Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus
Abstract
1. Introduction
2. Hepatitis E Genome Organization
3. VLP-Based Vaccines for HEV Prevention
3.1. Expression of HEV ORF2 in E. Coli
3.2. Expression of HEV VLPs in the Baculovirus-Insect Cell System
3.2.1. Formation of VLPs from Animal HEV Sequences in the Baculovirus-Insect Cell System
3.2.2. HEV VLPs as a Platform for Foreign Epitopes
3.3. Expression of ORF2 as VLPs in Plants
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kamar, N.; Bendall, R.; Legrand-Abravanel, F.; Xia, N.-S.; Ijaz, S.; Izopet, J.; Dalton, H.R. Hepatitis E. Lancet 2012, 379, 2477–2488. [Google Scholar] [CrossRef]
- Holla, R.P.; Ahmad, I.; Ahmad, Z.; Jameel, S. Molecular virology of hepatitis E virus. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2013; pp. 3–14. [Google Scholar]
- Meng, X.-J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011, 161, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Subhadra, S.; Singh, B.; Panda, B. Hepatitis E virus: The current scenario. Int. J. Infect. Dis. 2013, 17, e228–e233. [Google Scholar] [CrossRef] [PubMed]
- Panda, S.K.; Thakral, D.; Rehman, S. Hepatitis E virus. Rev. Med Virol. 2007, 17, 151–180. [Google Scholar] [CrossRef]
- Khuroo, M.S.; Teli, M.R.; Skidmore, S.; Sofi, M.A.; Khuroo, M.I. Incidence and severity of viral hepatitis in pregnancy. Am. J. Med. 1981, 70, 252–255. [Google Scholar] [CrossRef]
- Khuroo, M.; Kamili, S. Aetiology, clinical course and outcome of sporadic acute viral hepatitis in pregnancy. J. Viral Hepat. 2003, 10, 61–69. [Google Scholar] [CrossRef]
- Jilani, N.; Das, B.C.; Husain, S.A.; Baweja, U.K.; Chattopadhya, D.; Gupta, R.K.; Sardana, S.; Kar, P. Hepatitis E virus infection and fulminant hepatic failure during pregnancy. J. Gastroenterol. Hepatol. 2007, 22, 676–682. [Google Scholar] [CrossRef]
- Allaire, M.; Bazille, C.; Selves, J.; Salamé, E.; Altieri, M. Hepatitis E virus infection mimicking acute graft rejection in a liver transplant recipient. Clin. Res. Hepatol. Gastroenterol. 2018, 42, e68–e71. [Google Scholar] [CrossRef]
- Behrendt, P.; Steinmann, E.; Manns, M.P.; Wedemeyer, H. The impact of hepatitis E in the liver transplant setting. J. Hepatol. 2014, 61, 1418–1429. [Google Scholar] [CrossRef]
- Marion, O.; Kamar, N. Hepatitis E Infections in Transplants. Emerg. Transpl. Infect. Clin. Chall. Implic. 2020, 1–18. [Google Scholar] [CrossRef]
- Pischke, S.; Peron, J.-M.; von Wulffen, M.; von Felden, J.; Höner zu Siederdissen, C.; Fournier, S.; Lütgehetmann, M.; Iking-Konert, C.; Bettinger, D.; Thimme, R. Chronic hepatitis e in rheumatology and internal medicine patients: A retrospective multicenter european cohort study. Viruses 2019, 11, 186. [Google Scholar] [CrossRef] [PubMed]
- Tavitian, S.; Peron, J.-M.; Huguet, F.; Kamar, N.; Abravanel, F.; Beyne-Rauzy, O.; Oberic, L.; Faguer, S.; Alric, L.; Roussel, M. Ribavirin for chronic hepatitis prevention among patients with hematologic malignancies. Emerg. Infect. Dis. 2015, 21, 1466. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Juarez, A.; Lopez-Lopez, P.; Frias, M.; Rivero, A. Hepatitis E infection in HIV-infected patients. Front. Microbiol. 2019, 10, 1425. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Sharma, B.C.; Sarin, S.K. Hepatitis E virus as an etiology of acute exacerbation of previously unrecognized asymptomatic patients with hepatitis B virus-related chronic liver disease. J. Gastroenterol. Hepatol. 2008, 23, 883–887. [Google Scholar] [CrossRef] [PubMed]
- Gérolami, R.; Moal, V.; Colson, P. Chronic hepatitis E with cirrhosis in a kidney-transplant recipient. N. Engl. J. Med. 2008, 358, 859–860. [Google Scholar] [CrossRef]
- Mclean, B.N.; Gulliver, J.; Dalton, H.R. Hepatitis E virus and neurological disorders. Pract. Neurol. 2017, 17, 282–288. [Google Scholar] [CrossRef]
- Abravanel, F.; Pique, J.; Couturier, E.; Nicot, F.; Dimeglio, C.; Lhomme, S.; Chiabrando, J.; Saune, K.; Péron, J.-M.; Kamar, N. Acute hepatitis E in French patients and neurological manifestations. J. Infect. 2018, 77, 220–226. [Google Scholar] [CrossRef]
- Kamar, N.; Weclawiak, H.; Guilbeau-Frugier, C.; Legrand-Abravanel, F.; Cointault, O.; Ribes, D.; Esposito, L.; Cardeau-Desangles, I.; Guitard, J.; Sallusto, F. Hepatitis E virus and the kidney in solid-organ transplant patients. Transplantation 2012, 93, 617–623. [Google Scholar] [CrossRef]
- Leaf, R.K.; O‘Brien, K.L.; Leaf, D.E.; Drews, R.E. Autoimmune hemolytic anemia in a young man with acute hepatitis E infection. Am. J. Hematol. 2017, 92, E77–E79. [Google Scholar] [CrossRef]
- Jaroszewicz, J.; Flisiak, R.; Kalinowska, A.; Wierzbicka, I.; Prokopowicz, D. Acute hepatitis E complicated by acute pancreatitis: A case report and literature review. Pancreas 2005, 30, 382–384. [Google Scholar] [CrossRef]
- Atsama, M.A.; Atangana, P.J.A.; Noah, D.N.; Moundipa, P.F.; Pineau, P.; Njouom, R. Hepatitis E virus infection as a promoting factor for hepatocellular carcinoma in Cameroon: Preliminary observations. Int. J. Infect. Dis. 2017, 64, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.-C.; Liu, C.-J.; Chang, C.T.; Su, T.-H.; Yang, W.-T.; Tsai, C.-H.; Chen, C.-L.; Yang, H.-C.; Liu, C.-H.; Chen, P.-J. HEV superinfection accelerates disease progression in patients with chronic HBV infection and increases mortality in those with cirrhosis. J. Hepatol. 2020, 72, 1105–1111. [Google Scholar] [CrossRef] [PubMed]
- Shukla, P.; Nguyen, H.; Faulk, K.; Mather, K.; Torian, U.; Engle, R.; Emerson, S. Adaptation of a genotype 3 hepatitis E virus to efficient growth in cell culture depended on an inserted human gene segment acquired by recombination. J. Virol. 2012, 86, 5697–5707. [Google Scholar] [CrossRef] [PubMed]
- Schofield, D.; Glamann, J.; Emerson, S.; Purcell, R. Identification by phage display and characterization of two neutralizing chimpanzee monoclonal antibodies to the hepatitis E virus capsid protein. J. Virol. 2000, 74, 5548–5555. [Google Scholar] [CrossRef]
- Roldão, A.; Mellado, M.C.M.; Castilho, L.R.; Carrondo, M.J.; Alves, P.M. Virus-like particles in vaccine development. Expert Rev. Vaccines 2010, 9, 1149–1176. [Google Scholar] [CrossRef] [PubMed]
- Noad, R.; Roy, P. Virus-like particles as immunogens. Trends Microbiol. 2003, 11, 438–444. [Google Scholar] [CrossRef]
- Grgacic, E.V.; Anderson, D.A. Virus-like particles: Passport to immune recognition. Methods 2006, 40, 60–65. [Google Scholar] [CrossRef]
- Cox, R.G.; Erickson, J.J.; Hastings, A.K.; Becker, J.C.; Johnson, M.; Craven, R.E.; Tollefson, S.J.; Boyd, K.L.; Williams, J.V. Human metapneumovirus virus-like particles induce protective B and T cell responses in a mouse model. J. Virol. 2014, 88, 6368–6379. [Google Scholar] [CrossRef]
- Rts, S.; Agnandji, S.T.; Lell, B.; Fernandes, J.F.; Abossolo, B.P.; Methogo, B.; Kabwende, A.L.; Adegnika, A.A.; Mordmueller, B.; Issifou, S. A phase 3 trial of RTS, S/AS01 malaria vaccine in African infants. N. Engl. J. Med. 2012, 367, 2284–2295. [Google Scholar]
- Scotti, N.; Rybicki, E.P. Virus-like particles produced in plants as potential vaccines. Expert Rev. Vaccines 2013, 12, 211–224. [Google Scholar] [CrossRef]
- Almeida, J.; Edwards, D.C.; Brand, C.; Heath, T. Formation of virosomes from influenza subunits and liposomes. Lancet 1975, 306, 899–901. [Google Scholar] [CrossRef]
- Keating, G.M.; Noble, S. Recombinant hepatitis B vaccine (Engerix-B®). Drugs 2003, 63, 1021–1051. [Google Scholar] [CrossRef] [PubMed]
- Monie, A.; Hung, C.-F.; Roden, R.; Wu, T.C. Cervarix™: A vaccine for the prevention of HPV 16, 18-associated cervical cancer. Biol. Targets Ther. 2008, 2, 107. [Google Scholar]
- Venters, C.; Graham, W.; Cassidy, W. Recombivax-HB: Perspectives past, present and future. Expert Rev. Vaccines 2004, 3, 119–129. [Google Scholar] [CrossRef]
- Shi, L.; Sings, H.; Bryan, J.; Wang, B.; Wang, Y.; Mach, H.; Kosinski, M.; Washabaugh, M.; Sitrin, R.; Barr, E. GARDASIL®: Prophylactic human papillomavirus vaccine development–from bench top to bed-side. Clin. Pharmacol. Ther. 2007, 81, 259–264. [Google Scholar] [CrossRef]
- Crevar, C.J.; Ross, T.M. Elicitation of protective immune responses using a bivalent H5N1 VLP vaccine. Virol. J. 2008, 5, 1. [Google Scholar] [CrossRef]
- Treanor, J.J.; Atmar, R.L.; Frey, S.E.; Gormley, R.; Chen, W.H.; Ferreira, J.; Goodwin, R.; Borkowski, A.; Clemens, R.; Mendelman, P.M. A novel intramuscular bivalent norovirus virus-like particle vaccine candidate—Reactogenicity, safety, and immunogenicity in a phase 1 trial in healthy adults. J. Infect. Dis. 2014, 210, 1763–1771. [Google Scholar] [CrossRef]
- Smith, D.B.; Simmonds, P.; Jameel, S.; Emerson, S.U.; Harrison, T.J.; Meng, X.-J.; Okamoto, H.; Van der Poel, W.H.; Purdy, M.A.; Members of the International Committee on the Taxonomy of Viruses Hepeviridae Study Group. Consensus proposals for classification of the family Hepeviridae. J. Gen. Virol. 2014, 95, 2223–2232. [Google Scholar] [CrossRef]
- Takahashi, M.; Nishizawa, T.; Nagashima, S.; Jirintai, S.; Kawakami, M.; Sonoda, Y.; Suzuki, T.; Yamamoto, S.; Shigemoto, K.; Ashida, K. Molecular characterization of a novel hepatitis E virus (HEV) strain obtained from a wild boar in Japan that is highly divergent from the previously recognized HEV strains. Virus Res. 2014, 180, 59–69. [Google Scholar] [CrossRef]
- Woo, P.; Lau, S.; Teng, J.; Tsang, A.; Joseph, M.; Wong, E.; Tang, Y.; Sivakumar, S.; Xie, J.; Bai, R. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014, 20, e8. [Google Scholar] [CrossRef]
- Reyes, G.R.; Purdy, M.A.; Kim, J.P.; Ka-Cheung, L.; Young, L.M. Isolation of a cDNA from the virus responsible for enterically transmitted non-A, non-B hepatitis. Science 1990, 247, 1335. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.W.; Smith, M.M.; Guerra, M.E.; Huang, C.-C.; Bradley, D.W.; Fry, K.E.; Reyes, G.R. Hepatitis E virus (HEV): Molecular cloning and sequencing of the full-length viral genome. Virology 1991, 185, 120–131. [Google Scholar] [CrossRef]
- Graff, J.; Torian, U.; Nguyen, H.; Emerson, S.U. A bicistronic subgenomic mRNA encodes both the ORF2 and ORF3 proteins of hepatitis E virus. J. Virol. 2006, 80, 5919–5926. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Holla, R.P.; Jameel, S. Molecular virology of hepatitis E virus. Virus Res. 2011, 161, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Cao, D.; Meng, X.-J. Molecular biology and replication of hepatitis E virus. Emerg. Microbes Infect. 2012, 1, e17. [Google Scholar] [CrossRef] [PubMed]
- Kannan, H.; Fan, S.; Patel, D.; Bossis, I.; Zhang, Y.-J. The hepatitis E virus open reading frame 3 product interacts with microtubules and interferes with their dynamics. J. Virol. 2009, 83, 6375–6382. [Google Scholar] [CrossRef]
- Ding, Q.; Heller, B.; Capuccino, J.M.; Song, B.; Nimgaonkar, I.; Hrebikova, G.; Contreras, J.E.; Ploss, A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA 2017, 114, 1147–1152. [Google Scholar] [CrossRef]
- Jameel, S.; Zafrullah, M.; Ozdener, M.H.; Panda, S.K. Expression in animal cells and characterization of the hepatitis E virus structural proteins. J. Virol. 1996, 70, 207–216. [Google Scholar] [CrossRef]
- He, S.; Miao, J.; Zheng, Z.; Wu, T.; Xie, M.; Tang, M.; Zhang, J.; Ng, M.-H.; Xia, N. Putative receptor-binding sites of hepatitis E virus. J. Gen. Virol. 2008, 89, 245–249. [Google Scholar] [CrossRef]
- Kalia, M.; Chandra, V.; Rahman, S.A.; Sehgal, D.; Jameel, S. Heparan sulfate proteoglycans are required for cellular binding of the hepatitis E virus ORF2 capsid protein and for viral infection. J. Virol. 2009, 83, 12714–12724. [Google Scholar] [CrossRef]
- Xing, L.; Wang, J.C.; Li, T.-C.; Yasutomi, Y.; Lara, J.; Khudyakov, Y.; Schofield, D.; Emerson, S.U.; Purcell, R.H.; Takeda, N. Spatial configuration of hepatitis E virus antigenic domain. J. Virol. 2011, 85, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Torresi, J.; Li, F.; Locarnini, S.A.; Anderson, D.A. Only the non-glycosylated fraction of hepatitis E virus capsid (open reading frame 2) protein is stable in mammalian cells. J. Gen. Virol. 1999, 80, 1185–1188. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Kato, K.; Li, T.; Takeda, N.; Miyamura, T.; Hammar, L.; Cheng, R.H. Recombinant hepatitis E capsid protein self-assembles into a dual-domain T= 1 particle presenting native virus epitopes. Virology 1999, 265, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Guu, T.S.; Liu, Z.; Ye, Q.; Mata, D.A.; Li, K.; Yin, C.; Zhang, J.; Tao, Y.J. Structure of the hepatitis E virus-like particle suggests mechanisms for virus assembly and receptor binding. Proc. Natl. Acad. Sci. USA 2009, 106, 12992–12997. [Google Scholar] [CrossRef]
- Xing, L.; Li, T.-C.; Mayazaki, N.; Simon, M.N.; Wall, J.S.; Moore, M.; Wang, C.-Y.; Takeda, N.; Wakita, T.; Miyamura, T. Structure of hepatitis E virion-sized particle reveals an RNA-dependent viral assembly pathway. J. Biol. Chem. 2010, 285, 33175–33183. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, F.; Zhang, L.; Harrison, T.J.; Huang, W.; Zhao, C.; Kong, W.; Jiang, C.; Wang, Y. Hepatitis E virus produced from cell culture has a lipid envelope. PLoS ONE 2015, 10, e0132503. [Google Scholar] [CrossRef]
- Yin, X.; Ying, D.; Lhomme, S.; Tang, Z.; Walker, C.M.; Xia, N.; Zheng, Z.; Feng, Z. Origin, antigenicity, and function of a secreted form of ORF2 in hepatitis E virus infection. Proc. Natl. Acad. Sci. USA 2018, 115, 4773–4778. [Google Scholar] [CrossRef]
- Montpellier, C.; Wychowski, C.; Sayed, I.M.; Meunier, J.-C.; Saliou, J.-M.; Ankavay, M.; Bull, A.; Pillez, A.; Abravanel, F.; Helle, F. Hepatitis E virus lifecycle and identification of 3 forms of the ORF2 capsid protein. Gastroenterology 2018, 154, 211–223. [Google Scholar] [CrossRef]
- Fu, R.M.; Decker, C.C.; Dao Thi, V.L. Cell culture models for hepatitis E virus. Viruses 2019, 11, 608. [Google Scholar] [CrossRef]
- Rogee, S.; Talbot, N.; Caperna, T.; Bouquet, J.M.; Barnaud, E.; Pavio, N. New models of hepatitis E virus replication in human and porcine hepatocyte cell lines. J. Gen. Virol. 2013, 94, 549–558. [Google Scholar] [CrossRef]
- Capelli, N.; Marion, O.; Dubois, M.; Allart, S.; Bertrand-Michel, J.; Lhomme, S.; Abravanel, F.; Izopet, J.; Chapuy-Regaud, S. Vectorial release of hepatitis E virus in polarized human hepatocytes. J. Virol. 2019, 93, 93. [Google Scholar] [CrossRef] [PubMed]
- Capelli, N.; Dubois, M.; Pucelle, M.; Da Silva, I.; Lhomme, S.; Abravanel, F.; Chapuy-Regaud, S.; Izopet, J. Optimized hepatitis E virus (HEV) culture and its application to measurements of HEV infectivity. Viruses 2020, 12, 139. [Google Scholar] [CrossRef] [PubMed]
- Thi, V.L.D.; Wu, X.; Belote, R.L.; Andreo, U.; Takacs, C.N.; Fernandez, J.P.; Vale-Silva, L.A.; Prallet, S.; Decker, C.C.; Fu, R.M. Stem cell-derived polarized hepatocytes. Nat. Commun. 2020, 11, 1–13. [Google Scholar]
- Kamar, N.; Marion, O.; Abravanel, F.; Izopet, J.; Dalton, H.R. Extrahepatic manifestations of hepatitis E virus. Liver Int. 2016, 36, 467–472. [Google Scholar] [CrossRef]
- Meng, J.; Dai, X.; Chang, J.C.; Lopareva, E.; Pillot, J.; Fields, H.A.; Khudyakov, Y.E. Identification and characterization of the neutralization epitope (s) of the hepatitis E virus. Virology 2001, 288, 203–211. [Google Scholar] [CrossRef]
- Yamashita, T.; Mori, Y.; Miyazaki, N.; Cheng, R.H.; Yoshimura, M.; Unno, H.; Shima, R.; Moriishi, K.; Tsukihara, T.; Li, T.C. Biological and immunological characteristics of hepatitis E virus-like particles based on the crystal structure. Proc. Natl. Acad. Sci. USA 2009, 106, 12986–12991. [Google Scholar] [CrossRef]
- Zhou, Y.-H.; Purcell, R.H.; Emerson, S.U. An ELISA for putative neutralizing antibodies to hepatitis E virus detects antibodies to genotypes 1, 2, 3, and 4. Vaccine 2004, 22, 2578–2585. [Google Scholar] [CrossRef]
- Li, F.; Zhuang, H.; Kolivas, S.; Locarnini, S.A.; Anderson, D.A. Persistent and transient antibody responses to hepatitis E virus detected by western immunoblot using open reading frame 2 and 3 and glutathione S-transferase fusion proteins. J. Clin. Microbiol. 1994, 32, 2060–2066. [Google Scholar] [CrossRef]
- Li, S.-W.; Zhao, Q.; Wu, T.; Chen, S.; Zhang, J.; Xia, N.-S. The development of a recombinant hepatitis E vaccine HEV 239. Hum. Vaccines Immunother. 2015, 11, 908–914. [Google Scholar] [CrossRef]
- Shrestha, M.P.; Scott, R.M.; Joshi, D.M.; Mammen, M.P., Jr.; Thapa, G.B.; Thapa, N.; Myint, K.S.A.; Fourneau, M.; Kuschner, R.A.; Shrestha, S.K. Safety and efficacy of a recombinant hepatitis E vaccine. N. Engl. J. Med. 2007, 356, 895–903. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Ng, M.H.; Xia, N.; Lau, S.; Che, X.; Chau, T.; Lai, S.; Im, S.W. Conformational antigenic determinants generated by interactions between a bacterially expressed recombinant peptide of the hepatitis E virus structural protein. J. Med. Virol. 2001, 64, 125–132. [Google Scholar] [CrossRef]
- Li, T.-C.; Yamakawa, Y.; Suzuki, K.; Tatsumi, M.; Razak, M.; Uchida, T.; Takeda, N.; Miyamura, T. Expression and self-assembly of empty virus-like particles of hepatitis E virus. J. Virol. 1997, 71, 7207–7213. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Lin, S.-Q.; Gao, Y.; Li, M.; Luo, W.-X.; Zhang, J.; Xia, N.-S. Expression of ORF2 partial gene of hepatitis E virus in tomatoes and immunoactivity of expression products. World J. Gastroenterol. 2003, 9, 2211–2215. [Google Scholar] [CrossRef] [PubMed]
- Li, S.W.; Zhang, J.; Li, Y.M.; Ou, S.H.; Huang, G.Y.; He, Z.Q.; Sheng, X.G.; Xian, Y.L.; Pang, S.Q.; Ng, M.H. A bacterially expressed particulate hepatitis E vaccine: Antigenicity, immunogenicity and protectivity on primates. Vaccine 2005, 23, 2893–2901. [Google Scholar] [CrossRef] [PubMed]
- Li, T.-C.; Takeda, N.; Miyamura, T.; Matsuura, Y.; Wang, J.C.; Engvall, H.; Hammar, L.; Xing, L.; Cheng, R.H. Essential elements of the capsid protein for self-assembly into empty virus-like particles of hepatitis E virus. J. Virol. 2005, 79, 12999–13006. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Tang, X.; Seetharaman, J.; Yang, C.; Gu, Y.; Zhang, J.; Du, H.; Shih, J.W.K.; Hew, C.-L.; Sivaraman, J. Dimerization of hepatitis E virus capsid protein E2s domain is essential for virus–host interaction. PLoS Pathog 2009, 5, e1000537. [Google Scholar] [CrossRef] [PubMed]
- Im, S.W.; Zhang, J.Z.; Zhuang, H.; Che, X.Y.; Zhu, W.F.; Xu, G.M.; Li, K.; Xia, N.S.; Ng, M.H. A bacterially expressed peptide prevents experimental infection of primates by the hepatitis E virus. Vaccine 2001, 19, 3726–3732. [Google Scholar] [CrossRef]
- Zhang, J.; Gu, Y.; Sheng, X.G.; Li, S.W.; He, Z.Q.; Huang, G.Y.; Zhuang, H.; Ng, M.H.; Xia, N.S. Analysis of hepatitis E virus neutralization sites using monoclonal antibodies directed against a virus capsid protein. Vaccine 2005, 23, 2881–2892. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, M.; Pan, H.; Lin, Z.; Wang, K.; Weng, Z.; Zhu, Y.; Xin, L.; Zhang, J.; Li, S. Robust manufacturing and comprehensive characterization of recombinant hepatitis E virus-like particles in Hecolin®. Vaccine 2014, 32, 4039–4050. [Google Scholar] [CrossRef]
- Wu, T.; Wu, X.-L.; Ou, S.-H.; Lin, C.-X.; Cheng, T.; Li, S.-W.; Ng, M.H.; Zhang, J.; Xia, N.-S. Difference of T cell and B cell activation in two homologous proteins with similar antigenicity but great distinct immunogenicity. Mol. Immunol. 2007, 44, 3261–3266. [Google Scholar] [CrossRef]
- Rudolf, M.P.; Fausch, S.C.; Da Silva, D.M.; Kast, W.M. Human dendritic cells are activated by chimeric human papillomavirus type-16 virus-like particles and induce epitope-specific human T cell responses in vitro. J. Immunol. 2001, 166, 5917–5924. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Liu, C.-B.; Li, R.-C.; Li, Y.-M.; Zheng, Y.-J.; Li, Y.-P.; Luo, D.; Pan, B.-B.; Nong, Y.; Ge, S.-X. Randomized-controlled phase II clinical trial of a bacterially expressed recombinant hepatitis E vaccine. Vaccine 2009, 27, 1869–1874. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.-C.; Zhang, J.; Zhang, X.-F.; Zhou, C.; Wang, Z.-Z.; Huang, S.-J.; Wang, H.; Yang, C.-L.; Jiang, H.-M.; Cai, J.-P. Efficacy and safety of a recombinant hepatitis E vaccine in healthy adults: A large-scale, randomised, double-blind placebo-controlled, phase 3 trial. Lancet 2010, 376, 895–902. [Google Scholar] [CrossRef]
- Park, S.B. Hepatitis E vaccine debuts: Success of Chinese biotech partnership raises hopes for prevention of overlooked diseases. Nature 2012, 491, 21–23. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Zhang, X.-F.; Huang, S.-J.; Wu, T.; Hu, Y.-M.; Wang, Z.-Z.; Wang, H.; Jiang, H.-M.; Wang, Y.-J.; Yan, Q. Long-term efficacy of a hepatitis E vaccine. N. Engl. J. Med. 2015, 372, 914–922. [Google Scholar] [CrossRef]
- Yu, X.-Y.; Chen, Z.-P.; Wang, S.-Y.; Pan, H.-R.; Wang, Z.-F.; Zhang, Q.-F.; Shen, L.-Z.; Zheng, X.-P.; Yan, C.-F.; Lu, M. Safety and immunogenicity of hepatitis E vaccine in elderly people older than 65 years. Vaccine 2019, 37, 4581–4586. [Google Scholar] [CrossRef]
- Wu, T.; Huang, S.-J.; Zhu, F.-C.; Zhang, X.-F.; Ai, X.; Yan, Q.; Wang, Z.-Z.; Yang, C.-L.; Jiang, H.-M.; Liu, X.-H. Immunogenicity and safety of hepatitis E vaccine in healthy hepatitis B surface antigen positive adults. Hum. Vaccines Immunother. 2013, 9, 2474–2479. [Google Scholar] [CrossRef]
- Zaman, K.; Dudman, S.; Stene-Johansen, K.; Qadri, F.; Yunus, M.; Sandbu, S.; Gurley, E.S.; Overbo, J.; Julin, C.H.; Dembinski, J.L. HEV study protocol: Design of a cluster-randomised, blinded trial to assess the safety, immunogenicity and effectiveness of the hepatitis E vaccine HEV 239 (Hecolin) in women of childbearing age in rural Bangladesh. BMJ Open 2020, 10, e033702. [Google Scholar] [CrossRef]
- Cao, Y.-F.; Tao, H.; Hu, Y.-M.; Shi, C.-B.; Wu, X.; Liang, Q.; Chi, C.-P.; Li, L.; Liang, Z.-L.; Meng, J.-H. A phase 1 randomized open-label clinical study to evaluate the safety and tolerability of a novel recombinant hepatitis E vaccine. Vaccine 2017, 35, 5073–5080. [Google Scholar] [CrossRef]
- Wen, J.; Behloul, N.; Dai, X.; Dong, C.; Liang, J.; Zhang, M.; Shi, C.; Meng, J. Immunogenicity difference between two hepatitis E vaccines derived from genotype 1 and 4. Antivir. Res. 2016, 128, 36–42. [Google Scholar] [CrossRef]
- Zheng, M.; Jiang, J.; Zhang, X.; Wang, N.; Wang, K.; Li, Q.; Li, T.; Lin, Q.; Wang, Y.; Yu, H. Characterization of capsid protein (p495) of hepatitis E virus expressed in Escherichia coli and assembling into particles in vitro. Vaccine 2018, 36, 2104–2111. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.-X.; Lee, M.Y.-T.; Ng, J.M.-H.; Chye, M.-L.; Yip, W.-K.; Zee, S.-Y.; Lam, E. A truncated hepatitis E virus ORF2 protein expressed in tobacco plastids is immunogenic in mice. World J. Gastroenterol. WJG 2006, 12, 306. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Maloney, B.J.; Takeda, N.; Suzaki, Y.; Ami, Y.; Li, T.C.; Miyamura, T.; Arntzen, C.J.; Mason, H.S. Challenges in creating a vaccine to prevent hepatitis E. Vaccine 2005, 23, 1870–1874. [Google Scholar] [CrossRef]
- Mazalovska, M.; Varadinov, N.; Koynarski, T.; Minkov, I.; Teoharov, P.; Lomonossoff, G.P.; Zahmanova, G. Detection of serum antibodies to hepatitis E virus based on HEV genotype 3 ORF2 capsid protein expressed in nicotiana benthamiana. Ann. Lab. Med. 2017, 37, 313–319. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Li, T.-C.; Yoshimatsu, K.; Yasuda, S.P.; Arikawa, J.; Koma, T.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Hoa, N.T.; Yamashiro, T. Characterization of self-assembled virus-like particles of rat hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2011, 92, 2830–2837. [Google Scholar] [CrossRef]
- Yang, T.; Kataoka, M.; Ami, Y.; Suzaki, Y.; Kishida, N.; Shirakura, M.; Imai, M.; Asanuma, H.; Takeda, N.; Wakita, T. Characterization of self-assembled virus-like particles of ferret hepatitis E virus generated by recombinant baculoviruses. J. Gen. Virol. 2013, 94, 2647–2656. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Kataoka, M.; Liu, Z.; Takeda, N.; Wakita, T.; Li, T.-C. Characterization of self-assembled virus-like particles of dromedary camel hepatitis e virus generated by recombinant baculoviruses. Virus Res. 2015, 210, 8–17. [Google Scholar] [CrossRef]
- Li, T.-C.; Kataoka, M.; Takahashi, K.; Yoshizaki, S.; Kato, T.; Ishii, K.; Takeda, N.; Mishiro, S.; Wakita, T. Generation of hepatitis E virus-like particles of two new genotypes G5 and G6 and comparison of antigenic properties with those of known genotypes. Vet. Microbiol. 2015, 178, 150–157. [Google Scholar] [CrossRef]
- Kost, T.A.; Condreay, J.P. Recombinant baculoviruses as expression vectors for insect and mammalian cells. Curr. Opin. Biotechnol. 1999, 10, 428–433. [Google Scholar] [CrossRef]
- Berger, I.; Fitzgerald, D.J.; Richmond, T.J. Baculovirus expression system for heterologous multiprotein complexes. Nat. Biotechnol. 2004, 22, 1583–1587. [Google Scholar] [CrossRef]
- Van Oers, M.M.; Vlak, J.M. Baculovirus expression system. In Encyclopedia of Life Sciences; John Wiley & Sons Ltd: Chichester, UK, 2008; pp. 1–3. [Google Scholar]
- Vicente, T.; Roldão, A.; Peixoto, C.; Carrondo, M.J.; Alves, P.M. Large-scale production and purification of VLP-based vaccines. J. Invertebr. Pathol. 2011, 107, S42–S48. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.; Ferris, D.G.; Jenkins, D.; Schuind, A.; Zahaf, T.; Innis, B.; Naud, P.; De Carvalho, N.S. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: A randomised controlled trial. Lancet 2004, 364, 1757–1765. [Google Scholar] [CrossRef]
- Harper, D.M.; Franco, E.L.; Wheeler, C.M.; Moscicki, A.-B.; Romanowski, B.; Roteli-Martins, C.M.; Jenkins, D.; Schuind, A.; Clemens, S.A.C.; Dubin, G. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: Follow-up from a randomised control trial. Lancet 2006, 367, 1247–1255. [Google Scholar] [CrossRef]
- López-Macías, C.; Ferat-Osorio, E.; Tenorio-Calvo, A.; Isibasi, A.; Talavera, J.; Arteaga-Ruiz, O.; Arriaga-Pizano, L.; Hickman, S.P.; Allende, M.; Lenhard, K. Safety and immunogenicity of a virus-like particle pandemic influenza A (H1N1) 2009 vaccine in a blinded, randomized, placebo-controlled trial of adults in Mexico. Vaccine 2011, 29, 7826–7834. [Google Scholar] [CrossRef]
- Tacket, C.O.; Sztein, M.B.; Losonsky, G.A.; Wasserman, S.S.; Estes, M.K. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin. Immunol. 2003, 108, 241–247. [Google Scholar] [CrossRef]
- De Diego, A.C.P.; Athmaram, T.N.; Stewart, M.; Rodríguez-Sánchez, B.; Sánchez-Vizcaíno, J.M.; Noad, R.; Roy, P. Characterization of protection afforded by a bivalent virus-like particle vaccine against bluetongue virus serotypes 1 and 4 in sheep. PLoS ONE 2011, 6, e26666. [Google Scholar]
- Metz, S.W.; Gardner, J.; Geertsema, C.; Le, T.T.; Goh, L.; Vlak, J.M.; Suhrbier, A.; Pijlman, G.P. Effective chikungunya virus-like particle vaccine produced in insect cells. PLoS Negl. Trop. Dis. 2013, 7, e2124. [Google Scholar] [CrossRef]
- Robinson, R.A.; Burgess, W.H.; Emerson, S.U.; Leibowitz, R.S.; Sosnovtseva, S.A.; Tsarev, S.; Purcell, R.H. Structural characterization of recombinant hepatitis E virus ORF2 proteins in baculovirus-infected insect cells. Protein Expr. Purif. 1998, 12, 75–84. [Google Scholar] [CrossRef]
- Tsarev, S.A.; Tsareva, T.S.; Emerson, S.U.; Yarbough, P.O.; Legters, L.J.; Moskal, T.; Purcell, R.H. Infectivity titration of a prototype strain of hepatitis E virus in cynomolgus monkeys. J. Med. Virol. 1994, 43, 135–142. [Google Scholar] [CrossRef]
- Tsarev, S.A.; Tsareva, T.S.; Emerson, S.U.; Govindarajan, S.; Shapiro, M.; Gerin, J.L.; Purcell, R.H. Recombinant vaccine against hepatitis E: Dose response and protection against heterologous challenge. Vaccine 1997, 15, 1834–1838. [Google Scholar] [CrossRef]
- Park, S.B. Hepatitis E vaccine debuts. Nature 2012, 491, 21–22. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.; Singh, P.; Ochoa, W.; Manayani, D.J.; Manchester, M.; Schneemann, A.; Reddy, V.S. Characterization of polymorphism displayed by the coat protein mutants of tomato bushy stunt virus. Virology 2006, 349, 222–229. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Raj, V.S.; Smits, S.L.; Pas, S.D.; Provacia, L.; Moorman-Roest, H.; Osterhaus, A.; Haagmans, B.L. Novel hepatitis E virus in ferrets, the Netherlands. Emerg. Infect. Dis. 2012, 18, 1369–1370. [Google Scholar] [CrossRef] [PubMed]
- Johne, R.; Heckel, G.; Plenge-Bonig, A.; Kindler, E.; Maresch, C.; Reetz, J.; Schielke, A.; Ulrich, R.G. Novel hepatitis E virus genotype in Norway rats, Germany. Emerg. Infect. Dis. 2010, 16, 1452–1455. [Google Scholar] [CrossRef]
- Li, T.-C.; Takeda, N.; Miyamura, T. Oral administration of hepatitis E virus-like particles induces a systemic and mucosal immune response in mice. Vaccine 2001, 19, 3476–3484. [Google Scholar] [CrossRef]
- Li, T.-C.; Suzaki, Y.; Ami, Y.; Dhole, T.N.; Miyamura, T.; Takeda, N. Protection of cynomolgus monkeys against HEV infection by oral administration of recombinant hepatitis E virus-like particles. Vaccine 2004, 22, 370–377. [Google Scholar] [CrossRef]
- Niikura, M.; Takamura, S.; Kim, G.; Kawai, S.; Saijo, M.; Morikawa, S.; Kurane, I.; Li, T.-C.; Takeda, N.; Yasutomi, Y. Chimeric recombinant hepatitis E virus-like particles as an oral vaccine vehicle presenting foreign epitopes. Virology 2002, 293, 273–280. [Google Scholar] [CrossRef]
- Shima, R.; Li, T.C.; Sendai, Y.; Kataoka, C.; Mori, Y.; Abe, T.; Takeda, N.; Okamoto, T.; Matsuura, Y. Production of hepatitis E virus-like particles presenting multiple foreign epitopes by co-infection of recombinant baculoviruses. Sci. Rep. 2016, 6, 21638. [Google Scholar] [CrossRef]
- Sijmons, P.C.; Dekker, B.M.; Schrammeijer, B.; Verwoerd, T.C.; Van Den Elzen, P.J.; Hoekema, A. Production of correctly processed human serum albumin in transgenic plants. Nat. Biotechnol. 1990, 8, 217–221. [Google Scholar] [CrossRef]
- Twyman, R.M.; Schillberg, S.; Fischer, R. Transgenic plants in the biopharmaceutical market. Expert Opin. Emerg. Drugs 2005, 10, 185–218. [Google Scholar] [CrossRef]
- Rybicki, E.P. Plant-produced vaccines: Promise and reality. Drug Discov. Today 2009, 14, 16–24. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.K.; Sharma, M.K. Plants as bioreactors: Recent developments and emerging opportunities. Biotechnol. Adv. 2009, 27, 811–832. [Google Scholar] [CrossRef]
- Sala, F.; Rigano, M.M.; Barbante, A.; Basso, B.; Walmsley, A.M.; Castiglione, S. Vaccine antigen production in transgenic plants: Strategies, gene constructs and perspectives. Vaccine 2003, 21, 803–808. [Google Scholar] [CrossRef]
- Staub, J.M.; Garcia, B.; Graves, J.; Hajdukiewicz, P.T.; Hunter, P.; Nehra, N.; Paradkar, V.; Schlittler, M.; Carroll, J.A.; Spatola, L. High-yield production of a human therapeutic protein in tobacco chloroplasts. Nat. Biotechnol. 2000, 18, 333. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Lomonossoff, G.P. Extremely high-level and rapid transient protein production in plants without the use of viral replication. Plant Physiol. 2008, 148, 1212–1218. [Google Scholar] [CrossRef] [PubMed]
- Sainsbury, F.; Thuenemann, E.C.; Lomonossoff, G.P. pEAQ: Versatile expression vectors for easy and quick transient expression of heterologous proteins in plants. Plant Biotechnol. J. 2009, 7, 682–693. [Google Scholar] [CrossRef] [PubMed]
- Zahmanova, G.G.; Mazalovska, M.; Takova, K.H.; Toneva, V.T.; Minkov, I.N.; Mardanova, E.S.; Ravin, N.V.; Lomonossoff, G.P. Rapid high-yield transient expression of swine hepatitis E ORF2 capsid proteins in nicotiana benthamiana plants and production of chimeric hepatitis E virus-like particles bearing the M2e influenza epitope. Plants 2020, 9, 29. [Google Scholar] [CrossRef]
- Rein, D.B.; Stevens, G.A.; Theaker, J.; Wittenborn, J.S.; Wiersma, S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology 2012, 55, 988–997. [Google Scholar] [CrossRef]
- Meng, X.-J. Zoonotic and foodborne transmission of hepatitis E virus. In Seminars in Liver Disease; Thieme Medical Publishers: New York, NY, USA, 2013; pp. 41–49. [Google Scholar]
- Yazaki, Y.; Mizuo, H.; Takahashi, M.; Nishizawa, T.; Sasaki, N.; Gotanda, Y.; Okamoto, H. Sporadic acute or fulminant hepatitis E in Hokkaido, Japan, may be food-borne, as suggested by the presence of hepatitis E virus in pig liver as food. J. Gen. Virol. 2003, 84, 2351–2357. [Google Scholar] [CrossRef]
- Kamar, N.; Mansuy, J.M.; Cointault, O.; Selves, J.; Abravanel, F.; Danjoux, M.; Otal, P.; Esposito, L.; Durand, D.; Izopet, J. Hepatitis E virus-related cirrhosis in kidney-and kidney–pancreas-transplant recipients. Am. J. Transplant. 2008, 8, 1744–1748. [Google Scholar] [CrossRef]
 ), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
 ), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.
), with a small multifunctional protein encoded by ORF3. Three different capsid proteins have been discovered in vitro during infection, i.e., gORF2-glycosylated, iORF2-infectious and cORF2-cleaved ORF2.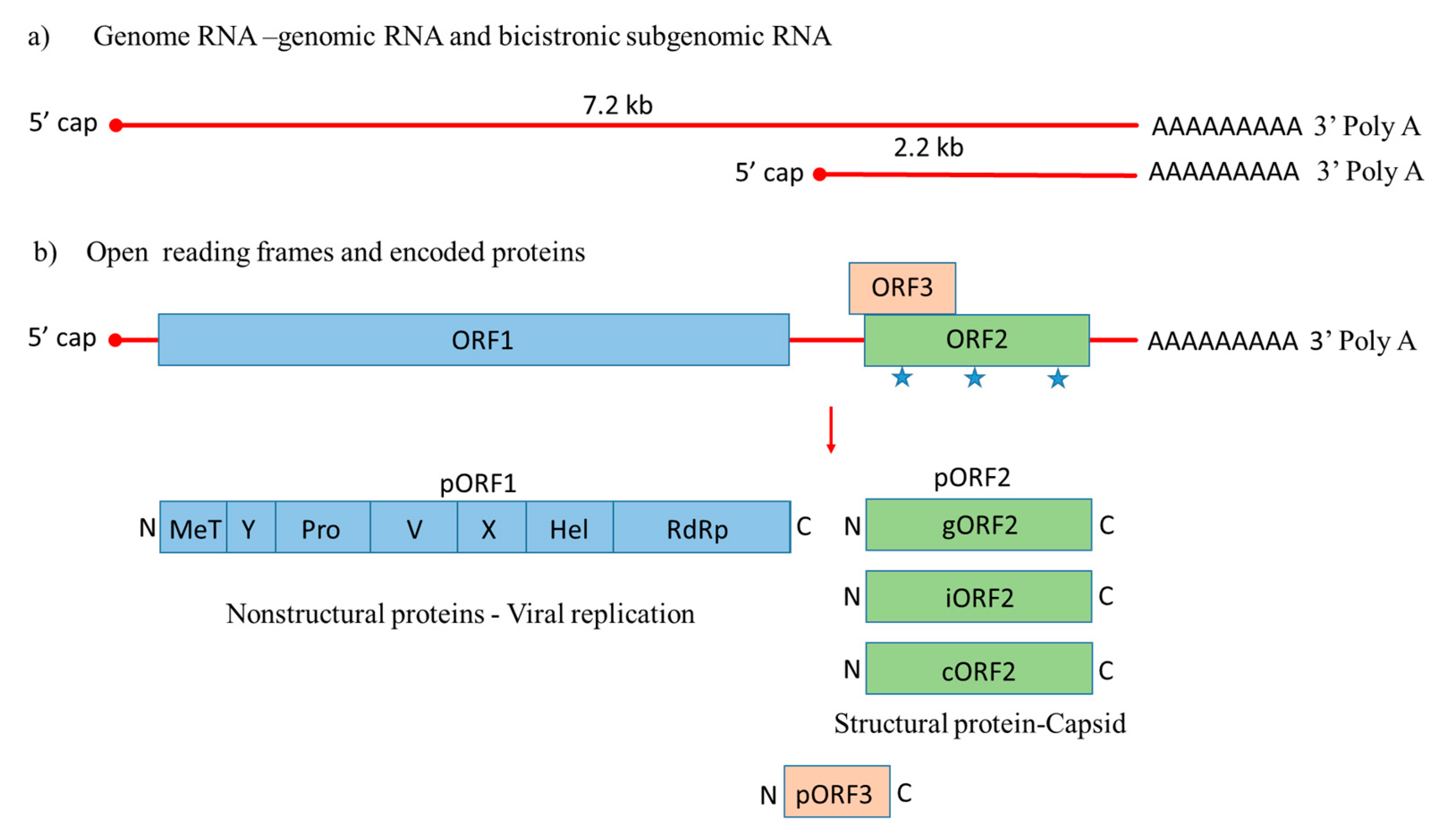
| Organism | HEV ORF2 | Molecular Weight | VLPs | T Number | RNA | Remarks | Ref: |
|---|---|---|---|---|---|---|---|
| Bacteria E. coli | E2s (459–606 aa) | 16 kDa | HEV 239 (p239) 20–30 nm | n/a | n/a | Hecolin® licensed vaccine only in China | [77] |
| E2 (394–606 aa) | 23 kDa | [72] | |||||
| p239 (368–606 aa) | 30 kDa | [80] | |||||
| P179 (439–617 aa) | 20 kDa | [90] | |||||
| p495 (112–606 aa) | 53 kDa | [92] | |||||
| Transgenic tomato plants | E2 (394–606 aa) | 23 kDa | Limited assembly of VLPs | n/a | n/a | No success so far in production of VLPs in plants | [74] |
| Tobacco plastids | E2 (394–606 aa) | 23 kDa | [93] | ||||
| Transgenic plants | 112–660 aa 112–608 aa | 54 kDa | [94] | ||||
| Nicotiana benthamiana | 110–610 aa | 56 kDa | [95] | ||||
| Baculovirus-Insect cells system | 112–660 aa (genotype 1) | 58, 50 kDa | 50 kDa VLPs ~23 nm | T = 1 | No | Expression of the whole ORF2 does not form VLPs | [73] |
| Tn5 cell line | Rat 110–660 aa | 58, 53 kDa | 53 kDa VLPs ~24, 35 nm | T = 1; T = 3 | No | [96] | |
| Ferret 112–613 aa | 53 kDa | VLPs ~24 nm | T = 1 | No | [97] | ||
| Camel 13–610 aa | 70, 64, 53, 40 kDa; | 64 kDa VLPs ~35 nm | T = 3 | Yes | For VLPs formation N-terminal truncation is needed. | [98] | |
| 111–610 aa | 58 и 53 kDa | 53 kDa VLPs ~24 nm | T = 1 | No | |||
| Wild boar 112–660aa | 58 and 53 kDa; | 53 kDa VLPs ~24 nm | T = 1 | No | [99] | ||
| 13–660 aa | 71, 64, 53, 40 kDa | 64 kDa VLPs ~35 nm | T = 3 | Yes |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mazalovska, M.; Kouokam, J.C. Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses 2020, 12, 826. https://doi.org/10.3390/v12080826
Mazalovska M, Kouokam JC. Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses. 2020; 12(8):826. https://doi.org/10.3390/v12080826
Chicago/Turabian StyleMazalovska, Milena, and J. Calvin Kouokam. 2020. "Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus" Viruses 12, no. 8: 826. https://doi.org/10.3390/v12080826
APA StyleMazalovska, M., & Kouokam, J. C. (2020). Progress in the Production of Virus-Like Particles for Vaccination against Hepatitis E Virus. Viruses, 12(8), 826. https://doi.org/10.3390/v12080826





