Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Considerations for the Commencement of the Study
2.2. Study Design and Patient Recruitment
2.3. Dengue NS1 Antigen and Serology Tests
2.4. Molecular Detection for DENV, Other Arboviruses, and Typhoid Fever
2.5. DENV Isolation in Cell Culture
2.6. Determination of DENV Genotype by Envelope Gene Sequencing
2.7. DENV Phylogenetic Reconstruction and Evolutionary Analysis
2.8. Statistical Analysis
3. Results
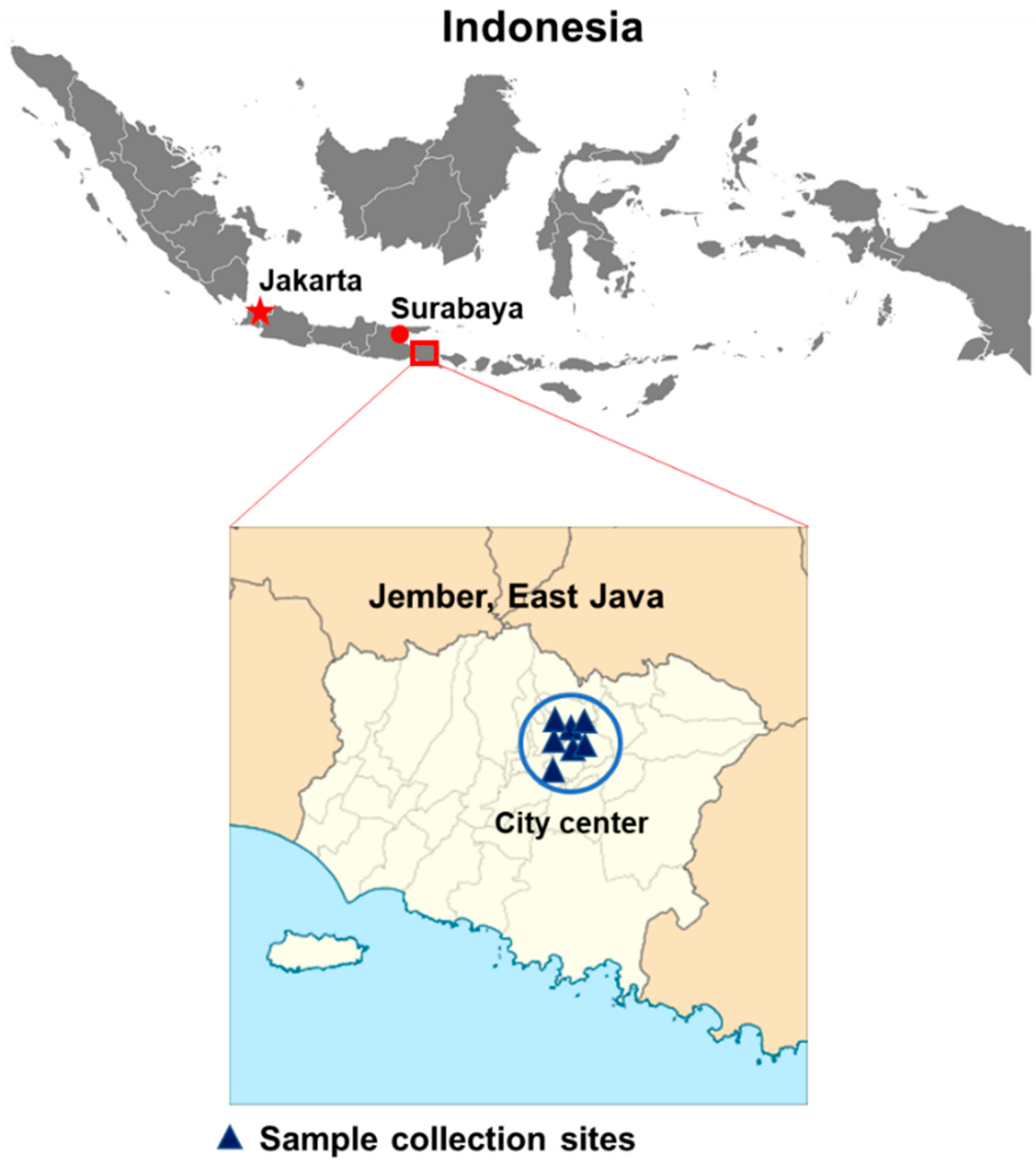
3.1. Sample Collection and Demographical Data
3.2. Clinical Examination of Dengue-Suspected Patients
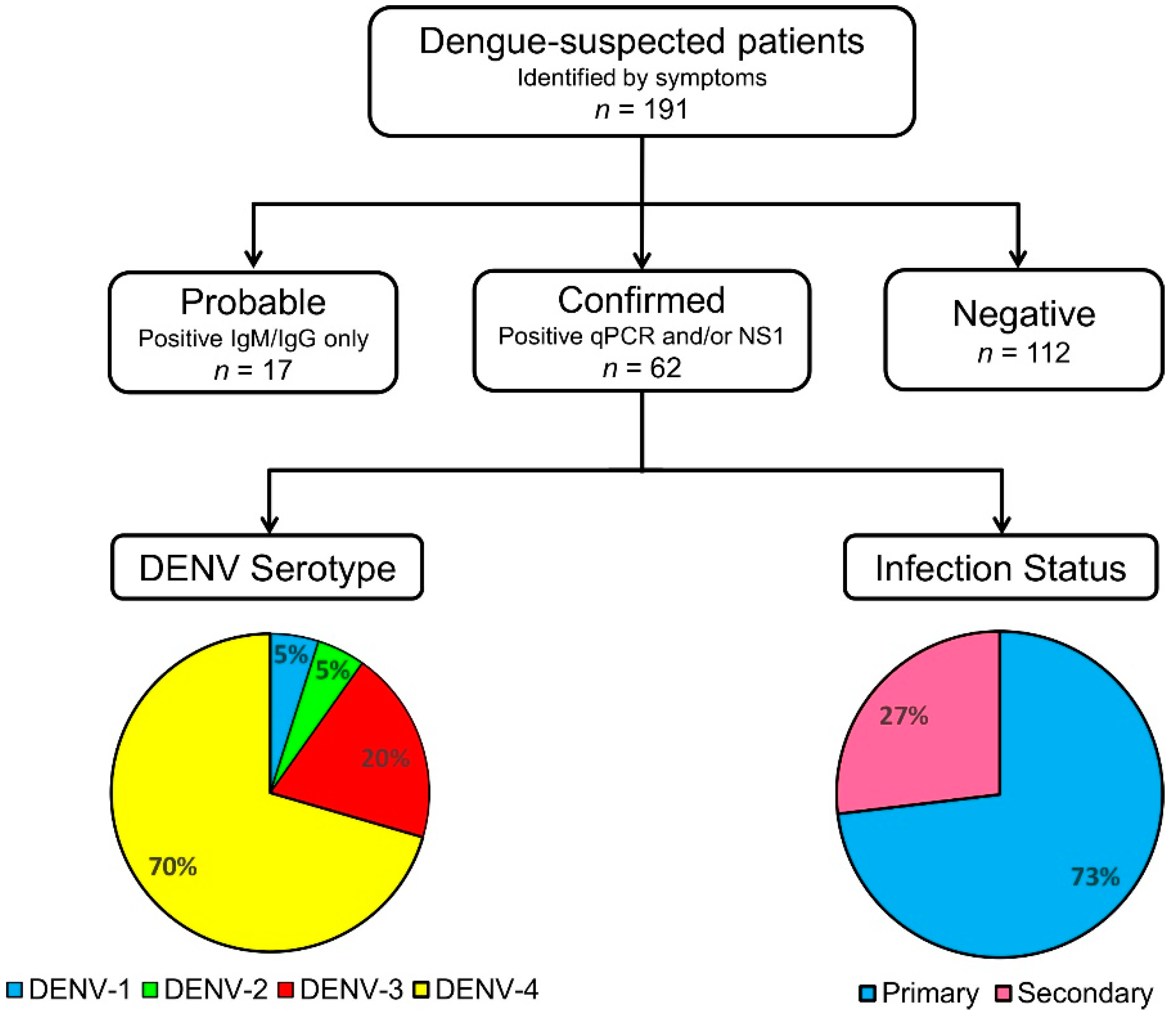
3.3. Dengue Serological Testing and Case Confirmation
3.4. DENV Circulating in Jember
3.5. DENV Serotypes and Their Clinical Correlates
3.6. Non-DENV Arboviruses and Other Pathogens Circulating Jember
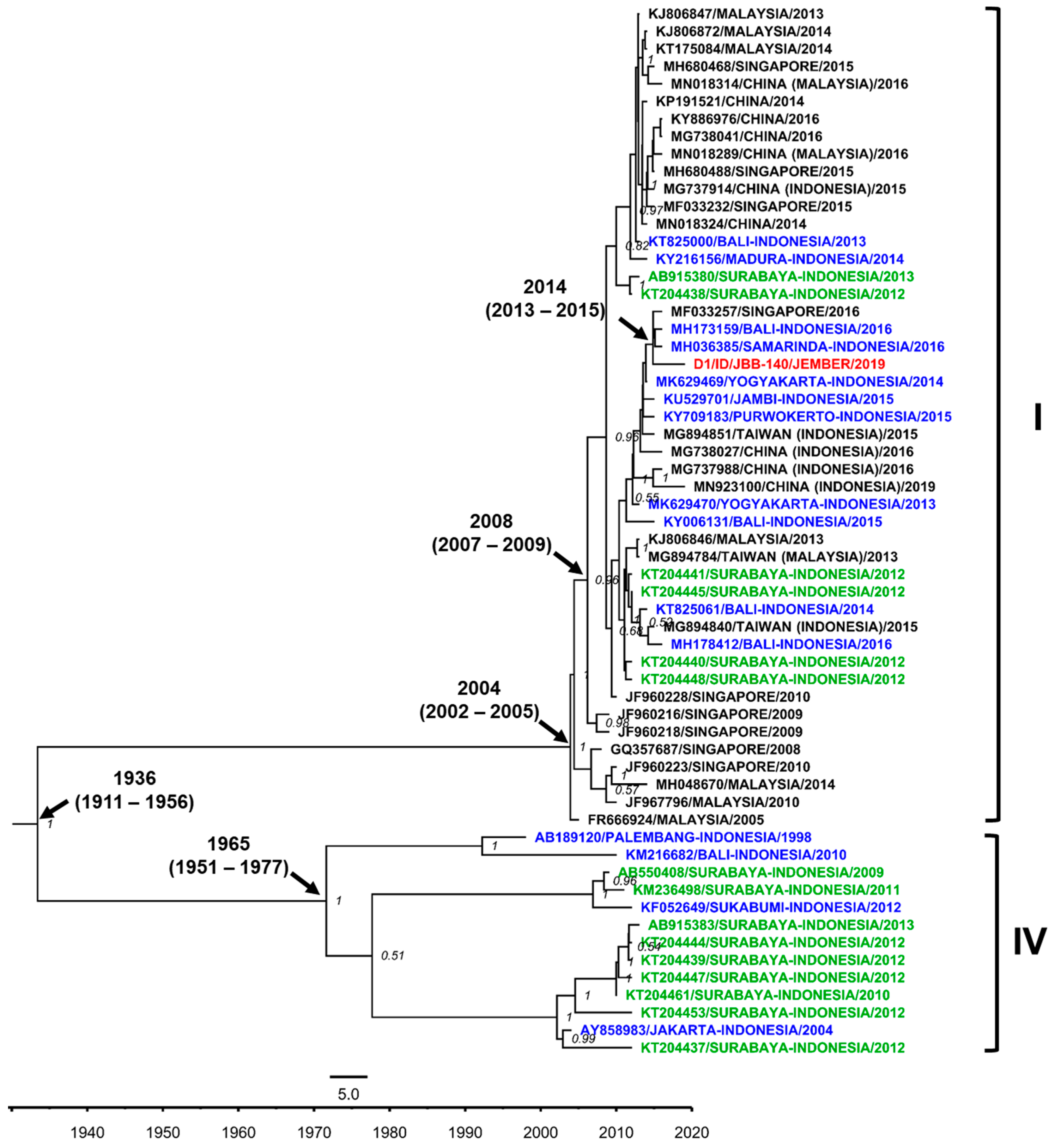
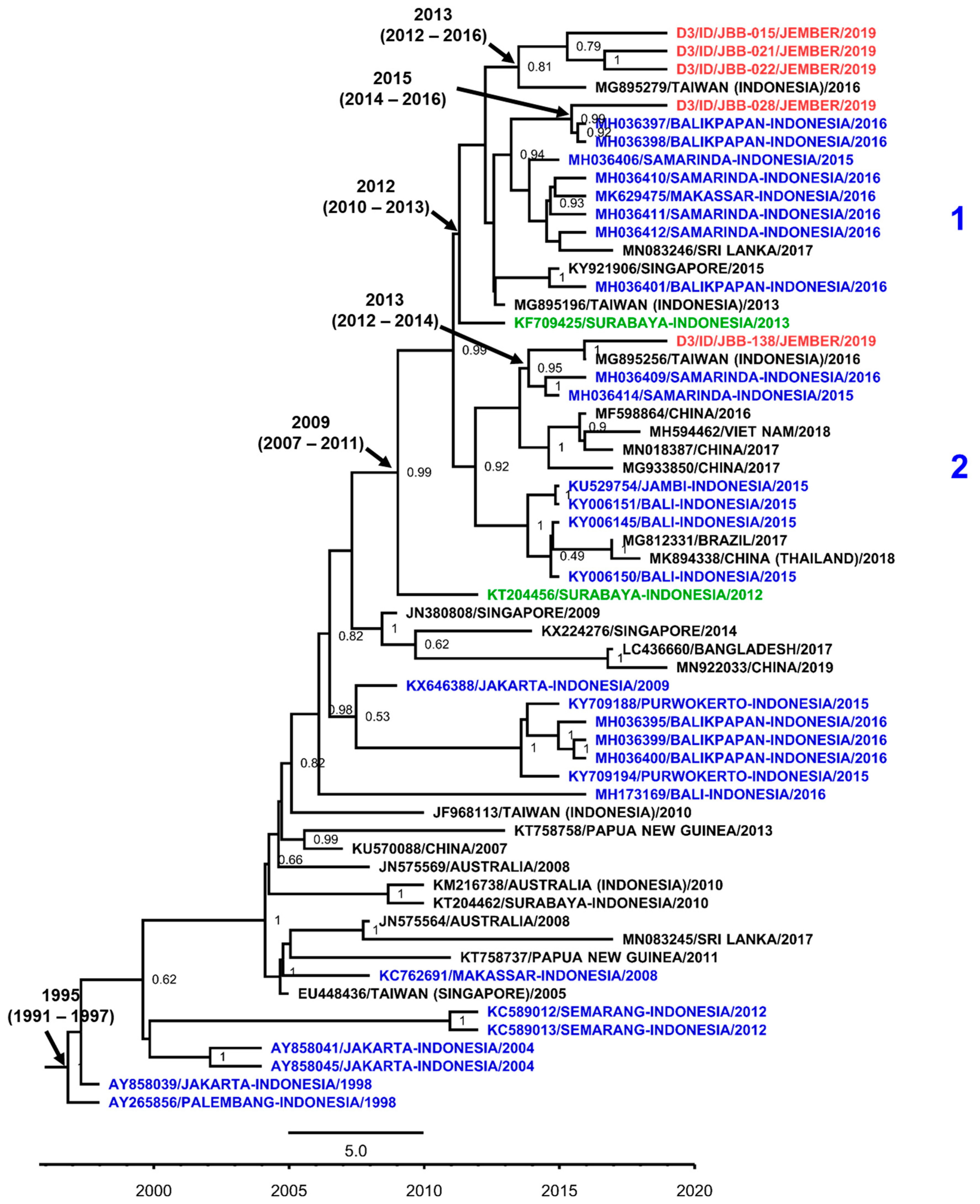
3.7. DENV Genotypes in Jember
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wei, K.; Li, Y. Global evolutionary history and spatio-temporal dynamics of dengue virus type 2. Sci. Rep. 2017, 7, 45505. [Google Scholar] [CrossRef] [PubMed]
- Henchal, E.A.; Putnak, J.R. The dengue viruses. Clin. Microbiol. Rev. 1990, 3, 376–396. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martínez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8, S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J. Dengue and dengue hemorrhagic fever. Clin. Microbiol. Rev. 1998, 11, 480–496. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Comprehensive Guidelines for Prevention and Control of Dengue and Dengue Haemorrhagic Fever; Revised and Expanded; WHO Regional Office for South-East Asia: New Delhi, India, 2011; ISBN 978-92-9022-387-0. [Google Scholar]
- Holmes, E.C.; Twiddy, S.S. The origin, emergence and evolutionary genetics of dengue virus. Infect. Genet. Evol. 2003, 3, 19–28. [Google Scholar] [CrossRef]
- Rico-Hesse, R. Microevolution and virulence of dengue viruses. Adv. Virus Res. 2003, 59, 315–341. [Google Scholar]
- Weaver, S.C.; Vasilakis, N. Molecular Evolution of Dengue Viruses: Contributions of Phylogenetics to Understanding the History and Epidemiology of the Preeminent Arboviral Disease. Infect. Genet. Evol. 2009, 9, 523–540. [Google Scholar] [CrossRef]
- Sang, S.; Liu-Helmersson, J.; Quam, M.B.M.; Zhou, H.; Guo, X.; Wu, H.; Liu, Q. The evolutionary dynamics of DENV 4 genotype I over a 60-year period. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef]
- Sumarmo. Dengue haemorrhagic fever in Indonesia. Southeast Asian J. Trop. Med. Public Health 1987, 18, 269–274. [Google Scholar]
- Ministry of Health of the Republic of Indonesia Indonesia Health Profile 2018; Ministry of Health: Jakarta, Indonesia, 2019.
- Kotaki, T.; Yamanaka, A.; Mulyatno, K.C.; Churrotin, S.; Labiqah, A.; Sucipto, T.H.; Soegijanto, S.; Kameoka, M.; Konishi, E. Continuous dengue type 1 virus genotype shifts followed by co-circulation, clade shifts and subsequent disappearance in Surabaya, Indonesia, 2008–2013. Infect. Genet. Evol. 2014, 28, 48–54. [Google Scholar] [CrossRef]
- Kotaki, T.; Yamanaka, A.; Mulyatno, K.C.; Churrotin, S.; Sucipto, T.H.; Labiqah, A.; Ahwanah, N.L.F.; Soegijanto, S.; Kameoka, M.; Konishi, E. Divergence of the dengue virus type 2 Cosmopolitan genotype associated with two predominant serotype shifts between 1 and 2 in Surabaya, Indonesia, 2008–2014. Infect. Genet. Evol. 2016, 37, 88–93. [Google Scholar] [CrossRef] [PubMed]
- Wardhani, P.; Aryati, A.; Yohan, B.; Trimarsanto, H.; Setianingsih, T.Y.; Puspitasari, D.; Arfijanto, M.V.; Bramantono, B.; Suharto, S.; Sasmono, R.T. Clinical and virological characteristics of dengue in Surabaya, Indonesia. PLoS ONE 2017, 12, e0178443. [Google Scholar] [CrossRef] [PubMed]
- Sucipto, T.H.; Kotaki, T.; Mulyatno, K.C.; Churrotin, S.; Labiqah, A.; Soegijanto, S.; Kameoka, M. Phylogenetic Analysis of Dengue Virus in Bangkalan, Madura Island, East Java Province, Indonesia. J. Trop. Med. 2018, 2018, 8127093. [Google Scholar] [CrossRef] [PubMed]
- Klungthong, C.; Putnak, R.; Mammen, M.P.; Li, T.; Zhang, C. Molecular genotyping of dengue viruses by phylogenetic analysis of the sequences of individual genes. J. Virol. Methods 2008, 154, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Sasmono, R.T.; Wahid, I.; Trimarsanto, H.; Yohan, B.; Wahyuni, S.; Hertanto, M.; Yusuf, I.; Mubin, H.; Ganda, I.J.; Latief, R.; et al. Genomic analysis and growth characteristic of dengue viruses from Makassar, Indonesia. Infect. Genet. Evol. 2015, 32, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Gallichotte, E.N.; Baric, T.J.; Nivarthi, U.; Delacruz, M.J.; Graham, R.; Widman, D.G.; Yount, B.L.; Durbin, A.P.; Whitehead, S.S.; de Silva, A.M.; et al. Genetic Variation between Dengue Virus Type 4 Strains Impacts Human Antibody Binding and Neutralization. Cell Rep. 2018, 25, 1214–1224. [Google Scholar] [CrossRef]
- Harapan, H.; Michie, A.; Yohan, B.; Shu, P.Y.; Mudatsir, M.; Sasmono, R.T.; Imrie, A. Dengue viruses circulating in Indonesia: A systematic review and phylogenetic analysis of data from five decades. Rev. Med. Virol. 2019, 29, e2037. [Google Scholar] [CrossRef]
- Lestari, C.S.W.; Yohan, B.; Yunita, A.; Meutiawati, F.; Hayati, R.F.; Trimarsanto, H.; Sasmono, R.T. Phylogenetic and evolutionary analyses of dengue viruses isolated in Jakarta, Indonesia. Virus Genes 2017, 53, 778–788. [Google Scholar] [CrossRef]
- Dewi, B.E.; Naiggolan, L.; Putri, D.H.; Rachmayanti, N.; Albar, S.; Indriastuti, N.T.; Sjamsuridzal, W.; Sudiro, T.M. Characterization of dengue virus serotype 4 infection in Jakarta, Indonesia. Southeast Asian J. Trop. Med. Public Health 2014, 45, 53–61. [Google Scholar]
- Harapan, H.; Michie, A.; Mudatsir, M.; Nusa, R.; Yohan, B.; Wagner, A.L.; Sasmono, R.T.; Imrie, A. Chikungunya virus infection in Indonesia: A systematic review and evolutionary analysis. BMC Infect. Dis. 2019, 19, 243. [Google Scholar] [CrossRef]
- Priyamvada, L.; Hudson, W.; Ahmed, R.; Wrammert, J. Humoral cross-reactivity between Zika and dengue viruses: Implications for protection and pathology. Emerg. Microbes Infect. 2017, 6, e33. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Nisalak, A.; Kalayanarooj, S.; Solomon, T.; Dung, N.M.; Cuzzubbo, A.; Devine, P.L. Evaluation of a Rapid Immunochromatographic Test for Diagnosis of Dengue Virus Infection. J. Clin. Microbiol. 1998, 36, 234–238. [Google Scholar] [CrossRef] [PubMed]
- Sasmono, R.T.; Dhenni, R.; Yohan, B.; Pronyk, P.; Hadinegoro, S.R.; Soepardi, E.J.; Ma’roef, C.; Satari, H.; Menzies, H.; Hawley, W.; et al. Endemicity of Zika virus in Indonesia: Serological evidence in pediatric population. Int. J. Infect. Dis. 2019, 79, 13–14. [Google Scholar] [CrossRef]
- BPS-Statistics of Jember Regency. Jember Regency in Figures 2020 Delivering Data to Inform Development Planning; BPS-Statistics of Jember Regency: Jember, Indonesia, 2020; ISBN 978-602-71310-3-3.
- Kuno, G.; Chang, G.J.; Tsuchiya, K.R.; Karabatsos, N.; Cropp, C.B. Phylogeny of the genus Flavivirus. J. Virol. 1998, 72, 73–83. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Escalante, A.A.; Pujol, F.H.; Ludert, J.E.; Tovar, D.; Salas, R.A.; Liprandi, F. Diversity and evolution of the envelope gene of dengue virus type 1. Virology 2002, 303, 110–119. [Google Scholar] [CrossRef]
- Twiddy, S.S.; Farrar, J.J.; Vinh Chau, N.; Wills, B.; Gould, E.A.; Gritsun, T.; Lloyd, G.; Holmes, E.C. Phylogenetic relationships and differential selection pressures among genotypes of dengue-2 virus. Virology 2002, 298, 63–72. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Lewis, J.G.; Gubler, D.J.; Trent, D.W. Molecular evolution and epidemiology of dengue-3 viruses. J. Gen. Virol. 1994, 75, 65–75. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Gubler, D.J.; Trent, D.W. Molecular evolution and phylogeny of dengue-4 viruses. J. Gen. Virol. 1997, 78, 2279–2284. [Google Scholar] [CrossRef]
- Bouckaert, R.; Vaughan, T.G.; Barido-Sottani, J.; Duchêne, S.; Fourment, M.; Gavryushkina, A.; Heled, J.; Jones, G.; Kühnert, D.; De Maio, N.; et al. BEAST 2.5: An advanced software platform for Bayesian evolutionary analysis. PLoS Comput. Biol. 2019, 15, e1006650. [Google Scholar] [CrossRef]
- Costa, R.L.; Voloch, C.M.; Schrago, C.G. Comparative evolutionary epidemiology of dengue virus serotypes. Infect. Genet. Evol. 2012, 12, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Sasmono, R.T.; Kalalo, L.P.; Trismiasih, S.; Denis, D.; Yohan, B.; Hayati, R.F.; Haryanto, S. Multiple introductions of dengue virus strains contribute to dengue outbreaks in East Kalimantan, Indonesia, in 2015–2016. Virol. J. 2019, 16, 93. [Google Scholar] [CrossRef] [PubMed]
- Masyeni, S.; Yohan, B.; Somia, I.K.A.; Myint, K.S.A.; Sasmono, R.T. Dengue infection in international travellers visiting Bali, Indonesia. J. Travel. Med. 2018, 25. [Google Scholar] [CrossRef] [PubMed]
- Utama, I.M.S.; Lukman, N.; Sukmawati, D.D.; Alisjahbana, B.; Alam, A.; Murniati, D.; Utama, I.M.G.D.L.; Puspitasari, D.; Kosasih, H.; Laksono, I.; et al. Dengue viral infection in Indonesia: Epidemiology, diagnostic challenges, and mutations from an observational cohort study. PLoS Negl. Trop. Dis. 2019, 13, e0007785. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, S.; Hayati, R.F.; Yohan, B.; Sijabat, L.; Sihite, I.F.; Fahri, S.; Meutiawati, F.; Halim, J.A.N.; Halim, S.N.; Soebandrio, A.; et al. The molecular and clinical features of dengue during outbreak in Jambi, Indonesia in 2015. Pathog. Glob. Health 2016, 110, 119–129. [Google Scholar] [CrossRef] [PubMed]
- Kusmintarsih, E.S.; Hayati, R.F.; Turnip, O.N.; Yohan, B.; Suryaningsih, S.; Pratiknyo, H.; Denis, D.; Sasmono, R.T. Molecular characterization of dengue viruses isolated from patients in Central Java, Indonesia. J. Infect. Public Health 2017. [Google Scholar] [CrossRef]
- Megawati, D.; Masyeni, S.; Yohan, B.; Lestarini, A.; Hayati, R.F.; Meutiawati, F.; Suryana, K.; Widarsa, T.; Budiyasa, D.G.; Budiyasa, N.; et al. Dengue in Bali: Clinical characteristics and genetic diversity of circulating dengue viruses. PLoS Negl. Trop. Dis. 2017, 11, e0005483. [Google Scholar] [CrossRef]
- Nusa, R.; Prasetyowati, H.; Meutiawati, F.; Yohan, B.; Trimarsanto, H.; Setianingsih, Y.; Sasmono, R.T. Molecular surveillance of dengue in Sukabumi, West Java province, Indonesia. J. Infect. Dev. Ctries 2014, 8, 733–741. [Google Scholar] [CrossRef]
- Kosasih, H.; de Mast, Q.; Widjaja, S.; Sudjana, P.; Antonjaya, U.; Ma’roef, C.; Riswari, S.F.; Porter, K.R.; Burgess, T.H.; Alisjahbana, B.; et al. Evidence for endemic chikungunya virus infections in Bandung, Indonesia. PLoS Negl. Trop. Dis. 2013, 7, e2483. [Google Scholar] [CrossRef]
- Capeding, M.R.; Chua, M.N.; Hadinegoro, S.R.; Hussain, I.I.H.M.; Nallusamy, R.; Pitisuttithum, P.; Rusmil, K.; Thisyakorn, U.; Thomas, S.J.; Huu Tran, N.; et al. Dengue and other common causes of acute febrile illness in Asia: An active surveillance study in children. PLoS Negl. Trop. Dis. 2013, 7, e2331. [Google Scholar] [CrossRef]
- Guzman, M.G.; Alvarez, M.; Halstead, S.B. Secondary infection as a risk factor for dengue hemorrhagic fever/dengue shock syndrome: An historical perspective and role of antibody-dependent enhancement of infection. Arch. Virol. 2013, 158, 1445–1459. [Google Scholar] [CrossRef] [PubMed]
- Oishi, K.; Inoue, S.; Cinco, M.T.D.D.; Dimaano, E.M.; Alera, M.T.P.; Alfon, J.A.R.; Abanes, F.; Cruz, D.J.M.; Matias, R.R.; Matsuura, H.; et al. Correlation between increased platelet-associated IgG and thrombocytopenia in secondary dengue virus infections. J. Med. Virol. 2003, 71, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Ong, S.H.; Yip, J.T.; Chen, Y.L.; Liu, W.; Harun, S.; Lystiyaningsih, E.; Heriyanto, B.; Beckett, C.G.; Mitchell, W.P.; Hibberd, M.L.; et al. Periodic re-emergence of endemic strains with strong epidemic potential-a proposed explanation for the 2004 Indonesian dengue epidemic. Infect. Genet. Evol. 2008, 8, 191–204. [Google Scholar] [CrossRef] [PubMed]
- Fahri, S.; Yohan, B.; Trimarsanto, H.; Sayono, S.; Hadisaputro, S.; Dharmana, E.; Syafruddin, D.; Sasmono, R.T. Molecular Surveillance of Dengue in Semarang, Indonesia Revealed the Circulation of an Old Genotype of Dengue Virus Serotype-1. PLoS Negl. Trop. Dis. 2013, 7, e2354. [Google Scholar] [CrossRef] [PubMed]
- Forshey, B.M.; Reiner, R.C.; Olkowski, S.; Morrison, A.C.; Espinoza, A.; Long, K.C.; Vilcarromero, S.; Casanova, W.; Wearing, H.J.; Halsey, E.S.; et al. Incomplete Protection against Dengue Virus Type 2 Re-infection in Peru. PLoS Negl. Trop. Dis. 2016, 10, e0004398. [Google Scholar] [CrossRef]
- Waggoner, J.J.; Balmaseda, A.; Gresh, L.; Sahoo, M.K.; Montoya, M.; Wang, C.; Abeynayake, J.; Kuan, G.; Pinsky, B.A.; Harris, E. Homotypic Dengue Virus Reinfections in Nicaraguan Children. J. Infect. Dis. 2016, 214, 986–993. [Google Scholar] [CrossRef]
- Soo, K.M.; Khalid, B.; Ching, S.M.; Chee, H.Y. Meta-Analysis of Dengue Severity during Infection by Different Dengue Virus Serotypes in Primary and Secondary Infections. PLoS ONE 2016, 11, e0154760. [Google Scholar] [CrossRef]
- Vicente, C.R.; Herbinger, K.H.; Fröschl, G.; Malta Romano, C.; de Souza Areias Cabidelle, A.; Cerutti Junior, C. Serotype influences on dengue severity: A cross-sectional study on 485 confirmed dengue cases in Vitória, Brazil. BMC Infect. Dis. 2016, 16, 320. [Google Scholar] [CrossRef]
- Yung, C.F.; Lee, K.S.; Thein, T.L.; Tan, L.K.; Gan, V.C.; Wong, J.G.X.; Lye, D.C.; Ng, L.C.; Leo, Y.S. Dengue serotype-specific differences in clinical manifestation, laboratory parameters and risk of severe disease in adults, Singapore. Am. J. Trop. Med. Hyg. 2015, 92, 999–1005. [Google Scholar] [CrossRef]



| Symptoms (n = 191) | n (%) |
|---|---|
| Headache | 159 (83.2) |
| Myalgia | 100 (52.4) |
| Vomiting | 80 (41.9) |
| Stomachache | 73 (38.2) |
| Arthralgia | 69 (36.1) |
| Retro-orbital Pain | 47 (24.6) |
| Bleeding manifestation | 27 (14.1) |
| Rash | 13 (6.8) |
| Positive Tourniquet Test | 10 (5.2) |
| Altered Consciousness | 0 (0) |
| Ascites | 0 (0) |
| Pleural Effusion | 0 (0) |
| Parameters | Infection Status (n = 79) | p-Value a | |
|---|---|---|---|
| Primary (n = 58) | Secondary (n = 21) | ||
| Age, Median (IQR) | 19.5 (8–28.5) | 19 (9–26) | 0.665 b |
| Age groups | 1.000 | ||
| Children (<20 y.o) | 28 | 10 | |
| Adults (≥20 y.o) | 30 | 11 | |
| Gender | 0.111 | ||
| Male | 33 | 7 | |
| Female | 25 | 14 | |
| Clinical manifestation | 0.005 | ||
| DF | 48 | 10 | |
| DHF | 10 | 11 | |
| Hematology data, Median (IQR) | |||
| Platelet (×103/µL) | 172 (105.3–223) | 57 (35–85) | <0.001 b |
| Hematocrit (%) | 41.1 (38.3–44.8) | 42.9 (41.3–46.0) | 0.052 b |
| WBC (×103/µL) | 4.3 (3.2–7.3) | 3.6 (2.7–5.6) | 0.502 b |
| Parameters | DENV Serotype (n = 61) | p-Value | |||
|---|---|---|---|---|---|
| DENV-1 (n = 3) | DENV-2 (n = 3) | DENV-3 (n = 12) | DENV-4 (n = 43) | ||
| Age, Median (IQR) | 8 | 11 | 20 | 20 | 0.336 a |
| (7–12.5) | (7.2–20) | (11.8–22.3) | (11.5–30.5) | ||
| Gender | 0.143 b | ||||
| Male | 3 | 1 | 4 | 25 | |
| Female | 0 | 2 | 8 | 18 | |
| Clinical manifestation | 0.011 b,c | ||||
| DF | 0 | 1 | 10 | 33 | |
| DHF | 3 | 2 | 2 | 10 | |
| Infection status | 0.004 b,d | ||||
| Primary | 1 | 1 | 12 | 36 | |
| Secondary | 2 | 2 | 0 | 6 | |
| Hematology, Median (IQR) | |||||
| Platelet (×103/µL) | 20 | 73 | 185 | 143 | 0.008 a,e |
| (15.5–20.5) | (53.5–86) | (118.8–194) | (76–227.5) | ||
| Hematocrit (%) | 2.90 | 2.60 | 3.65 | 4.64 | 0.529 a |
| (2.65–3.25) | (2.50–3.20) | (2.90–4.60) | (2.95–8.50) | ||
| WBC (×103/µL) | 47.5 | 44.4 | 42.0 | 41.5 | 0.204 a |
| (43.2–47.8) | (41.6–45.2) | (38.3–45.6) | (38.6–45.1) | ||
| n (%) | Dengue (n = 64) | Typhoid (n = 17) | Double Dengue-Typhoid (n = 15) | FUO (n = 95) | Pearson’s Chi Square Test |
|---|---|---|---|---|---|
| Headache | 50 (78.1) | 14 (82.4) | 14 (93.3) | 81 (85.3) | 0.404 |
| Retro-orbital Pain | 17 (26.6) | 4 (23.5) | 1 (6.7) | 25 (26.3) | 0.402 |
| Myalgia | 35 (54.7) | 9 (52.9) | 4 (26.7) | 52 (54.7) | 0.218 |
| Arthralgia | 25 (39.1) | 5 (29.4) | 2 (13.3) | 37 (38.9) | 0.225 |
| Rash | 3 (4.7) | 0 (0) | 0 (0) | 10 (10.5) | 0.182 |
| Stomachache | 27 (42.2) | 7 (41.2) | 4 (26.7) | 35 (36.8) | 0.713 |
| Vomiting | 23 (35.9) | 10 (58.8) | 6 (40.0) | 41 (43.2) | 0.382 |
| Bleeding | 8 (12.5) | 4 (23.5) | 3 (20.0) | 12 (12.6) | 0.577 |
| Positive Tourniquet | 11 (17.2) | 0 (0) | 2 (13.3) | 7 (7.4) | 0.097 |
| One-Way ANOVA | |||||
| Platelet (×103/µL) | 140 (71–223) | 174 (135–223) | 136 (74–181) | 171 (138–254) | 0.005 * |
| Hematocrit (%) | 42 (38.9–45.0) | 36 (34.2–41.0) | 42 (35.5–45.6) | 40 (36.6–44.0) | 0.249 |
| WBC (×103/µL) | 4.4 (2.85–7.13) | 5.6 (4.63–7.10) | 4.0 (3.25–6.05) | 5.6 (4.22–8.35) | 0.119 |
| No. | Sample ID | Serotype | Genotype | Age (y) | Manifestation | Accession No. |
|---|---|---|---|---|---|---|
| 1. | JBB-140 | DENV-1 | I | 8 | DHF | MT377728 |
| 2. | JBB-145 | DENV-2 | Cosmopolitan | 4 | DHF | MT377729 |
| 3. | JBB-015 | DENV-3 | I | 1 | DF | MT377730 |
| 4. | JBB-021 | DENV-3 | I | 8 | DF | MT377731 |
| 5. | JBB-022 | DENV-3 | I | 2 | DF | MT377732 |
| 6. | JBB-028 | DENV-3 | I | 21 | DF | MT377733 |
| 7. | JBB-138 | DENV-3 | I | 35 | DHF | MT377734 |
| 8. | JBB-004 | DENV-4 | II | 40 | DF | MT377735 |
| 9. | JBB-007 | DENV-4 | II | 16 | DHF | MT377736 |
| 10. | JBB-008 | DENV-4 | II | 26 | DF | MT377737 |
| 11. | JBB-023 | DENV-4 | II | 38 | DF | MT377738 |
| 12. | JBB-024 | DENV-4 | II | 7 | DF | MT377739 |
| 13. | JBB-037 | DENV-4 | II | 62 | DF | MT377740 |
| 14. | JBB-042 | DENV-4 | II | 19 | DHF | MT377741 |
| 15. | JBB-044 | DENV-4 | II | 18 | DF | MT377742 |
| 16. | JBB-045 | DENV-4 | II | 12 | DF | MT377743 |
| 17. | JBB-049 | DENV-4 | II | 18 | DF | MT377744 |
| 18. | JBB-050 | DENV-4 | II | 11 | DHF | MT377745 |
| 19. | JBB-051 | DENV-4 | II | 8 | DHF | MT377746 |
| 20. | JBB-055 | DENV-4 | II | 42 | DF | MT377747 |
| 21. | JBB-072 | DENV-4 | II | 5 | DF | MT377748 |
| 22. | JBB-136 | DENV-4 | II | 38 | DF | MT377749 |
| Serotype | DENV Tree | Jember DENV Isolate(s) | |||
|---|---|---|---|---|---|
| n | Tree Age (Years) | Median Rate × 10−4 (95% HPD) | n | Mean Rate (×10−4) ± STDEV | |
| DENV-1 | 60 | 83 | 7.7 (5.9–9.7) | 1 | 7.7 |
| DENV-2 | 60 | 41 | 6.5 (4.2–9.4) | 1 | 7.2 |
| DENV-3 | 60 | 24 | 11.2 (9.0–13.4) | 5 | 15.5 ± 4.2 |
| DENV-4 | 60 | 29 | 10.0 (8.2–11.8) | 15 | 9.9 ± 0.17 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aryati, A.; Wrahatnala, B.J.; Yohan, B.; Fanny, M.; Hakim, F.K.N.; Sunari, E.P.; Zuroidah, N.; Wardhani, P.; Santoso, M.S.; Husada, D.; et al. Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia. Viruses 2020, 12, 913. https://doi.org/10.3390/v12090913
Aryati A, Wrahatnala BJ, Yohan B, Fanny M, Hakim FKN, Sunari EP, Zuroidah N, Wardhani P, Santoso MS, Husada D, et al. Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia. Viruses. 2020; 12(9):913. https://doi.org/10.3390/v12090913
Chicago/Turabian StyleAryati, Aryati, Billy J. Wrahatnala, Benediktus Yohan, May Fanny, Faradila K. N. Hakim, Eka Putri Sunari, Nelly Zuroidah, Puspa Wardhani, Marsha S. Santoso, Dominicus Husada, and et al. 2020. "Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia" Viruses 12, no. 9: 913. https://doi.org/10.3390/v12090913
APA StyleAryati, A., Wrahatnala, B. J., Yohan, B., Fanny, M., Hakim, F. K. N., Sunari, E. P., Zuroidah, N., Wardhani, P., Santoso, M. S., Husada, D., Rohman, A., Tarmizi, S. N., Sievers, J. T. O., & Sasmono, R. T. (2020). Dengue Virus Serotype 4 Is Responsible for the Outbreak of Dengue in East Java City of Jember, Indonesia. Viruses, 12(9), 913. https://doi.org/10.3390/v12090913






