okabayashi, T.; Cnops, L.; Barbé, B.; Berg, R.V.D.; Bartholomeeusen, K.; Ariën, K.K.; Jacobs, J.; Bottieau, E.; Nakayama, E.E.; et al. Diagnostic accuracy of a rapid E1-antigen test for chikungunya virus infection in a reference setting. Clin. Microbiol. Infect. 2018, 24, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Jimena, B.; Wehner, S.; Harold, G.; Bakheit, M.; Frischmann, S.; Bekaert, M.; Faye, O.; Sall, A.A.; Weidmann, M. Development of a single-tube one-step RT-LAMP assay to detect the Chikungunya virus genome. PLoS Negl. Trop. Dis. 2018, 12, e0006448. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Salas, I.; Danis-Lozano, R.; Casas-Martínez, M.; Ulloa, A.; Bond, J.G.; Marina, C.F.; Lopez-Ordonez, T.; Elizondo-Quiroga, A.; Torres-Monzón, J.A.; Diaz-Gonzalez, E.E. Historical inability to control Aedes aegypti as a main contributor of fast dispersal of chikungunya outbreaks in Latin America. Antivir. Res. 2015, 124, 30–42. [Google Scholar] [CrossRef]
- World Health Organization. World Health Organization 2019. Available online: https://www.who.int (accessed on 17 April 2019).
- Staikowsky, F.; Talarmin, F.; Grivard, P.; Souab, A.; Schuffenecker, I.; Le Roux, K.; Lecuit, M.; Michault, A. Prospective Study of Chikungunya Virus Acute Infection in the Island of La Réunion during the 2005–2006 Outbreak. PLoS ONE 2009, 4, e7603. [Google Scholar] [CrossRef]
- De La Hoz, J.M.; Bayona, B.; Viloria, S.; Accini, J.L.; San-Juan-Vergara, H.; Viasus, D. Fatal cases of Chikungunya virus infection in Colombia: Diagnostic and treatment challenges. J. Clin. Virol. 2015, 69, 27–29. [Google Scholar] [CrossRef]
- Natrajan, M.S.; Rojas, A.; Waggoner, J.J. Beyond Fever and Pain: Diagnostic Methods for Chikungunya Virus. J. Clin. Microbiol. 2019, 57, 1–14. [Google Scholar] [CrossRef]

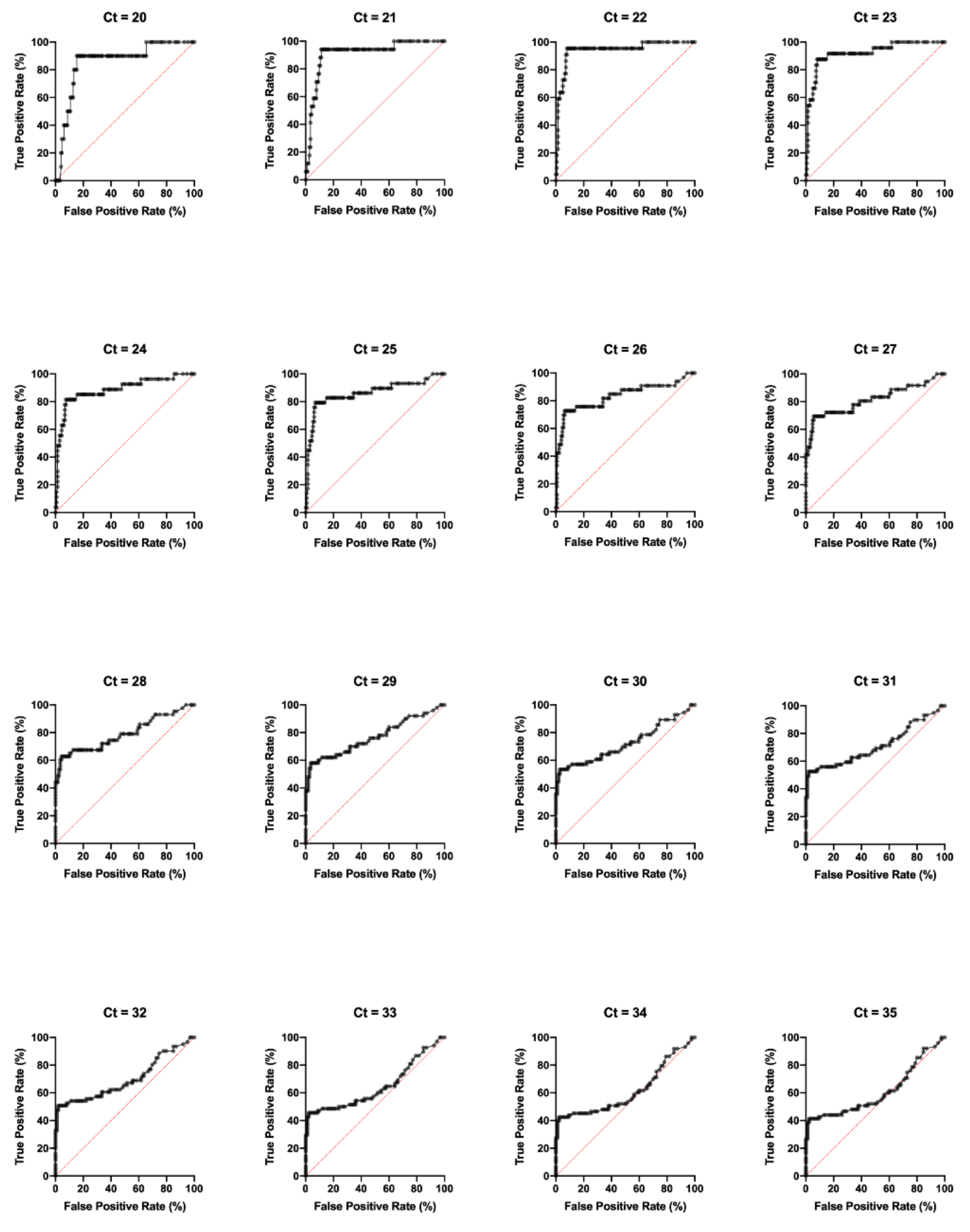

| CHIKV Combination A ELISA Receiver Operator Characteristic (ROC) Analysis (n = 160) | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ct Cutoff | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 | 30 | 31 | 32 | 33 | 34 | 35 |
| AUC | 0.86 | 0.91 | 0.94 | 0.92 | 0.89 | 0.86 | 0.83 | 0.81 | 0.79 | 0.77 | 0.72 | 0.71 | 0.70 | 0.65 | 0.61 | 0.61 |
| 95% CI | 0.74–0.97 | 0.84–0.99 | 0.89–1.00 | 0.86–0.98 | 0.80–0.97 | 0.77–0.95 | 0.74–0.93 | 0.72–0.91 | 0.70–0.88 | 0.68–0.86 | 0.63–0.82 | 0.62–0.81 | 0.61–0.79 | 0.55–0.74 | 0.52–0.71 | 0.52–0.70 |
| OD450 Cutoff | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 | 0.22 |
| Sensitivity (%) | 90.00 | 94.12 | 95.45 | 87.5 | 81.48 | 79.31 | 72.73 | 69.44 | 62.79 | 58.00 | 53.57 | 52.54 | 50.82 | 45.59 | 42.47 | 41.33 |
| Specificity (%) | 84.67 | 88.81 | 92.03 | 87.5 | 92.48 | 93.13 | 93.7 | 94.35 | 95.73 | 96.36 | 97.12 | 98.02 | 97.98 | 97.83 | 97.7 | 97.65 |
| N Total Positive | 150 | 143 | 138 | 136 | 133 | 131 | 127 | 124 | 117 | 110 | 104 | 101 | 99 | 92 | 87 | 85 |
| N Total Negative | 10 | 17 | 22 | 24 | 27 | 29 | 33 | 36 | 43 | 50 | 56 | 59 | 61 | 68 | 73 | 75 |
| A. | |||||
|---|---|---|---|---|---|
| CHIKV Combination A Lateral Flow Receiver Operator Characteristic (ROC) Analysis-Honduras (n = 19) | |||||
| Ct Cutoff | 20 | 21–24 | 25–26 | 27 | 28 |
| AUC | 0.96 | 0.95 | 0.94 | 1.00 | 0.81 |
| 95% CI | 0.88–1.00 | 0.85–1.00 | 0.83–1.00 | 1.00–1.00 | 0.61–1.000 |
| Lateral Flow Signal Intensity Cutoff | 0.82 | 0.75 | 0.67 | 0.57 | 0.57 |
| Sensitivity (%) | 100 | 100 | 100 | 100 | 62.50 |
| Specificity (%) | 92.31 | 91.67 | 90.91 | 100 | 100 |
| N Total Positive | 13 | 12 | 11 | 9 | 3 |
| N Total Negative | 6 | 7 | 8 | 10 | 16 |
| B. | |||||
| CHIKV Combination B Lateral Flow Receiver Operator Characteristic (ROC) Analysis-Honduras (n = 19) | |||||
| Ct Cutoff | 20 | 21–26 | 27 | 28 | |
| AUC | 0.94 | 0.93 | 1.00 | 0.81 | |
| 95% CI | 0.82–1.00 | 0.82–1.00 | 1.00–1.00 | 0.61–1.00 | |
| Lateral Flow Signal Intensity Cutoff | 0.72 | 0.53 | 0.53 | 0.53 | |
| Sensitivity (%) | 83.33 | 100 | 100 | 62.50 | |
| Specificity (%) | 92.31 | 75.00 | 100 | 100 | |
| N Total Positive | 13 | 12 | 9 | 3 | |
| N Total Negative | 6 | 7 | 10 | 16 | |
| C. | |||||
| CHIKV Combination B Lateral Flow Receiver Operator Characteristic (ROC) Analysis-Colombia (n = 10) | |||||
| Ct Cutoff | 18–19 | 20 | 21 | ||
| AUC | 0.86 | 1.00 | 0.78 | ||
| 95% CI | 0.60–1.00 | 1.00–1.00 | 0.51–1.00 | ||
| Lateral Flow Signal Intensity Cutoff | 0.63 | 0.59 | 0.56 | ||
| Sensitivity (%) | 100 | 100 | 77.78 | ||
| Specificity (%) | 85.71 | 100 | 100 | ||
| N Total Positive | 7 | 4 | 1 | ||
| N Total Negative | 3 | 6 | 9 | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Reddy, A.; Bosch, I.; Salcedo, N.; Herrera, B.B.; de Puig, H.; Narváez, C.F.; Caicedo-Borrero, D.M.; Lorenzana, I.; Parham, L.; García, K.; et al. Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas. Viruses 2020, 12, 971. https://doi.org/10.3390/v12090971
Reddy A, Bosch I, Salcedo N, Herrera BB, de Puig H, Narváez CF, Caicedo-Borrero DM, Lorenzana I, Parham L, García K, et al. Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas. Viruses. 2020; 12(9):971. https://doi.org/10.3390/v12090971
Chicago/Turabian StyleReddy, Ankita, Irene Bosch, Nol Salcedo, Bobby Brooke Herrera, Helena de Puig, Carlos F. Narváez, Diana María Caicedo-Borrero, Ivette Lorenzana, Leda Parham, Kimberly García, and et al. 2020. "Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas" Viruses 12, no. 9: 971. https://doi.org/10.3390/v12090971
APA StyleReddy, A., Bosch, I., Salcedo, N., Herrera, B. B., de Puig, H., Narváez, C. F., Caicedo-Borrero, D. M., Lorenzana, I., Parham, L., García, K., Mercado, M., Turca, A. M. R., Villar-Centeno, L. A., Gélvez-Ramírez, M., Ríos, N. A. G., Hiley, M., García, D., Diamond, M. S., & Gehrke, L. (2020). Development and Validation of a Rapid Lateral Flow E1/E2-Antigen Test and ELISA in Patients Infected with Emerging Asian Strain of Chikungunya Virus in the Americas. Viruses, 12(9), 971. https://doi.org/10.3390/v12090971





