pUC18-CpG Is an Effective Adjuvant for a Duck Tembusu Virus Inactivated Vaccine
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus Preparation
2.2. pUC18-CpG
2.3. Animals
2.4. Vaccine Preparation
2.5. Quantification of Serum Antibody Titers via HI Assays
2.6. Determination of Serum Cytokine Levels
2.7. Virus Challenge
2.8. Virus Isolation and RT-PCR
2.9. Statistical Analysis
3. Results
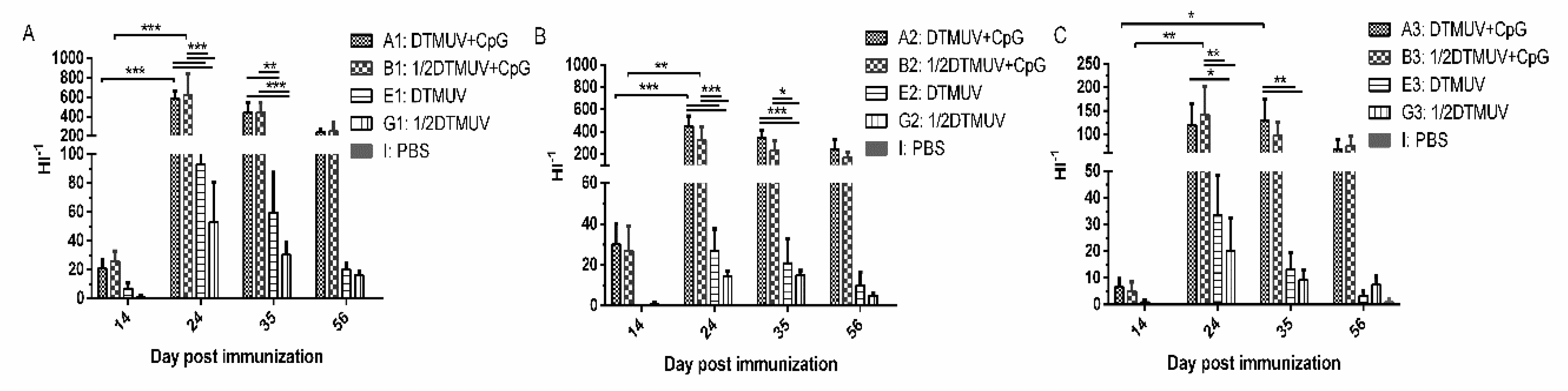
3.1. pUC18-CpG Enhanced Serum Antibody Responses
3.2. pUC18-CpG Enhanced both Th1- and Th2-Type Cytokine Production
3.3. pUC18-CpG Enhanced Protection Efficacy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Liu, P.; Lu, H.; Li, S.; Moureau, G.; Deng, Y.-Q.; Wang, Y.; Zhang, L.; Jiang, T.; De Lamballerie, X.; Qin, C.-F.; et al. Genomic and antigenic characterization of the newly emerging Chinese duck egg-drop syndrome flavivirus: Genomic comparison with Tembusu and Sitiawan viruses. J. Gen. Virol. 2012, 93, 2158–2170. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Li, S.; Hu, X.; Yu, X.; Wang, Y.; Liu, P.; Lu, X.; Zhang, G.; Hu, X.; Liu, D.; et al. Duck egg-drop syndrome caused by BYD virus, a new Tembusu-related flavivirus. PLoS ONE 2011, 6, e18106. [Google Scholar] [CrossRef] [PubMed]
- Ninvilai, P.; Nonthabenjawan, N.; Limcharoen, B.; Tunterak, W.; Oraveerakul, K.; Banlunara, W.; Amonsin, A.; Thontiravong, A. The presence of duck Tembusu virus in Thailand since 2007: A retrospective study. Transbound. Emerg. Dis. 2018, 65, 1208–1216. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Yu, X.; Yang, G.; Tang, Y.; Diao, Y. A Novel Diagnostic Method to Detect Duck Tembusu Virus: A Colloidal Gold-Based Immunochromatographic Assay. Front. Microbiol. 2018, 9, 1001. [Google Scholar] [CrossRef] [PubMed]
- Petz, L.N.; Turell, M.J.; Padilla, S.; Long, L.S.; Reinbold-Wasson, D.D.; Smith, D.R.; O’Guinn, M.L.; Melanson, V.R.; Lee, J.S. Development of conventional and real-time reverse transcription polymerase chain reaction assays to detect Tembusu virus in Culex tarsalis mosquitoes. Am. J. Trop. Med. Hyg. 2014, 91, 666–671. [Google Scholar] [CrossRef] [PubMed]
- Kardos, G.; Nagy, J.; Antal, M.; Bistyak, A.; Tenk, M.; Kiss, I. Development of a novel PCR assay specific for Riemerella anatipestifer. Lett. Appl. Microbiol. 2007, 44, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Cui, M.; Jia, R.; Huang, J.; Wu, X.; Hu, Z.; Zhang, X.; Wang, M.; Zhu, D.; Chen, S.; Liu, M.; et al. Analysis of the microRNA expression profiles in DEF cells infected with duck Tembusu virus. Infect. Genet. Evol. 2018, 63, 126–134. [Google Scholar] [CrossRef]
- Chen, S.; Wang, S.; Li, Z.; Lin, F.; Cheng, X.; Zhu, X.; Wang, J.; Chen, S.; Huang, M.; Zheng, M. Isolation and characterization of a Chinese strain of Tembusu virus from Hy-Line Brown layers with acute egg-drop syndrome in Fujian, China. Arch. Virol. 2014, 159, 1099–1107. [Google Scholar] [CrossRef]
- Liu, M.; Chen, S.; Chen, Y.; Liu, C.; Chen, S.; Yin, X.; Li, G.; Zhang, Y. Adapted Tembusu-like virus in chickens and geese in China. J. Clin. Microbiol. 2012, 50, 2807–2809. [Google Scholar] [CrossRef]
- Ti, J.; Zhang, L.; Li, Z.; Zhao, D.; Zhang, Y.; Li, F.; Diao, Y. Effect of age and inoculation route on the infection of duck Tembusu virus in Goslings. Vet. Microbiol. 2015, 181, 190–197. [Google Scholar] [CrossRef]
- He, Y.; Wang, A.; Chen, S.; Wu, Z.; Zhang, J.; Wang, M.; Jia, R.; Zhu, D.; Liu, M.; Yang, Q.; et al. Differential immune-related gene expression in the spleens of duck Tembusu virus-infected goslings. Vet. Microbiol. 2017, 212, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Ti, J.; Zhang, M.; Li, Z.; Li, X.; Diao, Y. Duck Tembusu Virus Exhibits Pathogenicity to Kunming Mice by Intracerebral Inoculation. Front. Microbiol. 2016, 7, 190. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Diao, Y.; Yu, C.; Gao, X.; Ju, X.; Xue, C.; Liu, X.; Ge, P.; Qu, J.; Zhang, D. Characterization of a Tembusu virus isolated from naturally infected house sparrows (Passer domesticus) in Northern China. Transbound. Emerg. Dis. 2013, 60, 152–158. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Gao, X.; Diao, Y.; Feng, Q.; Chen, H.; Liu, X.; Ge, P.; Yu, C. Tembusu virus in human, China. Transbound. Emerg. Dis. 2013, 60, 193–196. [Google Scholar] [CrossRef]
- Li, G.; Gao, X.; Xiao, Y.; Liu, S.; Peng, S.; Li, X.; Shi, Y.; Zhang, Y.; Yu, L.; Wu, X.; et al. Development of a live attenuated vaccine candidate against duck Tembusu viral disease. Virology 2014, 450, 233–242. [Google Scholar] [CrossRef]
- Tang, J.; Bi, Z.; Ding, M.; Yin, D.; Zhu, J.; Zhang, L.; Miao, Q.; Zhu, Y.; Wang, G.; Liu, G. Immunization with a suicidal DNA vaccine expressing the E glycoprotein protects ducklings against duck Tembusu virus. Virol. J. 2018, 15, 140. [Google Scholar] [CrossRef]
- Lin, J.; Liu, Y.; Wang, X.; Yang, B.; He, P.; Yang, Z.; Duan, H.; Xie, J.; Zou, L.; Zhao, J.; et al. Efficacy Evaluation of an Inactivated Duck Tembusu Virus Vaccine. Avian Dis. 2015, 59, 244–248. [Google Scholar] [CrossRef]
- Krieg, A.M.; Yi, A.K.; Matson, S.; Waldschmidt, T.J.; Bishop, G.A.; Teasdale, R.; Koretzky, G.A.; Klinman, D.M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature 1995, 374, 546–549. [Google Scholar] [CrossRef]
- Hartmann, G.; Krieg, A.M. Mechanism and function of a newly identified CpG DNA motif in human primary B cells. J. Immunol. 2000, 164, 944–953. [Google Scholar] [CrossRef]
- Prater, M.R.; Johnson, V.J.; Germolec, D.R.; Luster, M.I.; Holladay, S.D. Maternal treatment with a high dose of CpG ODN during gestation alters fetal craniofacial and distal limb development in C57BL/6 mice. Vaccine 2006, 24, 263–271. [Google Scholar] [CrossRef]
- Bode, C.; Zhao, G.; Steinhagen, F.; Kinjo, T.; Klinman, D.M. CpG DNA as a vaccine adjuvant. Expert Rev. Vaccines 2011, 10, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.Y.; Jia, H.; Zhang, Q.; Yuan, W.F.; Gang, Z.; Xin, T.; Zhu, H.F.; Sánchez-Vizcaíno, J.M. CpG-enriched plasmid enhances the efficacy of the traditional foot-and-mouth disease killed vaccine. Microbiol. Immunol. 2012, 56, 332–337. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.C.; Zhen, Y.; Su, Q.; Zhu, H.F.; Guo, X.Y.; Zhao, P. Efficacy of CpG-ODN and Freund’s immune adjuvants on antibody responses induced by chicken infectious anemia virus vp1, vp2, and vp3 subunit proteins. Poult. Sci. 2019, 98, 1121–1126. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Lin, Y.M.; Yen, T.Y.; Yang, W.J.; Chu, C.Y. CpG oligodeoxynucleotides containing GACGTT motifs enhance the immune responses elicited by a goose parvovirus vaccine in ducks. Vaccine 2010, 28, 7956–7962. [Google Scholar] [CrossRef]
- Kang, H.; Wang, H.; Yu, Q.; Yang, Q. A novel combined adjuvant strongly enhances mucosal and systemic immunity to low pathogenic avian influenza after oral immunization in ducks. Poult. Sci. 2013, 92, 1543–1551. [Google Scholar] [CrossRef]
- Zhang, Q.; Yu, K.; Yuan, W.; Xue, F.; Sun, H.; Zhu, H.F. Purification of large-scale plasmid DNAs by selective precipitation with cetyltrimethylammonium bromide. Sheng Wu Gong Cheng Xue Bao 2008, 24, 2117–2121. [Google Scholar]
- Nauta, J.J.P. Eliminating bias in the estimation of the geometric mean of HI titres. Biologicals 2006, 34, 183–186. [Google Scholar] [CrossRef]
- Committee for Proprietary Medicinal Products. Note for Guidance on Harmonisation of Requirements for Influenza Vaccines. Available online: http://www.emea.eu.int/pdfs/human/bwp/021496en.pdf (accessed on 4 October 2006).
- Malenovska, H. Virus quantitation by transmission electron microscopy, TCID50, and the role of timing virus harvesting: A case study of three animal viruses. J. Virol. Methods 2013, 191, 136–140. [Google Scholar] [CrossRef]
- Fulop, L.; Barrett, A.D.; Phillpotts, R.; Martin, K.; Leslie, D.; Titball, R.W. Rapid identification of flaviviruses based on conserved NS5 gene sequences. J. Virol. Methods 1993, 44, 179–188. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, Y.; Zhang, X.; Xu, D.; Dai, X.; Teng, Q.; Yan, L.; Zhou, J.; Ji, X.; Zhang, S.; et al. An infectious disease of ducks caused by a newly emerged Tembusu virus strain in mainland China. Virology 2011, 417, 1–8. [Google Scholar] [CrossRef]
- Tang, Y.; Diao, Y.; Chen, H.; Ou, Q.; Liu, X.; Gao, X.; Yu, C.; Wang, L. Isolation and genetic characterization of a tembusu virus strain isolated from mosquitoes in Shandong, China. Transbound. Emerg. Dis. 2015, 62, 209–216. [Google Scholar] [CrossRef]
- Klinman, D.M.; Klaschik, S.; Sato, T.; Tross, D. CpG oligonucleotides as adjuvants for vaccines targeting infectious diseases. Adv. Drug Deliv. Rev. 2009, 61, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.K.; Weiner, G.J. CpG oligonucleotides as immunotherapeutic adjuvants: Innovative applications and delivery strategies. Adv. Drug Deliv. Rev. 2009, 61, 193–194. [Google Scholar] [CrossRef] [PubMed]
- Peubez, I.; Chaudet, N.; Mignon, C.; Hild, G.; Husson, S.; Courtois, V.; Luca, K.D.; Speck, D.; Sodoyer, R. Antibiotic-free selection in E. coli: New considerations for optimal design and improved production. Microb. Cell Fact. 2010, 9, 65. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Hui, D.; Lu, H.Q.; Gu, X.X.; Tian, J.J. Development of an antibiotic-free plasmid selection system based on thymine auxotrophy in Lactococcus lactis. Ann. Microbio. 2015, 65, 1049–1055. [Google Scholar]
- Luke, J.; Carnes, A.E.; Hodgson, C.P.; Williams, J.A. Improved antibiotic-free DNA vaccine vectors utilizing a novel RNA based plasmid selection system. Vaccine 2009, 27, 6459. [Google Scholar] [CrossRef]
- Johnson, A. Antibiotic Resistance, Methods and Protocols; Humana Press: Totowa, NJ, USA, 2001. [Google Scholar]
- Prather, K.J.; Sagar, S.; Murphy, J.; Chartrain, M. Industrial scale production of plasmid DNA for vaccine and gene therapy: Plasmid design, production, and purification. Enzyme Microb. Technol. 2003, 33, 865–883. [Google Scholar] [CrossRef]
- Sato, Y.; Roman, M.; Tighe, H.; Lee, D.; Corr, M.; Nguyen, M.-D.; Silverman, G.J.; Lotz, M.; Carson, D.A.; Raz, E. Immunostimulatory DNA Sequences Necessary for Effective Intradermal Gene Immunization. Science 1996, 273, 352–354. [Google Scholar] [CrossRef]
- Guy, B.; Nougarede, N.; Begue, S.; Sanchez, V.; Souag, N.; Carre, M.; Chambonneau, L.; Morrisson, D.N.; Shaw, D.; Qiao, M. Cell-mediated immunity induced by chimeric tetravalent dengue vaccine in naive or flavivirus-primed subjects. Vaccine 2008, 26, 5721. [Google Scholar] [CrossRef]
- Lobigs, M.; Blanden, R.V.; Müllbacher, A. Flavivirus-Induced Up-regulation of MHC Class I Antigens; Implications for the Induction of CD8+ T-Cell-Mediated Autoimmunity. Immunol. Rev. 1996, 152, 5–19. [Google Scholar] [CrossRef]
- Croft, M.; Swain, S.L. B cell response to fresh and effector T helper cells: Role of cognate T-B interaction and the cytokines IL-2, IL-4, and IL-6. J. Immunol. 1991, 146, 4055–4064. [Google Scholar] [PubMed]
- Nakano, N.; Kikutani, H.; Kishimoto, T. Differential Effects of IL-2 and IL-6 on the Development of Three Distinct Precursor T-Cell Populations in the Thymus. Dev. Immunol. 2007, 1, 77. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buggins, A.G.S.; Patten, P.E.M.; Richards, J.; Orr, S.J.; Mufti, G.J.; Thomas, N.S.B.; Devereux, S. IL-6 production by B-CLL cells plays a critical role in the inhibition of T cell activation and proliferation and promotes a Th2 response in normal T lymphocytes. Blood 2006, 108, 2808. [Google Scholar] [CrossRef]


| Groups | Contents | Inactivated Viral Fluid Volume (mL) | pUC18-CpG (mg) | Aqueous Phase Volume (mL) | Oil Phase Volume (mL) | Vaccine Volume (mL) | |
|---|---|---|---|---|---|---|---|
| A | A1 | DTUMV + CpG | 10 | 3.2 | 10 | 30 | 40 |
| A2 | 10 | 6.4 | 10 | 30 | 40 | ||
| A3 | 10 | 12.8 | 10 | 30 | 40 | ||
| B | B1 | 1/2DTUMV + CpG | 5 | 3.2 | 10 | 30 | 40 |
| B2 | 5 | 6.4 | 10 | 30 | 40 | ||
| B3 | 5 | 12.8 | 10 | 30 | 40 | ||
| E | E1 | DTUMV | 10 | 0 | 10 | 30 | 40 |
| E2 | 10 | 0 | 10 | 30 | 40 | ||
| E3 | 10 | 0 | 10 | 30 | 40 | ||
| G | G1 | 1/2DTUMV | 5 | 0 | 10 | 30 | 40 |
| G2 | 5 | 0 | 10 | 30 | 40 | ||
| G3 | 5 | 0 | 10 | 30 | 40 | ||
| I | I | PBS | / | / | / | / | 40 |
| Groups | Contents | Inoculum Dose (mL) | Number of Ducks | Route of Immunizations | Times of Immunization | Purpose of the Experiment | ||
|---|---|---|---|---|---|---|---|---|
| A | A1 | DTUMV + CpG | 0.5 | Full | 11 | IM | 2 | Immune responses to vaccine formulation X |
| A2 | 0.25 | 1/2 | 6 | IM | 2 | |||
| A3 | 0.125 | 1/4 | 6 | IM | 2 | |||
| B | B1 | 1/2DTUMV + CpG | 0.5 | Full | 11 | IM | 2 | |
| B2 | 0.25 | 1/2 | 6 | IM | 2 | |||
| B3 | 0.125 | 1/4 | 6 | IM | 2 | |||
| E | E1 | DTUMV | 0.5 | Full | 11 | IM | 2 | |
| E2 | 0.25 | 1/2 | 6 | IM | 2 | |||
| E3 | 0.125 | 1/4 | 6 | IM | 2 | |||
| G | G1 | 1/2DTUMV | 0.5 | Full | 11 | IM | 2 | |
| G2 | 0.25 | 1/2 | 6 | IM | 2 | |||
| G3 | 0.125 | 1/4 | 6 | IM | 2 | |||
| I | I | PBS | / | 12 | IM | 2 | ||
| Groups | Dose | Protected/All | Protective Rate | PD50/0.5 mL | |
|---|---|---|---|---|---|
| A | A1 | Full | 6/6 | 100% | 4.0 |
| A2 | 1/2 | 6/6 | 100% | ||
| A3 | 1/4 | 3/6 | 50% | ||
| B | B1 | Full | 6/6 | 100% | 4.5 |
| B2 | 1/2 | 6/6 | 100% | ||
| B3 | 1/4 | 4/6 | 66.67% | ||
| E | E1 | Full | 4/6 | 66.67% | 1.4 |
| E2 | 1/2 | 1/6 | 16.67% | ||
| E3 | 1/4 | 1/6 | 16.67% | ||
| G | G1 | Full | 4/6 | 66.67% | 1.8 |
| G2 | 1/2 | 2/6 | 33.33% | ||
| G3 | 1/4 | 2/6 | 33.33% | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, X.; Wang, X.; Zhang, S.; Gao, X.; Fang, L.; Wang, X.; Lin, W.; Jia, H.; Guo, X.; Xin, T.; et al. pUC18-CpG Is an Effective Adjuvant for a Duck Tembusu Virus Inactivated Vaccine. Viruses 2020, 12, 238. https://doi.org/10.3390/v12020238
Ren X, Wang X, Zhang S, Gao X, Fang L, Wang X, Lin W, Jia H, Guo X, Xin T, et al. pUC18-CpG Is an Effective Adjuvant for a Duck Tembusu Virus Inactivated Vaccine. Viruses. 2020; 12(2):238. https://doi.org/10.3390/v12020238
Chicago/Turabian StyleRen, Xiao, Xiaolei Wang, Shan Zhang, Xintao Gao, Lichun Fang, Xixi Wang, Weidong Lin, Hong Jia, Xiaoyu Guo, Ting Xin, and et al. 2020. "pUC18-CpG Is an Effective Adjuvant for a Duck Tembusu Virus Inactivated Vaccine" Viruses 12, no. 2: 238. https://doi.org/10.3390/v12020238
APA StyleRen, X., Wang, X., Zhang, S., Gao, X., Fang, L., Wang, X., Lin, W., Jia, H., Guo, X., Xin, T., Zhu, H., Lin, J., & Hou, S. (2020). pUC18-CpG Is an Effective Adjuvant for a Duck Tembusu Virus Inactivated Vaccine. Viruses, 12(2), 238. https://doi.org/10.3390/v12020238





