Epstein–Barr Virus-Induced Post-Transplant Lymphoproliferative Disorder of the Central Nervous System Successfully Treated with Chemo-Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Patient Consent
2.2. RT-PCR for Assessing EBV Load
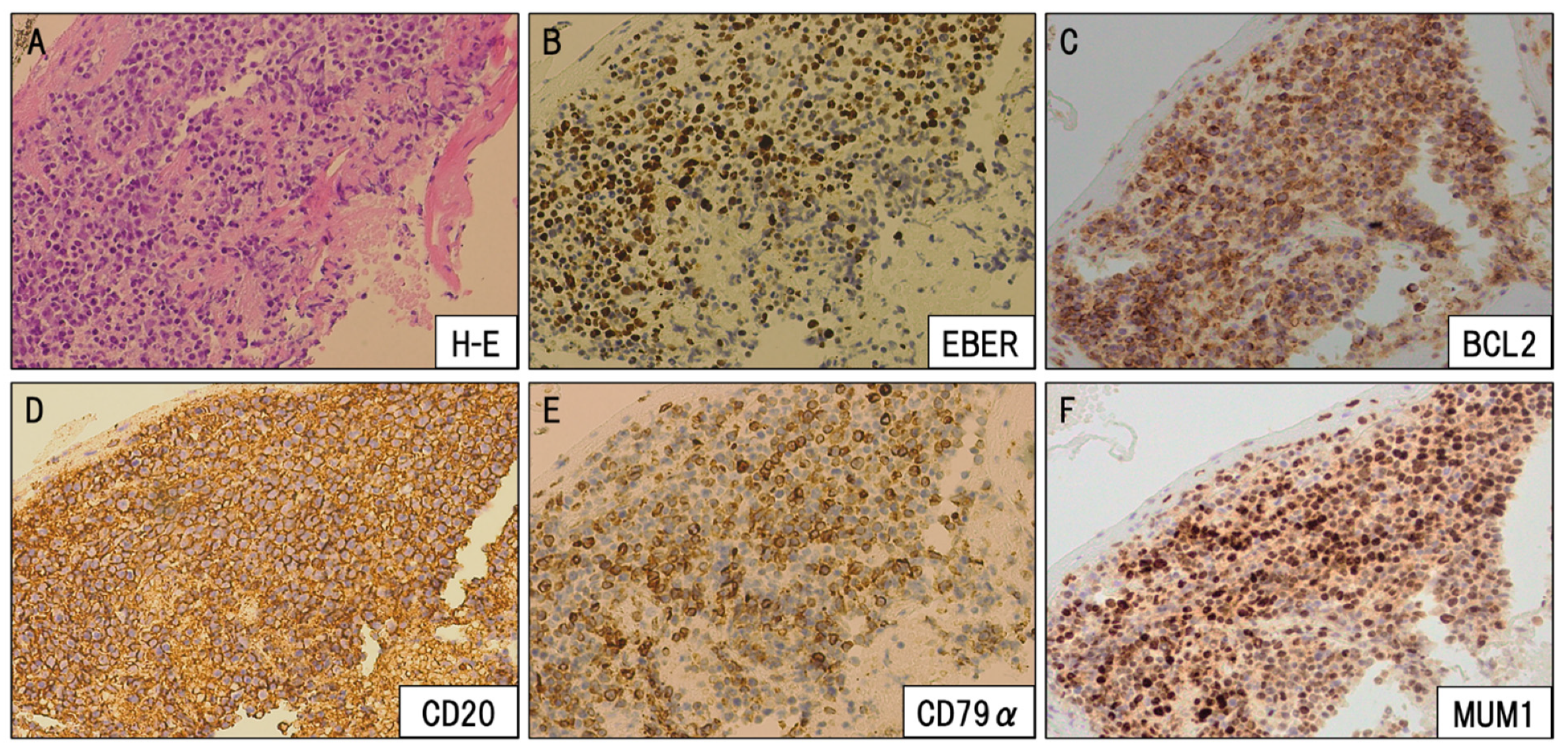
2.3. Tissue Processing, Staining, and Immunohistochemistry
2.4. Serological Studies
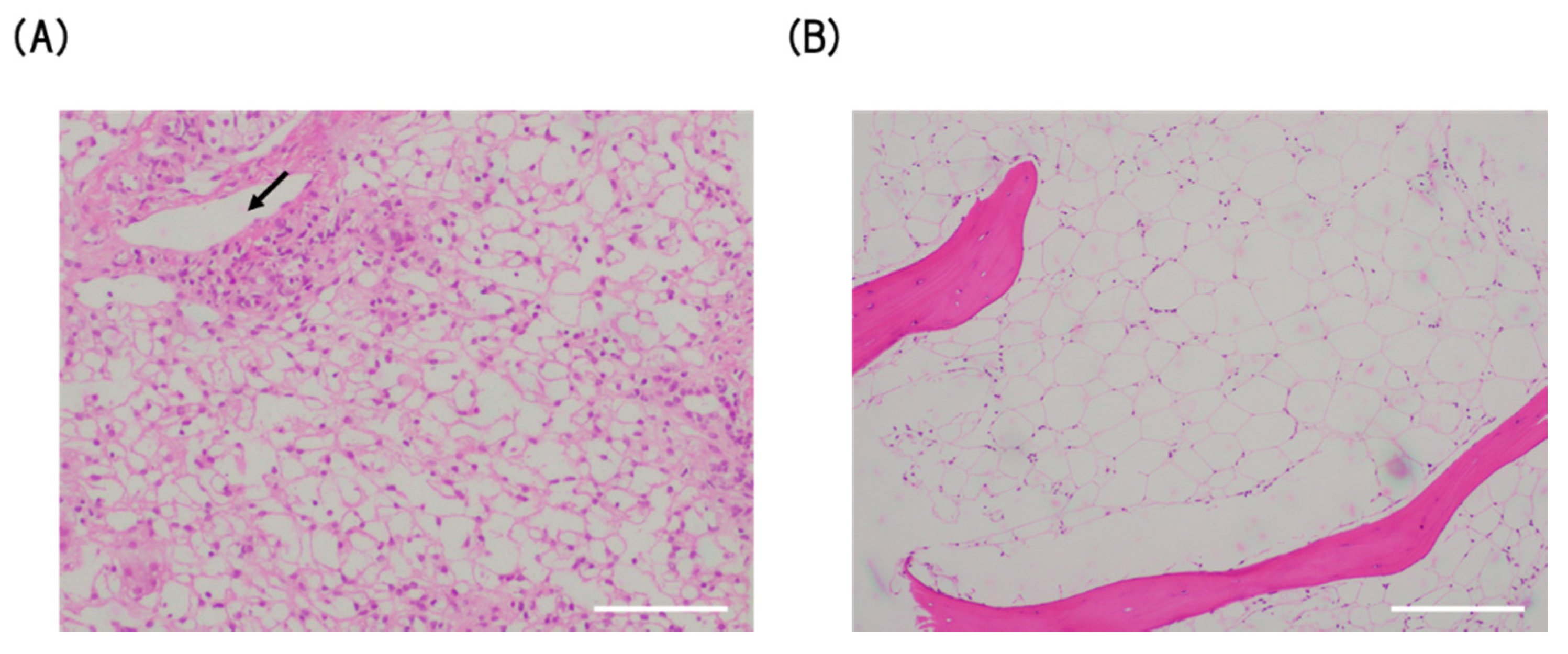
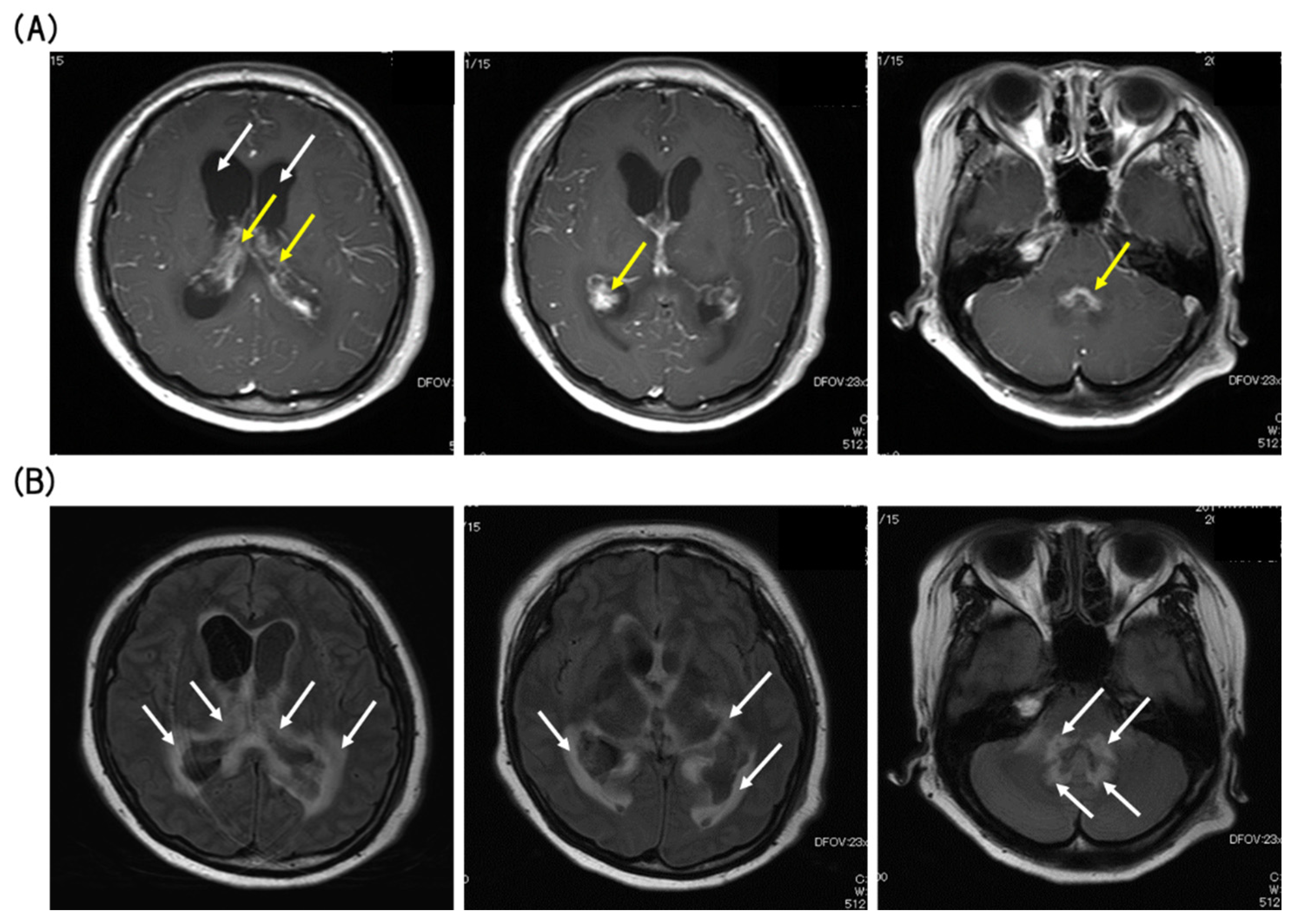
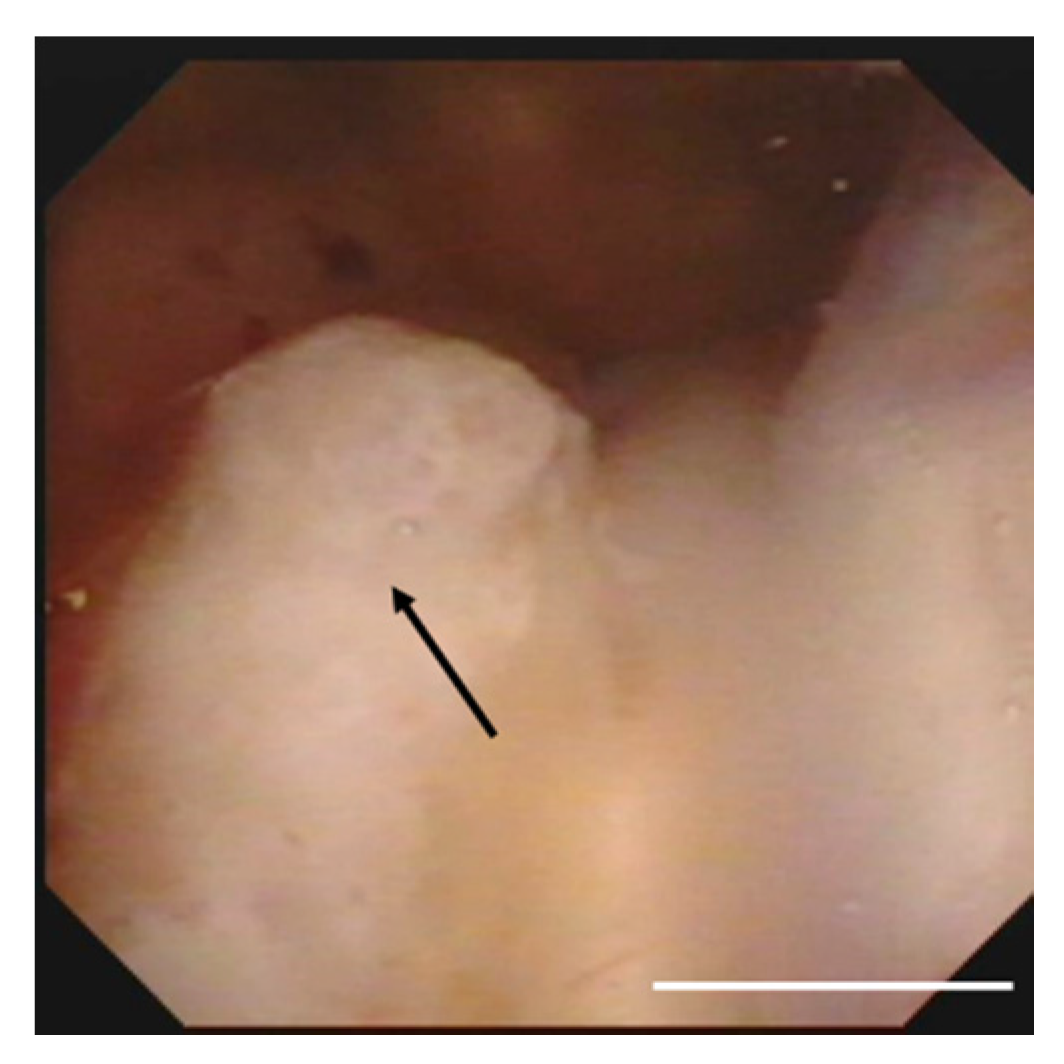
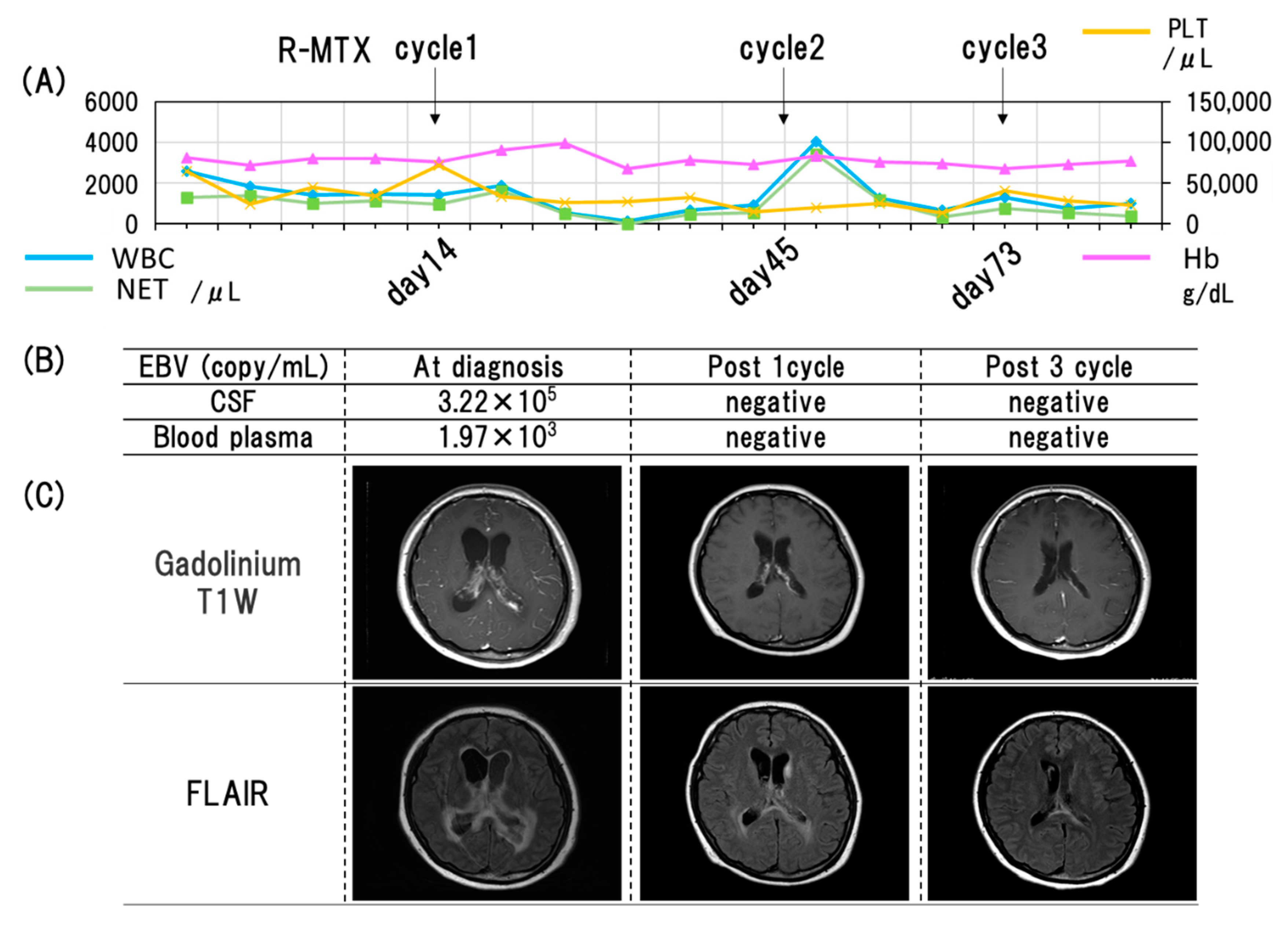
3. Case Presentation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Espinoza, J.L.; ElBadry, M.I.; Chonabayashi, K.; Yoshida, Y.; Katagiri, T.; Harada, K.; Nakagawa, N.; Zaimoku, Y.; Imi, T.; Takamatsu, H.; et al. Hematopoiesis by iPSC-derived hematopoietic stem cells of aplastic anemia that escape cytotoxic T-cell attack. Blood Adv. 2018, 2, 390–400. [Google Scholar] [CrossRef]
- Wang, L.; Liu, H. Pathogenesis of aplastic anemia. Hematology 2019, 24, 559–566. [Google Scholar] [CrossRef]
- Bacigalupo, A. How I treat acquired aplastic anemia. Blood 2017, 129, 1428–1436. [Google Scholar] [CrossRef]
- Kumar, R.; Bonfim, C.; George, B. Hematopoietic cell transplantation for aplastic anemia. Curr. Opin. Hematol. 2017, 24, 509–514. [Google Scholar] [CrossRef]
- DeStefano, C.B.; Desai, S.H.; Shenoy, A.G.; Catlett, J.P. Management of post-transplant lymphoproliferative disorders. Br. J. Haematol. 2018, 182, 330–343. [Google Scholar] [CrossRef] [PubMed]
- Nagle, S.J.; Reshef, R.; Tsai, D.E. Posttransplant Lymphoproliferative Disorder in Solid Organ and Hematopoietic Stem Cell Transplantation. Clin. Chest Med. 2017, 38, 771–783. [Google Scholar] [CrossRef] [PubMed]
- Ru, Y.; Chen, J.; Wu, D.-P. Epstein-Barr virus post-transplant lymphoproliferative disease (PTLD) after hematopoietic stem cell transplantation. Eur. J. Haematol. 2018, 101, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Dávila-Collado, R.; Jarquín-Durán, O.; Dong, L.T.; Espinoza, J.L. Epstein–Barr Virus and Helicobacter Pylori Co-Infection in Non-Malignant Gastroduodenal Disorders. Pathogens 2020, 9, 104. [Google Scholar] [CrossRef]
- Shannon-Lowe, C.; Rickinson, A.B. The Global Landscape of EBV-Associated Tumors. Front. Oncol. 2019, 9, 713. [Google Scholar] [CrossRef]
- Al Hamed, R.; Bazarbachi, A.H.; Mohty, M. Epstein-Barr virus-related post-transplant lymphoproliferative disease (EBV-PTLD) in the setting of allogeneic stem cell transplantation: A comprehensive review from pathogenesis to forthcoming treatment modalities. Bone Marrow Transplant. 2019, 55, 25–39. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Thye, L.S.; Habermann, T.M. Current Management Concepts: Primary Central Nervous System Lymphoma, Natural Killer T-Cell Lymphoma Nasal Type, and Post-transplant Lymphoproliferative Disorder. Am. Soc. Clin. Oncol. Educ. Book 2016, 35, e354–e366. [Google Scholar] [CrossRef] [PubMed]
- Grommes, C.; DeAngelis, L.M. Primary CNS Lymphoma. J. Clin. Oncol. 2017, 35, 2410–2418. [Google Scholar] [CrossRef] [PubMed]
- Martinez, O.M. Biomarkers for PTLD diagnosis and therapies. Pediatr. Nephrol. 2019, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Batchelor, T.T. Diagnosis and management of primary central nervous system lymphoma. Cancer 2017, 123, 4314–4324. [Google Scholar] [CrossRef]
- Kimura, H.; Morita, M.; Yabuta, Y.; Kuzushima, K.; Kato, K.; Kojima, S.; Matsuyama, T.; Morishima, T. Quantitative Analysis of Epstein-Barr Virus Load by Using a Real-Time PCR Assay. J. Clin. Microbiol. 1999, 37, 132–136. [Google Scholar] [CrossRef]
- Cannon, D.M.; Mohindra, P.; Gondi, V.; Kruser, T.J.; Kozak, K.R. Choroid plexus tumor epidemiology and outcomes: Implications for surgical and radiotherapeutic management. J. Neuro-Oncol. 2014, 121, 151–157. [Google Scholar] [CrossRef]
- Cecchi, P.C.; Billio, A.; Colombetti, V.; Rizzo, P.; Ricci, U.M.; Schwarz, A. Primary high-grade B-cell lymphoma of the choroid plexus. Clin. Neurol. Neurosurg. 2008, 110, 75–79. [Google Scholar] [CrossRef]
- Kelley, T.W.; Prayson, R.A.; Barnett, G.H.; Stevens, G.H.J.; Cook, J.R.; Hsi, E.D. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue arising in the lateral ventricle. Leuk. Lymphoma 2005, 46, 1423–1427. [Google Scholar] [CrossRef]
- Terasaki, M.; Abe, T.; Tajima, Y.; Fukushima, S.; Hirohata, M.; Shigemori, M. Primary choroid plexus T-cell lymphoma and multiple aneurysms in the CNS. Leuk. Lymphoma 2006, 47, 1680–1682. [Google Scholar] [CrossRef]
- Schwerk, C.; Tenenbaum, T.; Kim, K.S.; Schroten, H. The choroid plexus—A multi-role player during infectious diseases of the CNS. Front. Cell. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- De Castro, F.D.; Reis, F.; Guerra, J.G.G. Intraventricular mass lesions at magnetic resonance imaging: Iconographic essay—Part 1. Radiol. Bras. 2014, 47, 176–181. [Google Scholar] [PubMed]
- Fujimoto, A.; Hiramoto, N.; Yamasaki, S.; Inamoto, Y.; Ogata, M.; Sugio, Y.; Fukuda, T.; Uchida, N.; Ikegame, K.; Matsuoka, K.; et al. Low incidence of posttransplant lymphoproliferative disorder after allogeneic stem cell transplantation in patients with lymphoma treated with rituximab. Hematol. Oncol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Allen, U.D.; Preiksaitis, J.K.; The AST Infectious Diseases Community of Practice. Post-transplant lymphoproliferative disorders, Epstein-Barr virus infection, and disease in solid organ transplantation: Guidelines from the American Society of Transplantation Infectious Diseases Community of Practice. Clin. Transplant. 2019, 33, e13652. [Google Scholar] [PubMed]
- Kinch, A.; Hallböök, H.; Arvidson, J.; Sällström, K.; Bondeson, K.; Pauksens, K. Long-term outcome of Epstein–Barr virus DNAemia and PTLD with the use of preemptive rituximab following allogeneic HSCT. Leuk. Lymphoma 2017, 59, 1172–1179. [Google Scholar] [CrossRef]
- Van Besien, K.; Bachier-Rodriguez, L.; Satlin, M.; Brown, M.A.; Gergis, U.; Guarneri, D.; Hsu, J.; Phillips, A.A.; Mayer, S.A.; Singh, A.D.; et al. Prophylactic rituximab prevents EBV PTLD in haplo-cord transplant recipients at high risk. Leuk. Lymphoma 2019, 60, 1693–1696. [Google Scholar] [CrossRef]
- Naidoo, J.; Panday, H.; Jackson, S.; Grossman, S.A. Optimizing the Delivery of Antineoplastic Therapies to the Central Nervous System. Oncology (Williston Park) 2016, 30, 953–962. [Google Scholar]
- Petereit, H.-F.; Rubbert-Roth, A. Rituximab levels in cerebrospinal fluid of patients with neurological autoimmune disorders. Mult. Scler. J. 2008, 15, 189–192. [Google Scholar] [CrossRef]
- Batchelor, T.T.; Grossman, S.A.; Mikkelsen, T.; Ye, X.; Desideri, S.; Lesser, G.J. Rituximab monotherapy for patients with recurrent primary CNS lymphoma. Neurology 2011, 76, 929–930. [Google Scholar] [CrossRef]
- Ferreri, A.J.M.; Reni, M.; Foppoli, M.; Martelli, M.; A Pangalis, G.; Frezzato, M.; Cabras, M.G.; Fabbri, A.; Corazzelli, G.; Ilariucci, F.; et al. High-dose cytarabine plus high-dose methotrexate versus high-dose methotrexate alone in patients with primary CNS lymphoma: A randomised phase 2 trial. Lancet 2009, 374, 1512–1520. [Google Scholar] [CrossRef]
- Thiel, E.; Korfel, A.; Martus, P.; Kanz, L.; Griesinger, F.; Rauch, M.; Roth, A.; Hertenstein, B.; Von Toll, T.; Hundsberger, T.; et al. High-dose methotrexate with or without whole brain radiotherapy for primary CNS lymphoma (G-PCNSL-SG-1): A phase 3, randomised, non-inferiority trial. Lancet Oncol. 2010, 11, 1036–1047. [Google Scholar] [CrossRef]
- Hoang-Xuan, K.; Bessell, E.; Bromberg, J.; Hottinger, A.F.; Preusser, M.; Rudà, R.; Schlegel, U.; Siegal, T.; Soussain, C.; Abacioglu, U.; et al. Diagnosis and treatment of primary CNS lymphoma in immunocompetent patients: Guidelines from the European Association for Neuro-Oncology. Lancet Oncol. 2015, 16, e322–e332. [Google Scholar] [CrossRef]
- Biller, P.; Michaux, L.; De Pauw, L.; Camboni, A.; Mourad, M.; Kanaan, N. Post-transplant lymphoproliferative disorder after kidney transplantation: Time to adopt monitoring of Epstein-Barr virus? Acta Clin. Belg. 2014, 70, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Oertel, S.; Trappe, R.; Zeidler, K.; Babel, N.; Reinke, P.; Hummel, M.; Jonas, S.; Papp-Vary, M.; Subklewe, M.; Dörken, B.; et al. Epstein–Barr viral load in whole blood of adults with posttransplant lymphoproliferative disorder after solid organ transplantation does not correlate with clinical course. Ann. Hematol. 2006, 85, 478–484. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, H.; Rai, S.; Tanaka, H.; Espinoza, J.L.; Komori-Inoue, M.; Kakutani, H.; Minamoto, S.; Kumode, T.; Nakayama, S.; Taniguchi, Y.; et al. Epstein–Barr Virus-Induced Post-Transplant Lymphoproliferative Disorder of the Central Nervous System Successfully Treated with Chemo-Immunotherapy. Viruses 2020, 12, 416. https://doi.org/10.3390/v12040416
Inoue H, Rai S, Tanaka H, Espinoza JL, Komori-Inoue M, Kakutani H, Minamoto S, Kumode T, Nakayama S, Taniguchi Y, et al. Epstein–Barr Virus-Induced Post-Transplant Lymphoproliferative Disorder of the Central Nervous System Successfully Treated with Chemo-Immunotherapy. Viruses. 2020; 12(4):416. https://doi.org/10.3390/v12040416
Chicago/Turabian StyleInoue, Hiroaki, Shinya Rai, Hirokazu Tanaka, J. Luis Espinoza, Maiko Komori-Inoue, Hiroaki Kakutani, Shuji Minamoto, Takahiro Kumode, Shoko Nakayama, Yasuhiro Taniguchi, and et al. 2020. "Epstein–Barr Virus-Induced Post-Transplant Lymphoproliferative Disorder of the Central Nervous System Successfully Treated with Chemo-Immunotherapy" Viruses 12, no. 4: 416. https://doi.org/10.3390/v12040416
APA StyleInoue, H., Rai, S., Tanaka, H., Espinoza, J. L., Komori-Inoue, M., Kakutani, H., Minamoto, S., Kumode, T., Nakayama, S., Taniguchi, Y., Morita, Y., Okuda, T., Tatsumi, Y., Ashida, T., & Matsumura, I. (2020). Epstein–Barr Virus-Induced Post-Transplant Lymphoproliferative Disorder of the Central Nervous System Successfully Treated with Chemo-Immunotherapy. Viruses, 12(4), 416. https://doi.org/10.3390/v12040416





