Yellow Fever Virus Down-Regulates mRNA Expression of SOCS1 in the Initial Phase of Infection in Human Cell Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Lines and Viruses
2.2. Experimental Design
2.3. Plaque Assay
2.4. RT-qPCR
2.5. Western Blot Analysis
2.6. Statistical Analysis
3. Results
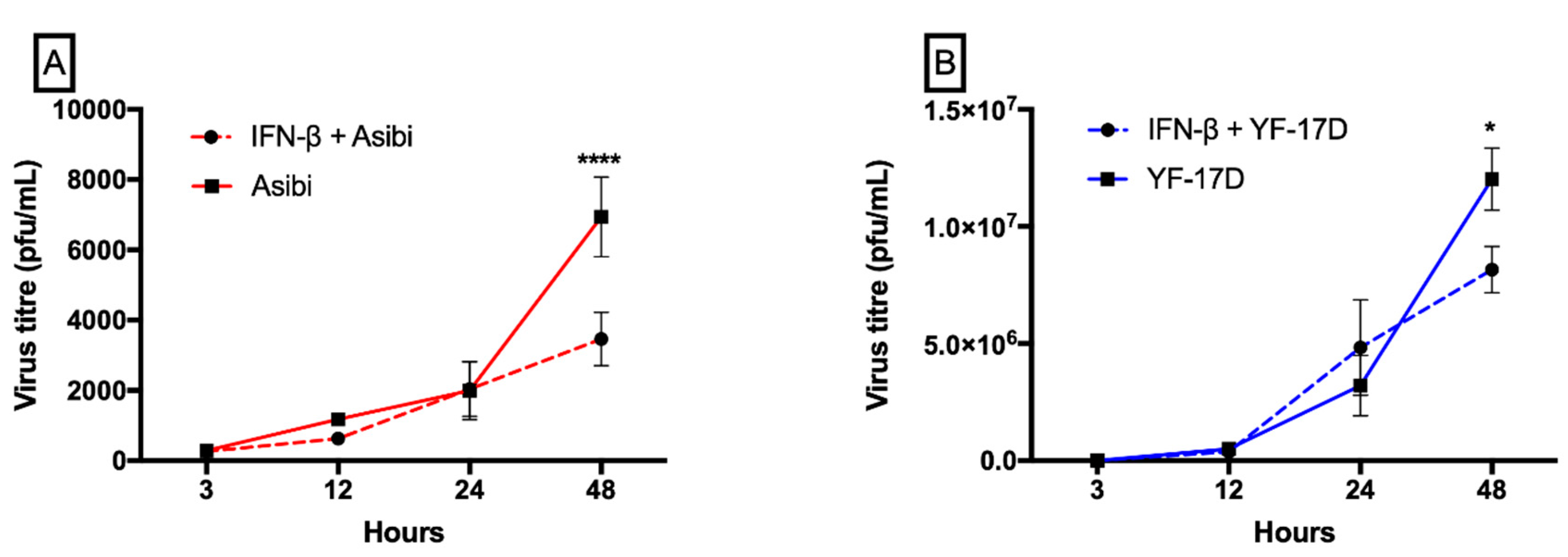
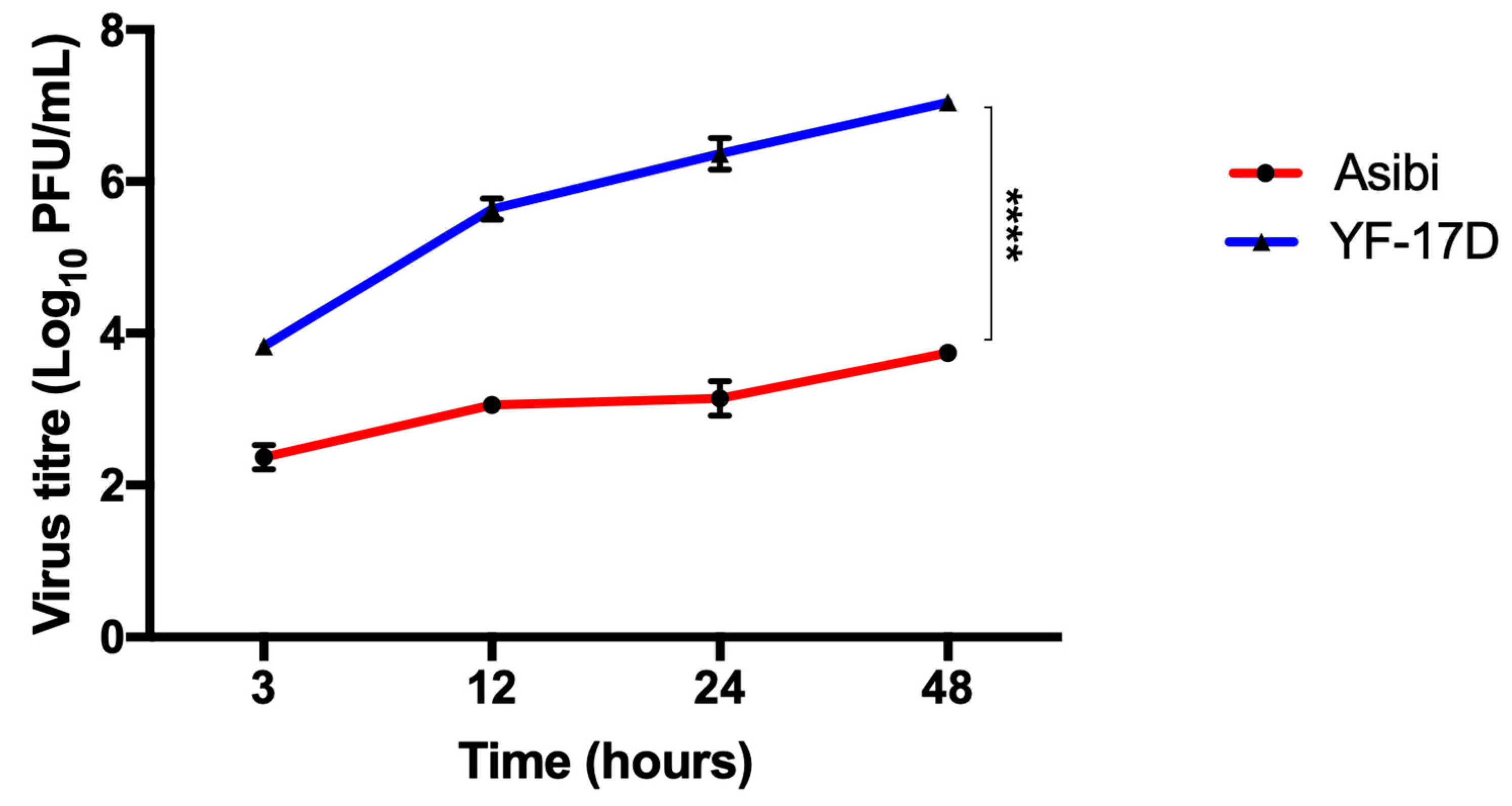
3.1. IFN-β Inhibits both YFV Strains at Later Time Points whilst YF-17D Replicates More Efficiently
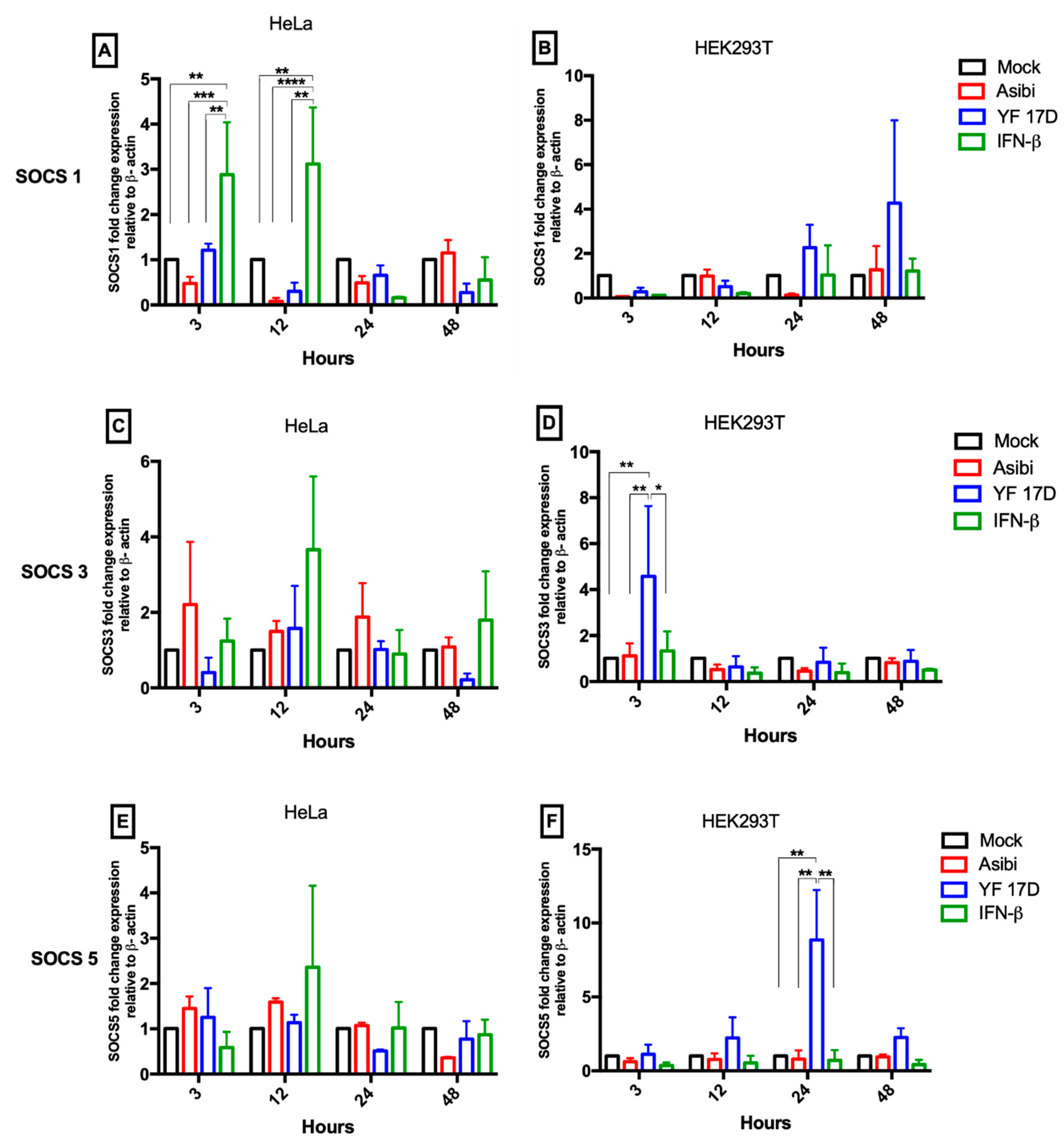
3.2. Expression Dynamics of SOCS Genes
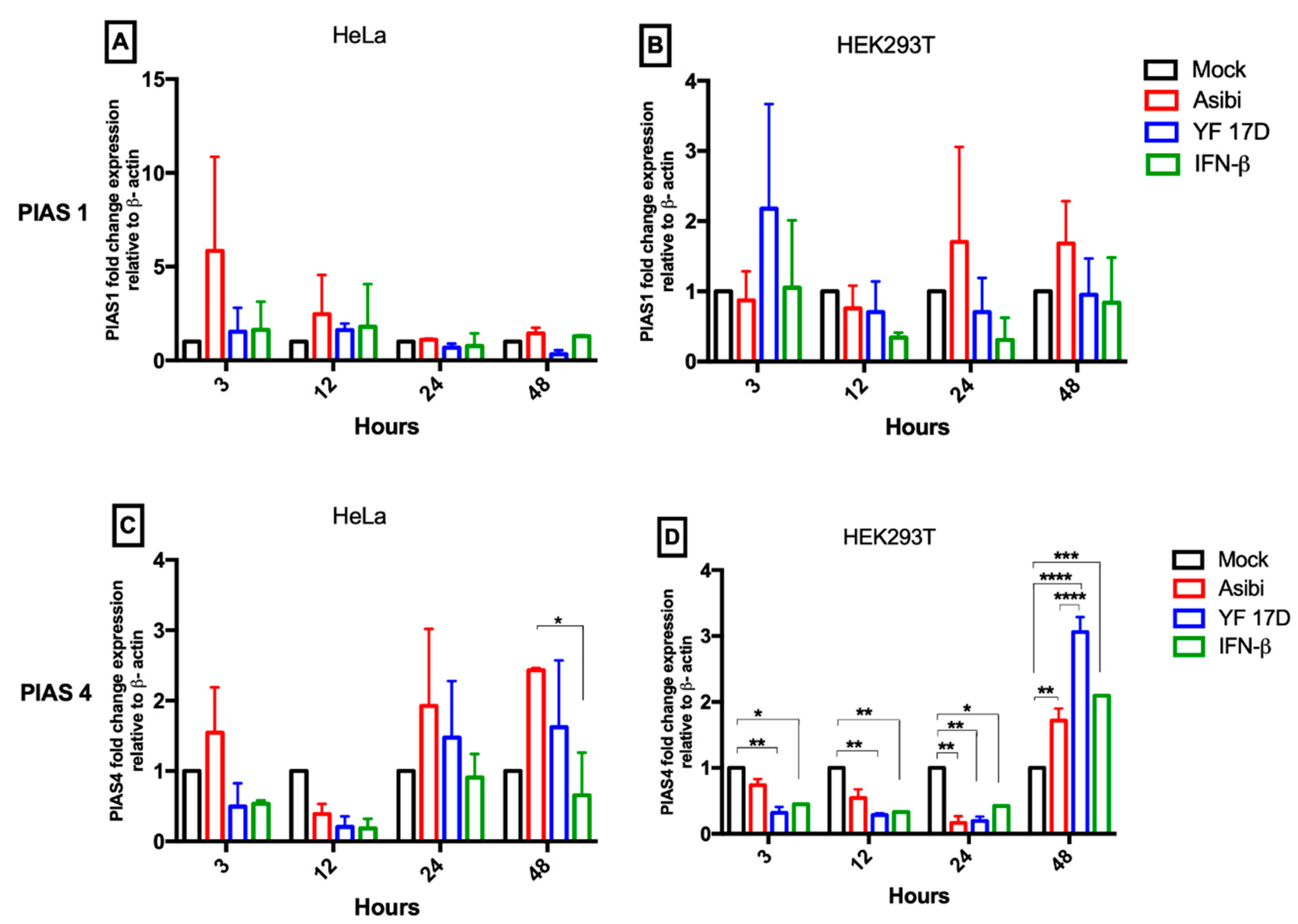
3.3. Expression Dynamics of PIAS Genes
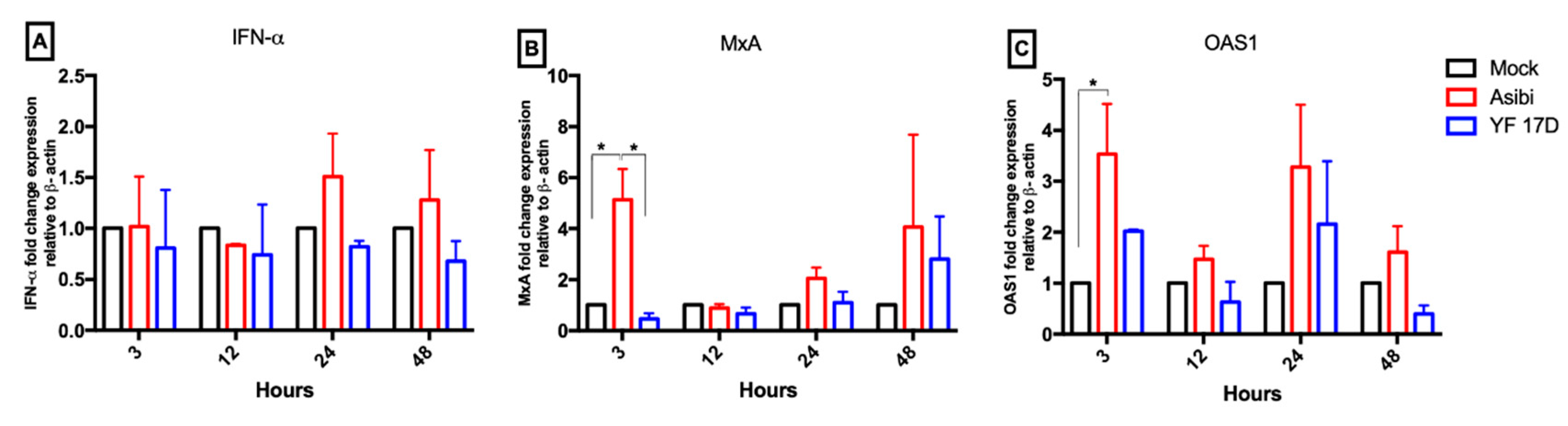
3.4. Expression Dynamics of Antiviral Molecules
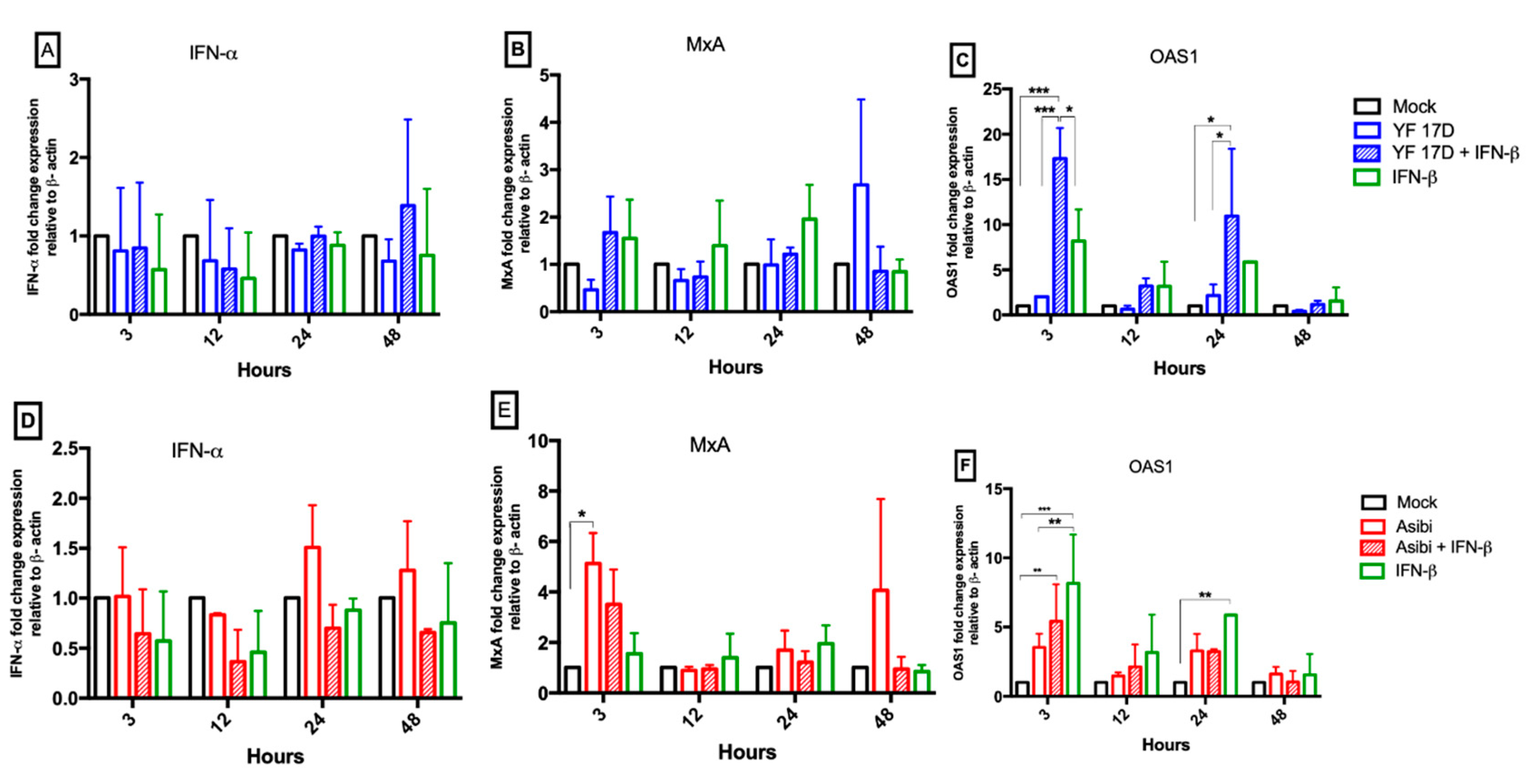
3.5. Effect of IFN-β on YFV Induction of Antiviral Molecules
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, J.; Chen, Z.J. Innate immune sensing and signaling of cytosolic nucleic acids. Annu. Rev. Immunol. 2014, 32, 461–488. [Google Scholar] [CrossRef] [PubMed]
- Schoggins, J.W.; MacDuff, D.A.; Imanaka, N.; Gainey, M.D.; Shrestha, B.; Eitson, J.L.; Mar, K.B.; Richardson, R.B.; Ratushny, A.V.; Litvak, V.; et al. Pan-Viral specificity of IFN-induced genes reveals new roles for cGAS in innate immunity. Nature 2014, 505, 691–695. [Google Scholar] [CrossRef] [PubMed]
- Yoshimura, A.; Naka, T.; Kubo, M. SOCS proteins, cytokine signalling and immune regulation. Nat. Rev. Immunol. 2007, 7, 454–465. [Google Scholar] [CrossRef]
- Shuai, K. Regulation of cytokine signaling pathways by PIAS proteins. Cell Res. 2006, 16, 196–202. [Google Scholar] [CrossRef]
- Akhtar, L.N.; Benveniste, E.N. Viral exploitation of host SOCS protein functions. J. Virol. 2011, 85, 1912–1921. [Google Scholar] [CrossRef] [PubMed]
- Kotaja, N.; Karvonen, U.; Janne, O.A.; Palvimo, J.J. PIAS proteins modulate transcription factors by functioning as SUMO-1 ligases. Mol. Cell. Biol. 2002, 22, 5222–5234. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, L.N.; Qin, H.; Muldowney, M.T.; Yanagisawa, L.L.; Kutsch, O.; Clements, J.E.; Benveniste, E.N. Suppressor of cytokine signaling 3 inhibits antiviral IFN-beta signaling to enhance HIV-1 replication in macrophages. J. Immunol. 2010, 185, 2393–2404. [Google Scholar] [CrossRef] [PubMed]
- Estrada-Jimenez, T.; Millan-Perez Pena, L.; Flores-Mendoza, L.; Sedeno-Monge, V.; Santos-Lopez, G.; Rosas-Murrieta, N.; Reyes-Carmona, S.; Teran-Cabanillas, E.; Hernandez, J.; Herrera-Camacho, I.; et al. Upregulation of the suppressors of cytokine signaling 1 and 3 is associated with arrest of phosphorylated-stat1 nuclear importation and reduced innate response in denguevirus-infected macrophages. Viral Immunol. 2016, 29, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Palma-Ocampo, H.K.; Flores-Alonso, J.C.; Vallejo-Ruiz, V.; Reyes-Leyva, J.; Flores-Mendoza, L.; Herrera-Camacho, I.; Rosas-Murrieta, N.H.; Santos-López, G. Interferon lambda inhibits dengue virus replication in epithelial cells. Virol. J. 2015, 12, 150. [Google Scholar] [CrossRef]
- Kundu, K.; Dutta, K.; Nazmi, A.; Basu, A. Japanese encephalitis virus infection modulates the expression of suppressors of cytokine signaling (SOCS) in macrophages: Implications for the hosts’ innate immune response. Cell. Immunol. 2013, 285, 100–110. [Google Scholar] [CrossRef]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; Goes de Jesus, J.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; du Plessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef] [PubMed]
- Theiler, M.; Smith, H.H. The use of yellow fever virus modified by in vitro cultivation for human immunization. J. Exp. Med. 1937, 65, 787–800. [Google Scholar] [CrossRef] [PubMed]
- Beck, A.S.; Barrett, A.D. Current status and future prospects of yellow fever vaccines. Expert Rev. Vaccines 2015, 14, 1479–1492. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R.E. Yellow fever vaccine-associated viscerotropic disease: Current perspectives. Drug Des. Dev. Ther. 2016, 10, 3345–3353. [Google Scholar] [CrossRef]
- World Health Organization. WHO position on the use of fractional doses—June 2017, addendum to vaccines and vaccination against yellow fever WHO: Position paper—June 2013. Vaccine 2017, 35, 5751–5752. [Google Scholar] [CrossRef] [PubMed]
- Monath, T.P. Yellow fever vaccine. Expert Rev. Vaccines 2005, 4, 553–574. [Google Scholar] [CrossRef]
- Monath, T.P.; Lee, C.K.; Julander, J.G.; Brown, A.; Beasley, D.W.; Watts, D.M.; Hayman, E.; Guertin, P.; Makowiecki, J.; Crowell, J.; et al. Inactivated yellow fever 17D vaccine: Development and nonclinical safety, immunogenicity and protective activity. Vaccine 2010, 28, 3827–3840. [Google Scholar] [CrossRef]
- Freire, M.S.; Mann, G.F.; Marchevsky, R.S.; Yamamura, A.M.; Almeida, L.F.; Jabor, A.V.; Malachias, J.M.; Coutinho, E.S.; Galler, R. Production of yellow fever 17DD vaccine virus in primary culture of chicken embryo fibroblasts: Yields, thermo and genetic stability, attenuation and immunogenicity. Vaccine 2005, 23, 2501–2512. [Google Scholar] [CrossRef][Green Version]
- Beck, A.; Tesh, R.B.; Wood, T.G.; Widen, S.G.; Ryman, K.D.; Barrett, A.D. Comparison of the live attenuated yellow fever vaccine 17D-204 strain to its virulent parental strain Asibi by deep sequencing. J. Infect. Dis. 2014, 209, 334–344. [Google Scholar] [CrossRef]
- Fernandez-Garcia, M.D.; Meertens, L.; Chazal, M.; Hafirassou, M.L.; Dejarnac, O.; Zamborlini, A.; Despres, P.; Sauvonnet, N.; Arenzana-Seisdedos, F.; Jouvenet, N.; et al. Vaccine and wild-type strains of yellow fever virus engage distinct entry mechanisms and differentially stimulate antiviral immune responses. mBio 2016, 7, e01956-15. [Google Scholar] [CrossRef]
- Diamond, M.S.; Harris, E. Interferon inhibits dengue virus infection by preventing translation of viral RNA through a PKR-independent mechanism. Virology 2001, 289, 297–311. [Google Scholar] [CrossRef] [PubMed]
- Keller, B.C.; Fredericksen, B.L.; Samuel, M.A.; Mock, R.E.; Mason, P.W.; Diamond, M.S.; Gale, M., Jr. Resistance to alpha/beta interferon is a determinant of West Nile virus replication fitness and virulence. J. Virol. 2006, 80, 9424–9434. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.; McArthur, M.A.; Cohen, M.; Jahrling, P.B.; Janosko, K.B.; Josleyn, N.; Kang, K.; Zhang, T.; Holbrook, M.R. Characterization of yellow fever virus infection of human and non-human primate antigen presenting cells and their interaction with CD4+ T Cells. PLoS Negl. Trop. Dis. 2016, 10, e0004709. [Google Scholar] [CrossRef] [PubMed]
- Akondy, R.S.; Johnson, P.L.; Nakaya, H.I.; Edupuganti, S.; Mulligan, M.J.; Lawson, B.; Miller, J.D.; Pulendran, B.; Antia, R.; Ahmed, R. Initial viral load determines the magnitude of the human CD8 T cell response to yellow fever vaccination. Proc. Natl. Acad. Sci. USA 2015, 112, 3050–3055. [Google Scholar] [CrossRef]
- Watson, A.M.; Lam, L.K.M.; Klimstra, W.B.; Ryman, K.D. The 17D-204 vaccine strain-induced protection against virulent yellow fever virus is mediated by humoral immunity and CD4+ but not CD8+ T Cells. PLoS Pathog. 2016, 12, e1005786. [Google Scholar] [CrossRef]
- Querec, T.D.; Akondy, R.S.; Lee, E.K.; Cao, W.; Nakaya, H.I.; Teuwen, D.; Pirani, A.; Gernert, K.; Deng, J.; Marzolf, B.; et al. Systems biology approach predicts immunogenicity of the yellow fever vaccine in humans. Nat. Immunol. 2009, 10, 116–125. [Google Scholar] [CrossRef]
- Seong, R.K.; Lee, J.K.; Shin, O.S. Zika virus-induction of the suppressor of cytokine signaling 1/3 contributes to the modulation of viral replication. Pathogens (Basel, Switzerland) 2020, 9, 163. [Google Scholar] [CrossRef]
- Gao, W.; Hou, M.; Liu, X.; Li, Z.; Yang, Y.; Zhang, W. Induction of SOCS expression by EV71 infection promotes EV71 replication. BioMed Res. Int. 2020, 2020, 2430640. [Google Scholar] [CrossRef]
- Steffensen, M.A.; Fenger, C.; Christensen, J.E.; Jørgensen, C.K.; Bassi, M.R.; Christensen, J.P.; Finsen, B.; Thomsen, A.R. Suppressors of cytokine signaling 1 and 3 are upregulated in brain resident cells in response to virus-induced inflammation of the central nervous system via at least two distinctive pathways. J. Virol. 2014, 88, 14090–14104. [Google Scholar] [CrossRef]
- Souma, Y.; Nishida, T.; Serada, S.; Iwahori, K.; Takahashi, T.; Fujimoto, M.; Ripley, B.; Nakajima, K.; Miyazaki, Y.; Mori, M.; et al. Antiproliferative effect of SOCS-1 through the suppression of STAT3 and p38 MAPK activation in gastric cancer cells. Int. J. Cancer 2012, 131, 1287–1296. [Google Scholar] [CrossRef]
- Laurent-Rolle, M.; Morrison, J.; Rajsbaum, R.; Macleod, J.M.; Pisanelli, G.; Pham, A.; Ayllon, J.; Miorin, L.; Martinez-Romero, C.; tenOever, B.R.; et al. The interferon signaling antagonist function of yellow fever virus NS5 protein is activated by type I interferon. Cell Host Microbe 2014, 16, 314–327. [Google Scholar] [CrossRef]
- ter Meulen, J.; Sakho, M.; Koulemou, K.; Magassouba, N.F.; Bah, A.; Preiser, W.; Daffis, S.; Klewitz, C.; Bae, H.-G.; Niedrig, M.; et al. Activation of the cytokine network and unfavorable outcome in patients with yellow fever. J. Infect. Dis. 2004, 190, 1821–1827. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Yokosawa, N.; Okabayashi, T.; Suzutani, T.; Fujii, N. Induction of suppressor of cytokine signaling-3 by herpes simplex virus type 1 confers efficient viral replication. Virology 2005, 338, 173–181. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Wang, S.; Sun, F.; Zheng, G.; Wu, T.; Du, Y.; Zhang, S.; Qian, J.; Sun, R. Inhibition of murine herpesvirus-68 replication by IFN-gamma in macrophages is counteracted by the induction of SOCS1 expression. PLoS Pathog. 2018, 14, e1007202. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, X.; Magri, A.; Hill, M.; Lai, A.G.; Kumar, A.; Rambhatla, S.B.; Donald, C.L.; Lopez-Clavijo, A.F.; Rudge, S.; Pinnick, K.; et al. The circadian clock components BMAL1 and REV-ERBalpha regulate flavivirus replication. Nat. Commun. 2019, 10, 377. [Google Scholar] [CrossRef] [PubMed]
| Name | 5′-Sequence-3′ |
|---|---|
| IFN-α F | TCGCCCTTTGCTTTACTGAT |
| IFN-α R | GGGTCTCAGGGAGATCACAG |
| MxA F | CAGTTGAGGGCAAGGAGTGT |
| MxA R | ATGCCAGGAACCCACATACG |
| OAS1 F | AGCAACAGTGCAGACGATGA |
| OAS1 R | TTGGCTCTGTGCCTTGAAGT |
| PIAS1 F | GCAGACTTGTCCATCCCCAA |
| PIAS1 R | ACTGGGTCAAAGTAAAAGCCT |
| PIAS4 F | CTGGCACTTCCCATACCTGT |
| PIAS4 R | GGGATGGGAGAAGGACTAGC |
| β-Actin F | ATGATATCGCCGCGCTCGTC |
| β-Actin R | CGCTCGGTGAGGATCTTCA |
| SOCS-1 F | AGACCCCTTCTCACCTCTTG |
| SOCS-1 R | CTGCACAGCAGAAAATAAAGC |
| SOCS-3 F | TCCCCCCAGAAGAGCCTATTAC |
| SOCS-3 R | TCCGACAGAGATGCTGAAGAGTG |
| SOCS-5 F | AGTCAAAGCCTCTCTTTTCC |
| SOCS-5 R | ACTGAACCTGACCGTACACATTTTTGGGCTAAATCTGA |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yakass, M.B.; Franco, D.; Quaye, O. Yellow Fever Virus Down-Regulates mRNA Expression of SOCS1 in the Initial Phase of Infection in Human Cell Lines. Viruses 2020, 12, 802. https://doi.org/10.3390/v12080802
Yakass MB, Franco D, Quaye O. Yellow Fever Virus Down-Regulates mRNA Expression of SOCS1 in the Initial Phase of Infection in Human Cell Lines. Viruses. 2020; 12(8):802. https://doi.org/10.3390/v12080802
Chicago/Turabian StyleYakass, Michael B., David Franco, and Osbourne Quaye. 2020. "Yellow Fever Virus Down-Regulates mRNA Expression of SOCS1 in the Initial Phase of Infection in Human Cell Lines" Viruses 12, no. 8: 802. https://doi.org/10.3390/v12080802
APA StyleYakass, M. B., Franco, D., & Quaye, O. (2020). Yellow Fever Virus Down-Regulates mRNA Expression of SOCS1 in the Initial Phase of Infection in Human Cell Lines. Viruses, 12(8), 802. https://doi.org/10.3390/v12080802






