Differential Detection of Encapsidated versus Unencapsidated Enterovirus RNA in Samples Containing Pancreatic Enzymes—Relevance for Diabetes Studies
Abstract
1. Introduction
2. Materials and Methods
2.1. Participating Virology Laboratories
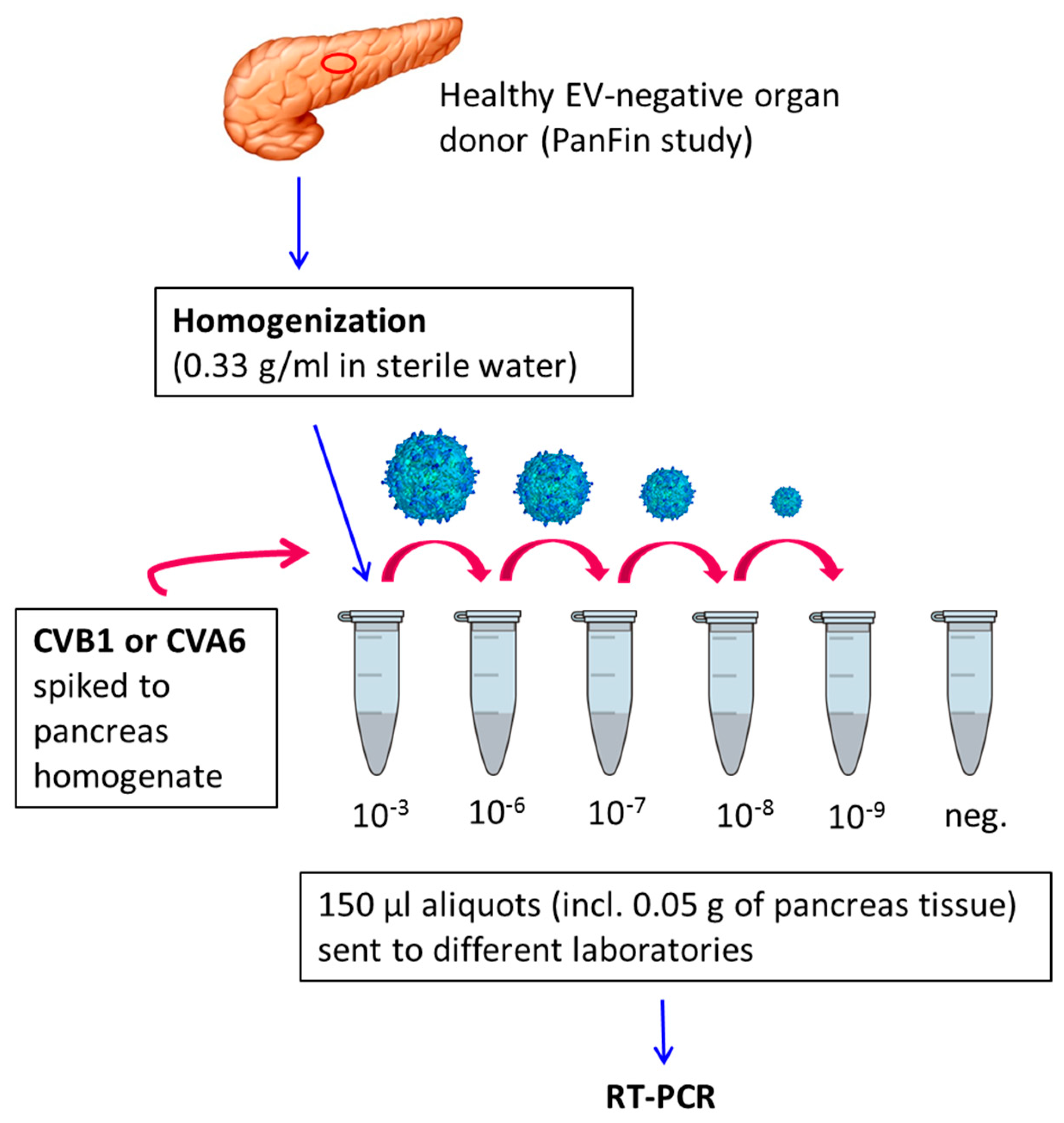
2.2. Preparation of Virus-Spiked Human Pancreas Homogenate (University of Tampere, Finland)
2.3. Virus Detection by RT-PCR
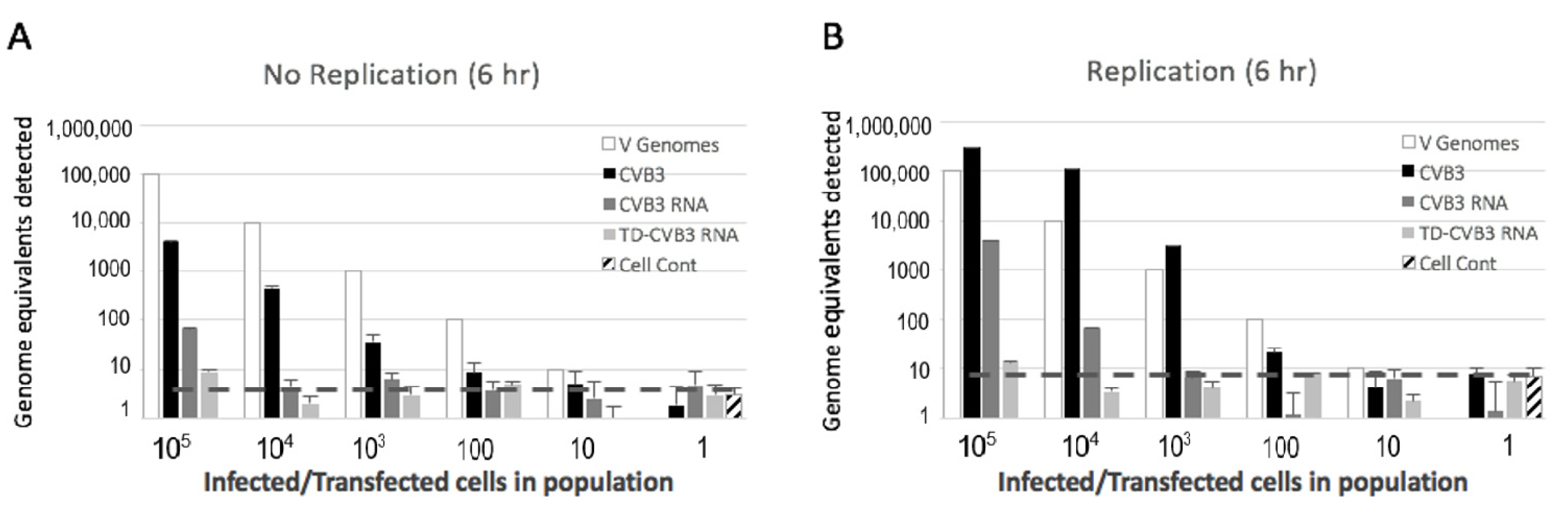
2.4. Preparation and Detection of Full-Length and Terminally Deleted Coxsackievirus B3 (Baylor College of Medicine, Houston, Texas)
3. Results
3.1. Sensitivity of Different RT-PCR Methods to Detect Enterovirus RNA
3.2. Detection of Encapsidated and Unencapsidated Enterovirus RNA
3.3. Detection of Replicating and Non-Replicating Enterovirus
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Morgan, N.G.; Richardson, S.J. Enteroviruses as causative agents in type 1 diabetes: loose ends or lost cause? Trends Endocrinol. Metab. 2014, 25, 611–619. [Google Scholar] [CrossRef] [PubMed]
- Krogvold, L.; Edwin, B.; Buanes, T.; Frisk, G.; Skog, O.; Anagandula, M.; Korsgren, O.; Undlien, D.; Eike, M.C.; Richardson, S.J.; et al. Detection of a low-grade enteroviral infection in the islets of Langerhans of living patients newly diagnosed with type 1 diabetes. Diabetes 2015, 64, 1682–1687. [Google Scholar] [CrossRef]
- Dotta, F.; Censini, S.; van Halteren, A.G.; Marselli, L.; Masini, M.; Dionisi, S.; Mosca, F.; Boggi, U.; Muda, A.O.; Del Prato, S.; et al. Coxsackie B4 virus infection of beta cells and natural killer cell insulitis in recent-onset type 1 diabetic patients. Proc. Natl. Acad. Sci. USA 2007, 104, 5115–5120. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Leete, P.; Dhayal, S.; Russell, M.A.; Oikarinen, M.; Laiho, J.E.; Svedin, E.; Lind, K.; Rosenling, T.; Chapman, N.; et al. Detection of enterovirus in the islet cells of patients with type 1 diabetes: what do we learn from immunohistochemistry? Reply to Hansson SF, Korsgren S, Ponten F et al [letter]. Diabetologia 2014, 57, 647–649. [Google Scholar] [CrossRef] [PubMed]
- Ylipaasto, P.; Klingel, K.; Lindberg, A.M.; Otonkoski, T.; Kandolf, R.; Hovi, T.; Roivainen, M. Enterovirus infection in human pancreatic islet cells, islet tropism in vivo and receptor involvement in cultured islet beta cells. Diabetologia 2004, 47, 225–239. [Google Scholar] [CrossRef] [PubMed]
- Richardson, S.J.; Leete, P.; Bone, A.J.; Foulis, A.K.; Morgan, N.G. Expression of the enteroviral capsid protein VP1 in the islet cells of patients with type 1 diabetes is associated with induction of protein kinase R and downregulation of Mcl-1. Diabetologia 2013, 56, 185–193. [Google Scholar] [CrossRef]
- Chapman, N.M.; Kim, K.S.; Drescher, K.M.; Oka, K.; Tracy, S. 5' terminal deletions in the genome of a coxsackievirus B2 strain occurred naturally in human heart. Virology 2008, 375, 480–491. [Google Scholar] [CrossRef]
- Tracy, S.; Smithee, S.; Alhazmi, A.; Chapman, N. Coxsackievirus can persist in murine pancreas by deletion of 5' terminal genomic sequences. J. Med. Virol. 2015, 87, 240–247. [Google Scholar] [CrossRef]
- Kim, K.S.; Tracy, S.; Tapprich, W.; Bailey, J.; Lee, C.K.; Kim, K.; Barry, W.H.; Chapman, N.M. 5'-Terminal deletions occur in coxsackievirus B3 during replication in murine hearts and cardiac myocyte cultures and correlate with encapsidation of negative-strand viral RNA. J. Virol. 2005, 79, 7024–7041. [Google Scholar] [CrossRef]
- Sharma, N.; O'Donnell, B.J.; Flanegan, J.B. 3'-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation. J. Virol. 2005, 79, 3565–3577. [Google Scholar] [CrossRef]
- Vogt, D.A.; Andino, R. An RNA element at the 5'-end of the poliovirus genome functions as a general promoter for RNA synthesis. PLoS Pathog. 2010, 6, e1000936. [Google Scholar] [CrossRef] [PubMed]
- Ertel, K.J.; Brunner, J.E.; Semler, B.L. Mechanistic consequences of hnRNP C binding to both RNA termini of poliovirus negative-strand RNA intermediates. J. Virol. 2010, 84, 4229–4242. [Google Scholar] [CrossRef] [PubMed]
- Andino, R.; Rieckhof, G.E.; Achacoso, P.L.; Baltimore, D. Poliovirus RNA synthesis utilizes an RNP complex formed around the 5'-end of viral RNA. EMBO J. 1993, 12, 3587–3598. [Google Scholar] [CrossRef] [PubMed]
- Nugent, C.I.; Johnson, K.L.; Sarnow, P.; Kirekegaard, K. Functional coupling between replication and packaging of poliovirus replicon RNA. J. Virol. 1999, 73, 427–435. [Google Scholar] [CrossRef]
- Augereau, C.; Lemaigre, F.P.; Jacquemin, P. Extraction of High-Quality RNA From Pancreatic Tissues for Gene Expression Studies. Anal. Biochem. 2016, 500, 60–62. [Google Scholar] [CrossRef]
- Kaddis, J.S.; Pugliese, A.; Atkinson, M.A. A Run on the Biobank: What Have We Learned About Type 1 Diabetes From the nPOD Tissue Repository? Curr. Opin. Endocrinol. Diabetes Obes. 2015, 22, 290–295. [Google Scholar] [CrossRef]
- Laiho, J.E.; Oikarinen, M.; Richardson, S.J.; Frisk, J.; Nyalwidhe, J.; Burch, T.C.; Morris, M.A.; Oikarinen, S.; Pugliese, A.; Dotta, F.; et al. Relative sensitivity of immunohistochemistry, multiple reaction monitoring mass spectrometry, in situ hybridization and PCR to detect Coxsackievirus B1 in A549 cells. J. Clin. Virol. 2016, 77, 21–28. [Google Scholar] [CrossRef]
- Tauriainen, S.; Salmela, K.; Rantala, I.; Knip, M.; Hyöty, H. Collecting high-quality pancreatic tissue for experimental study from organ donors with signs of beta-cell autoimmunity. Diabetes Metab. Res. Rev. 2010, 26, 585–592. [Google Scholar] [CrossRef]
- Lonnrot, M.; Sjoroos, M.; Salminen, K.; Maaronen, M.; Hyypiä, T.; Hyöty, H. Diagnosis of enterovirus and rhinovirus infections by RT-PCR and time-resolved fluorometry with lanthanide chelate labeled probes. J. Med. Virol. 1999, 59, 378–384. [Google Scholar] [CrossRef]
- Honkanen, H.; Oikarinen, S.; Pakkanen, O.; Ruokoranta, T.; Pulkki, M.M.; Laitinen, O.H.; Tauriainen, S.; Korpela, S.; Lappalainen, M.; Vuorinen, T.; et al. Human enterovirus 71 strains in the background population and in hospital patients in Finland. J. Clin. Virol. 2013, 56, 348–353. [Google Scholar] [CrossRef]
- Genoni, A.; Canducci, F.; Rossi, A.; Broccolo, B.; Chumakov, K.; Bono, G.; Salerno-Uriarte, J.; Salvatoni, A.; Pugliese, A.; Toniolo, A. Revealing Enterovirus Infection in Chronic Human Disorders: An Integrated Diagnostic Approach. Sci. Rep. 2017, 7, 5013. [Google Scholar] [CrossRef] [PubMed]
- Smithee, S.; Tracy, S.; Chapman, N.M. Mutational Disruption of cis-Acting Replication Element 2C in Coxsackievirus B3 Leads to 5′-Terminal Genomic Deletions. J. Virol. 2015, 89, 11761–11772. [Google Scholar] [CrossRef] [PubMed]
- Kempf, B.J.; Barton, D.J. Poliovirus 2A(Pro) increases viral mRNA and polysome stability coordinately in time with cleavage of eIF4G. J. Virol. 2008, 82, 5847–5859. [Google Scholar] [CrossRef] [PubMed]
- Vehik, K.; Fiske, S.W.; Logan, C.A.; Agardh, D.; Cilio, C.M.; Hagopian, W.; Simell, O.; Roivainen, M.; She, J.X.; Brieseet, T.; et al. Methods, Quality Control and Specimen Management in an International Multicentre Investigation of Type 1 Diabetes: TEDDY. Diabetes Metab. Res. Rev. 2013, 29, 557–567. [Google Scholar] [CrossRef] [PubMed]
- Feuer, R.; Ruller, C.M.; An, N.; Tabor-Godwin, J.M.; Rhoades, R.E.; Maciejewski, S.; Pagarigan, R.R.; Cornell, C.T.; Crocker, S.J.; Kiosseset, W.B.; et al. Viral persistence and chronic immunopathology in the adult central nervous system following Coxsackievirus infection during the neonatal period. J. Virol. 2009, 83, 9356–9369. [Google Scholar] [CrossRef]
- N'Guyen, Y.; Lesaffre, F.; Metz, D.; Tassan, S.; Saade, Y.; Boulagnon, C.; Fornes, P.; Renois, F.; Andreoletti, L. Enterovirus but not Parvovirus B19 is associated with idiopathic dilated cardiomyopathy and endomyocardial CD3, CD68, or HLA-DR expression. J. Med. Virol. 2017, 89, 55–63. [Google Scholar] [CrossRef]

| Laboratory | Method | EV Group | Forward Primer | Reverse Primer | Probe | Location in the Genome * |
|---|---|---|---|---|---|---|
| Houston | PCR 1 | 5′UTR-A-D | CGGCCCCTGAATGCGGCTAA | GAAACACGGACACCCAAAGTA | 449–563 | |
| Tampere | PCR 1 | 5′UTR-A-D | CGGCCCCTGAATGCGGCTAA | GAAACACGGACACCCAAAGTA | TAITCGGTTCCGCTGC | 449–563 |
| PCR 2 | 5′UTR-A-D | CGGCCCCTGAATGCGGCTAA | GAAACACGGACACCCAAAGTA | FAM-TCTGTGGCG GAA CCGACTA-TAMRA FAM-TCTGCAGCGGAA CCGACTA-TAMRA | 449–563 | |
| Varese | PCR 1 and 2 | 5′UTR-A | GTGTAGATCAGGTCGATGAGTCAC | ATTGTCACCATAAGCAGCCA | 306–597 | |
| 5′UTR-B | GACCAAGCACTTCTGTTACCC | GTCACCATAAGCAGCCAATATA | 161–594 | |||
| 5′UTR-C | GGTGTGAAGAGCCTATTGAGC | GATTGTCACCATAAGCAGCCA | 413–598 | |||
| 5′UTR-D | TGGTCCAGGCTGCGTT | AACACGGACACCCAAAGTAGT | 351–561 |
| Houston | Tampere | Varese | |||
|---|---|---|---|---|---|
| Sample | PCR 1 | PCR 1 | PCR 2 | PCR 1 | PCR 2 |
| CVB1 in water | 10−7 | 10−9 | 10−9 | 10−6 | n.a. |
| CVB1 in pancreas extract | 10−8 | 10−9 | 10−9 | 10−9 | 10−9 |
| CVB1 in pancreas extract, 20 h on ice | 10−8 | 10−8 | 10−8 | 10−9 | 10−9 |
| CVA6 in pancreas extract | 10−7 | 10−7 | 10−7 | 10−8 | 10−9 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oikarinen, M.; Bertolet, L.; Toniolo, A.; Oikarinen, S.; Laiho, J.E.; Pugliese, A.; Lloyd, R.E.; Hyöty, H.; the nPOD-V Study Group. Differential Detection of Encapsidated versus Unencapsidated Enterovirus RNA in Samples Containing Pancreatic Enzymes—Relevance for Diabetes Studies. Viruses 2020, 12, 747. https://doi.org/10.3390/v12070747
Oikarinen M, Bertolet L, Toniolo A, Oikarinen S, Laiho JE, Pugliese A, Lloyd RE, Hyöty H, the nPOD-V Study Group. Differential Detection of Encapsidated versus Unencapsidated Enterovirus RNA in Samples Containing Pancreatic Enzymes—Relevance for Diabetes Studies. Viruses. 2020; 12(7):747. https://doi.org/10.3390/v12070747
Chicago/Turabian StyleOikarinen, Maarit, Lori Bertolet, Antonio Toniolo, Sami Oikarinen, Jutta E. Laiho, Alberto Pugliese, Richard E. Lloyd, Heikki Hyöty, and the nPOD-V Study Group. 2020. "Differential Detection of Encapsidated versus Unencapsidated Enterovirus RNA in Samples Containing Pancreatic Enzymes—Relevance for Diabetes Studies" Viruses 12, no. 7: 747. https://doi.org/10.3390/v12070747
APA StyleOikarinen, M., Bertolet, L., Toniolo, A., Oikarinen, S., Laiho, J. E., Pugliese, A., Lloyd, R. E., Hyöty, H., & the nPOD-V Study Group. (2020). Differential Detection of Encapsidated versus Unencapsidated Enterovirus RNA in Samples Containing Pancreatic Enzymes—Relevance for Diabetes Studies. Viruses, 12(7), 747. https://doi.org/10.3390/v12070747