Acidic pH Triggers Lipid Mixing Mediated by Lassa Virus GP
Abstract
1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Plasmid DNA
2.3. Pseudotype Production
2.4. Virus Infectivity Assays
2.5. LASV GP Antibody Capture ELISA
2.6. LAMP1 Purification
2.7. Liposome Formation
2.8. Labeling Virions
2.9. Single-Virion Lipid Mixing Assay
3. Results
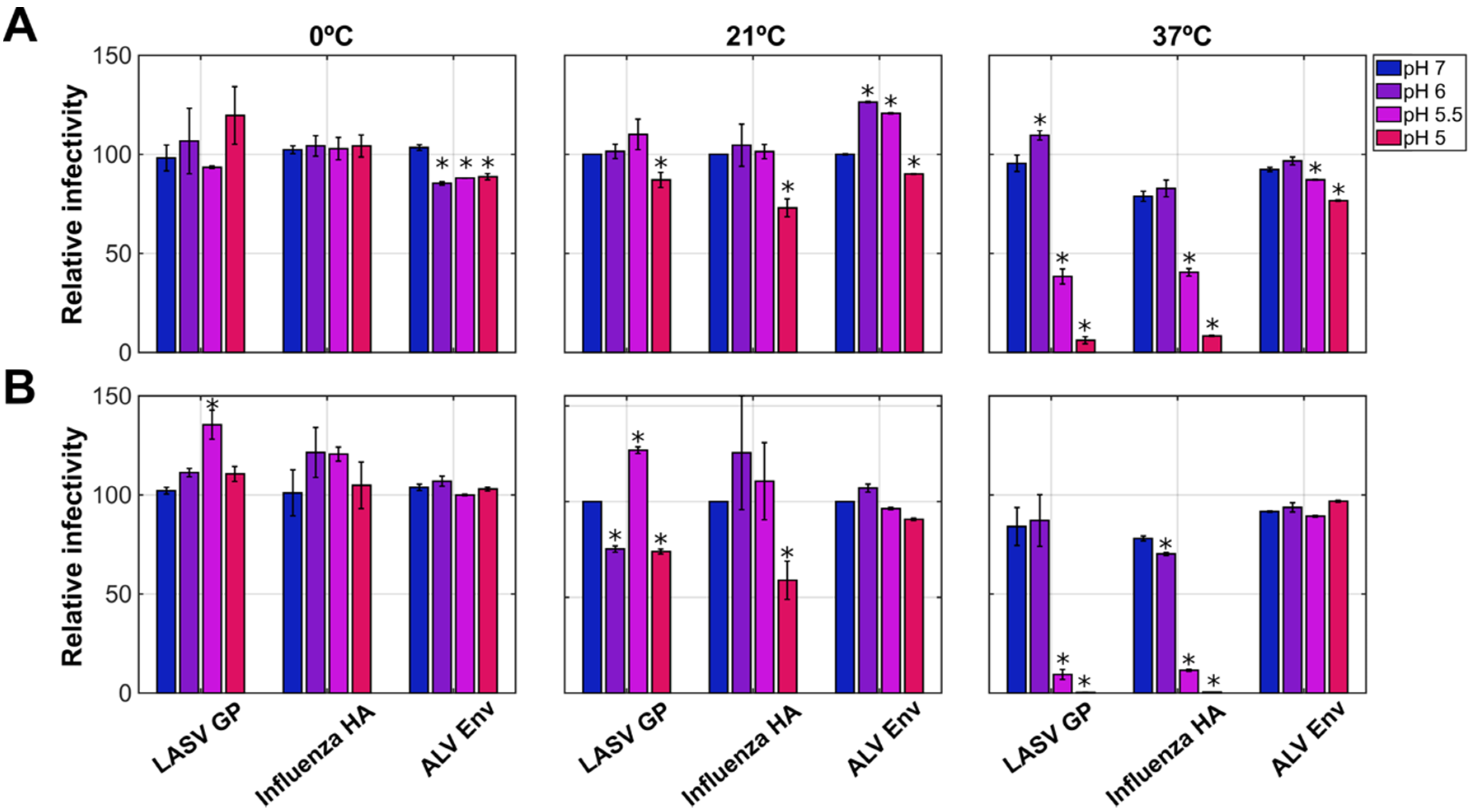
3.1. The pH Sensitivity of LASV GP
3.2. Acidic pH Triggers Loss of Recognition by Neutralizing Antibodies
3.3. Acidic pH Triggers Lipid Mixing Mediated by LASV GP
3.4. LAMP1 Increases the Kinetics of Lipid Mixing
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nigeria Centre for Disease Control. Lassa Fever Situation Report; NCDC: Abuja, Nigeria, 2020. [Google Scholar]
- Okogbenin, S.; Okoeguale, J.; Akpede, G.; Colubri, A.; Barnes, K.G.; Mehta, S.; Eifediyi, R.; Okogbo, F.; Eigbefoh, J.; Momoh, M.; et al. Retrospective cohort study of Lassa fever in pregnancy, Southern Nigeria. Emerg. Infect. Dis. 2019, 25, 1494–1500. [Google Scholar] [CrossRef] [PubMed]
- Mateer, E.J.; Huang, C.; Shehu, N.Y.; Paessler, S. Lassa fever–induced sensorineural hearing loss: A neglected public health and social burden. PLoS Neglect. Trop. D 2018, 12, e0006187. [Google Scholar] [CrossRef]
- Hastie, K.M.; Saphire, E.O. Lassa virus glycoprotein: Stopping a moving target. Curr. Opin. Virol. 2018, 31, 52–58. [Google Scholar] [CrossRef] [PubMed]
- Lenz, O.; ter Meulen, J.; Klenk, H.D.; Seidah, N.G.; Garten, W. The Lassa virus glycoprotein precursor GP-C is proteolytically processed by subtilase SKI-1/S1P. Proc. Natl. Acad. Sci. USA 2001, 98, 12701–12705. [Google Scholar] [CrossRef]
- Burri, D.J.; Pasquato, A.; da Palma, J.R.; Igonet, S.; Oldstone, M.B.A.; Kunz, S. The role of proteolytic processing and the stable signal peptide in expression of the Old World arenavirus envelope glycoprotein ectodomain. Virology 2013, 436, 127–133. [Google Scholar] [CrossRef]
- Israeli, H.; Cohen-Dvashi, H.; Shulman, A.; Shimon, A.; Diskin, R. Mapping of the Lassa virus LAMP1 binding site reveals unique determinants not shared by other old world arenaviruses. PLoS Pathog. 2017, 13, e1006337. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Henry, M.D.; Borrow, P.; Yamada, H.; Elder, J.H.; Ravkov, E.V.; Nichol, S.T.; Compans, R.W.; Campbell, K.P.; Oldstone, M.B.A. Identification of -dystroglycan as a receptor for lymphocytic choriomeningitis virus and Lassa fever virus. Science 1998, 282, 2079–2081. [Google Scholar] [CrossRef]
- Oppliger, J.; Torriani, G.; Herrador, A.; Kunz, S. Lassa virus cell entry via dystroglycan involves an unusual pathway of macropinocytosis. J. Virol. 2016, 90, 6412–6429. [Google Scholar] [CrossRef]
- Jae, L.T.; Brummelkamp, T.R. Emerging intracellular receptors for hemorrhagic fever viruses. Trends Microbiol. 2015, 23, 392–400. [Google Scholar] [CrossRef]
- Acciani, M.; Alston, J.T.; Zhao, G.; Reynolds, H.; Ali, A.M.; Xu, B.; Brindley, M.A. Mutational analysis of Lassa virus glycoprotein highlights regions required for alpha-dystroglycan utilization. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Cohen-Dvashi, H.; Cohen, N.; Israeli, H.; Diskin, R. Molecular mechanism for LAMP1 recognition by Lassa virus. J. Virol. 2015, 89, 7584–7592. [Google Scholar] [CrossRef] [PubMed]
- Hastie, K.M.; Zandonatti, M.A.; Kleinfelter, L.M.; Heinrich, M.L.; Rowland, M.M.; Chandran, K.; Branco, L.M.; Robinson, J.E.; Garry, R.F.; Saphire, E.O. Structural basis for antibody-mediated neutralization of Lassa virus. Science 2017, 356, 923–928. [Google Scholar] [CrossRef]
- Li, S.; Sun, Z.; Pryce, R.; Parsy, M.-L.; Fehling, S.K.; Schlie, K.; Siebert, C.A.; Garten, W.; Bowden, T.A.; Strecker, T.; et al. Acidic pH-induced conformations and LAMP1 binding of the Lassa virus glycoprotein spike. PLoS Pathog. 2016, 12, e1005418. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Agard, D.A. Influenza hemagglutinin: Kinetic control of protein function. Structure 1994, 2, 907–910. [Google Scholar] [CrossRef]
- Harrison, S.C. Viral membrane fusion. Virology 2015, 479, 498–507. [Google Scholar] [CrossRef] [PubMed]
- Igonet, S.; Vaney, M.-C.; Vonrhein, C.; Vonhrein, C.; Bricogne, G.; Stura, E.A.; Hengartner, H.; Eschli, B.; Rey, F.A. X-ray structure of the arenavirus glycoprotein GP2 in its postfusion hairpin conformation. Proc. Natl. Acad. Sci. USA 2011, 108, 19967–19972. [Google Scholar] [CrossRef]
- Eschli, B.; Quirin, K.; Wepf, A.; Weber, J.; Zinkernagel, R.; Hengartner, H. Identification of an N-terminal trimeric coiled-coil core within arenavirus glycoprotein 2 permits assignment to class I viral fusion proteins. J. Virol. 2006, 80, 5897–5907. [Google Scholar] [CrossRef]
- Shulman, A.; Katz, M.; Cohen-Dvashi, H.; Greenblatt, H.M.; Levy, Y.; Diskin, R. Variations in core packing of GP2 from old world mammarenaviruses in their post-fusion conformations affect membrane-fusion efficiencies. J. Mol. Biol. 2019. [Google Scholar] [CrossRef]
- Hulseberg, C.E.; Fénéant, L.; Szymańska, K.M.; White, J.M. Lamp1 increases the efficiency of Lassa virus Infection by promoting fusion in less acidic endosomal compartments. Mbio 2018, 9, 75. [Google Scholar] [CrossRef]
- Cohen-Dvashi, H.; Israeli, H.; Shani, O.; Katz, A.; Diskin, R. Role of LAMP1 binding and pH sensing by the spike Complex of Lassa virus. J. Virol. 2016, 90, 10329–10338. [Google Scholar] [CrossRef]
- Thomas, C.J.; Shankar, S.; Casquilho-Gray, H.E.; York, J.; Sprang, S.R.; Nunberg, J.H. Biochemical reconstitution of hemorrhagic-fever arenavirus envelope glycoprotein-mediated membrane fusion. PLoS ONE 2012, 7. [Google Scholar] [CrossRef] [PubMed]
- Borrow, P.; Oldstone, M.B.A. Mechanism of lymphocytic choriomeningitis virus entry into cells. Virology 1994, 198, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Glushakova, S.E.; Lukashevich, I.S. Early events in arenavirus replication are sensitive to lysosomotropic compounds. Arch. Virol. 1989, 104, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Rojek, J.M.; Sanchez, A.B.; Nguyen, N.T.; de la Torre, J.-C.; Kunz, S. Different mechanisms of cell entry by human-pathogenic Old World and New World arenaviruses. J. Virol. 2008, 82, 7677–7687. [Google Scholar] [CrossRef] [PubMed]
- Rojek, J.M.; Kunz, S. Cell entry by human pathogenic arenaviruses. Cell Microbiol. 2008, 10, 828–835. [Google Scholar] [CrossRef] [PubMed]
- Cosset, F.-L.; Marianneau, P.; Verney, G.; Gallais, F.; Tordo, N.; Pécheur, E.-I.; ter Meulen, J.; Deubel, V.; Bartosch, B. Characterization of Lassa virus cell entry and neutralization with Lassa virus pseudoparticles. J. Virol. 2009, 83, 3228–3237. [Google Scholar] [CrossRef]
- Klewitz, C.; Klenk, H.-D.; ter Meulen, J. Amino acids from both N-terminal hydrophobic regions of the Lassa virus envelope glycoprotein GP-2 are critical for pH-dependent membrane fusion and infectivity. J. Gen. Virol. 2007, 88, 2320–2328. [Google Scholar] [CrossRef]
- Simone, C.D.; Zandonatti, M.A.; Buchmeier, M.J. Acidic pH triggers LCMV membrane fusion activity and conformational change in the glycoprotein spike. Virology 1994, 198, 455–465. [Google Scholar] [CrossRef]
- Das, D.K.; Govindan, R.; Nikić-Spiegel, I.; Krammer, F.; Lemke, E.A.; Munro, J.B. Direct visualization of the conformational dynamics of single influenza hemagglutinin trimers. Cell 2018, 174, 926–937.e12. [Google Scholar] [CrossRef]
- Whitt, M.A. Generation of VSV pseudotypes using recombinant ΔG-VSV for studies on virus entry, identification of entry inhibitors, and immune responses to vaccines. J. Virol. Methods 2010, 169, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Robinson, J.E.; Hastie, K.M.; Cross, R.W.; Yenni, R.E.; Elliott, D.H.; Rouelle, J.A.; Kannadka, C.B.; Smira, A.A.; Garry, C.E.; Bradley, B.T.; et al. Most neutralizing human monoclonal antibodies target novel epitopes requiring both Lassa virus glycoprotein subunits. Nat. Commun. 2016, 7, 11544. [Google Scholar] [CrossRef] [PubMed]
- Floyd, D.L.; Ragains, J.R.; Skehel, J.J.; Harrison, S.C.; van Oijen, A.M. Single-particle kinetics of influenza virus membrane fusion. Proc. Natl. Acad. Sci. USA 2008, 105, 15382–15387. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C. Stability of infectious influenza A viruses to treatment at low pH and heating. Arch. Virol. 1985, 85, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Marzi, A.; Feldmann, F.; Geisbert, T.W.; Feldmann, H.; Safronetz, D. Vesicular stomatitis virus-based vaccines against Lassa and Ebola viruses. Emerg. Infect. Dis. 2015, 21, 305–307. [Google Scholar] [CrossRef]
- Skehel, J.J.; Wiley, D.C. Receptor binding and membrane fusion in virus entry: The influenza hemagglutinin. Annu. Rev. Biochem. 2000, 69, 531–569. [Google Scholar] [CrossRef]
- Ivanovic, T.; Choi, J.L.; Whelan, S.P.; van Oijen, A.M.; Harrison, S.C. Influenza-virus membrane fusion by cooperative fold-back of stochastically induced hemagglutinin intermediates. Elife 2013, 2, e00333. [Google Scholar] [CrossRef]
- Costello, D.A.; Lee, D.W.; Drewes, J.; Vasquez, K.A.; Kisler, K.; Wiesner, U.; Pollack, L.; Whittaker, G.R.; Daniel, S. Influenza virus-membrane fusion triggered by proton uncaging for single particle studies of fusion kinetics. Anal. Chem. 2012, 84, 8480–8489. [Google Scholar] [CrossRef]
- Chao, L.H.; Klein, D.E.; Schmidt, A.G.; Pena, J.M.; Harrison, S.C. Sequential conformational rearrangements in flavivirus membrane fusion. Elife 2014, 3. [Google Scholar] [CrossRef]
- Costello, D.A.; Millet, J.K.; Hsia, C.-Y.; Whittaker, G.R.; Daniel, S. Single particle assay of coronavirus membrane fusion with proteinaceous receptor-embedded supported bilayers. Biomaterials 2013, 34, 7895–7904. [Google Scholar] [CrossRef] [PubMed]
- Kim, I.S.; Jenni, S.; Stanifer, M.L.; Roth, E.; Whelan, S.P.J.; van Oijen, A.M.; Harrison, S.C. Mechanism of membrane fusion induced by vesicular stomatitis virus G protein. Proc. Natl. Acad. Sci. USA 2016, 114, E28–E36. [Google Scholar] [CrossRef]
- Huotari, J.; Helenius, A. Endosome maturation. EMBO J. 2011, 30, 3481–3500. [Google Scholar] [CrossRef] [PubMed]
- Carr, C.M.; Chaudhry, C.; Kim, P.S. Influenza hemagglutinin is spring-loaded by a metastable native conformation. Proc. Natl. Acad. Sci. USA 1997, 94, 14306–14313. [Google Scholar] [CrossRef] [PubMed]
- Das, D.K.; Bulow, U.; Diehl, W.E.; Durham, N.D.; Senjobe, F.; Chandran, K.; Luban, J.; Munro, J.B. Conformational changes in the Ebola virus membrane fusion machine induced by pH, Ca2+, and receptor binding. PLoS Biol. 2020, 18, e3000626. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bulow, U.; Govindan, R.; Munro, J.B. Acidic pH Triggers Lipid Mixing Mediated by Lassa Virus GP. Viruses 2020, 12, 716. https://doi.org/10.3390/v12070716
Bulow U, Govindan R, Munro JB. Acidic pH Triggers Lipid Mixing Mediated by Lassa Virus GP. Viruses. 2020; 12(7):716. https://doi.org/10.3390/v12070716
Chicago/Turabian StyleBulow, Uriel, Ramesh Govindan, and James B. Munro. 2020. "Acidic pH Triggers Lipid Mixing Mediated by Lassa Virus GP" Viruses 12, no. 7: 716. https://doi.org/10.3390/v12070716
APA StyleBulow, U., Govindan, R., & Munro, J. B. (2020). Acidic pH Triggers Lipid Mixing Mediated by Lassa Virus GP. Viruses, 12(7), 716. https://doi.org/10.3390/v12070716




