Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. Source and Isolation of Phages
2.3. Transmission Electron Microscopy
2.4. One-Step Growth Curve and Host Range Analysis
2.5. Stability Studies
2.6. Phage DNA Extraction and Genome Sequencing
2.7. Whole-Genome Bioinformatic Analysis
2.8. Expression, Purification, and Preparation of FITC-Labelled TFPs
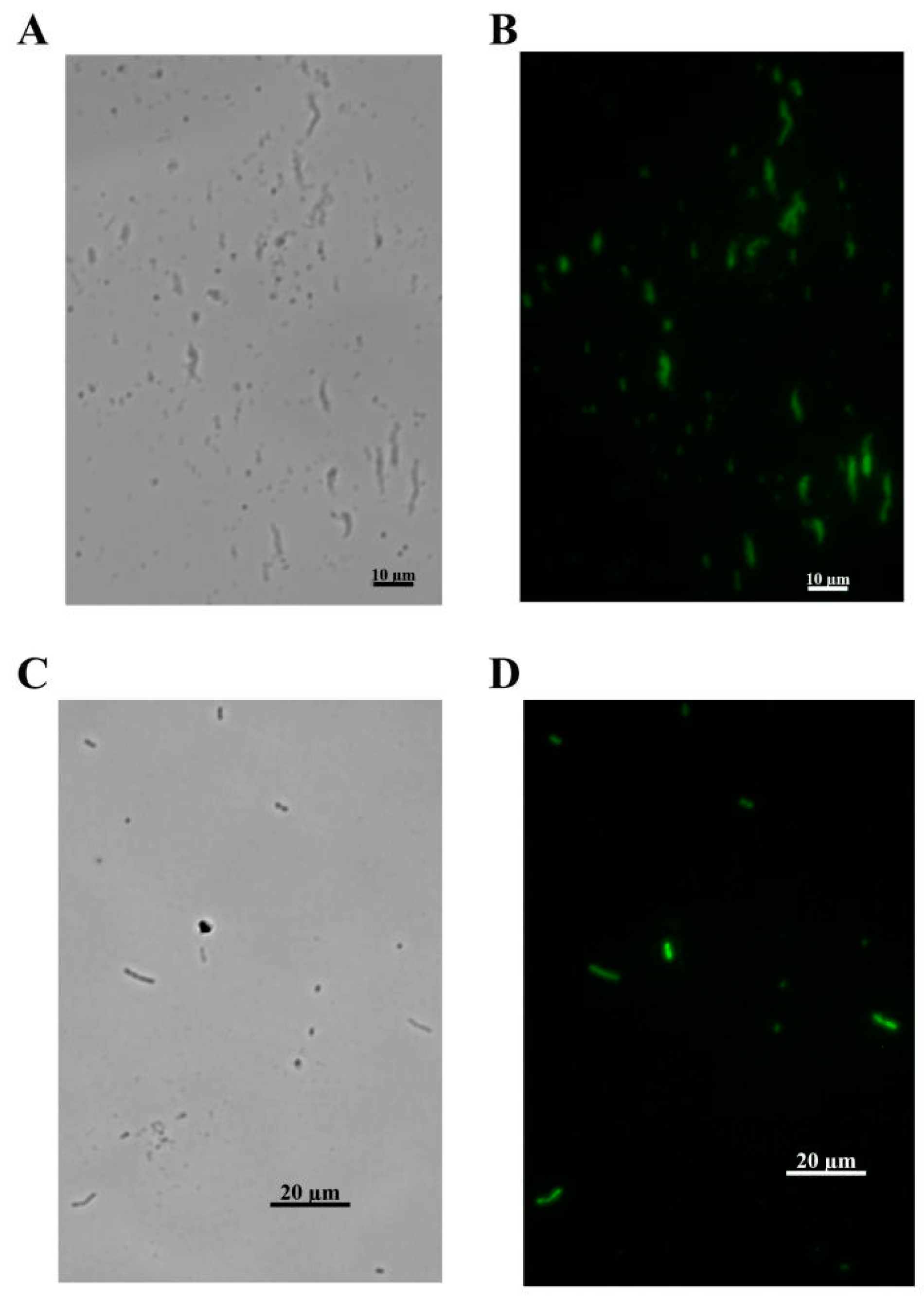
2.9. Immunofluorescence Imaging of FITC-Stained A. baumannii
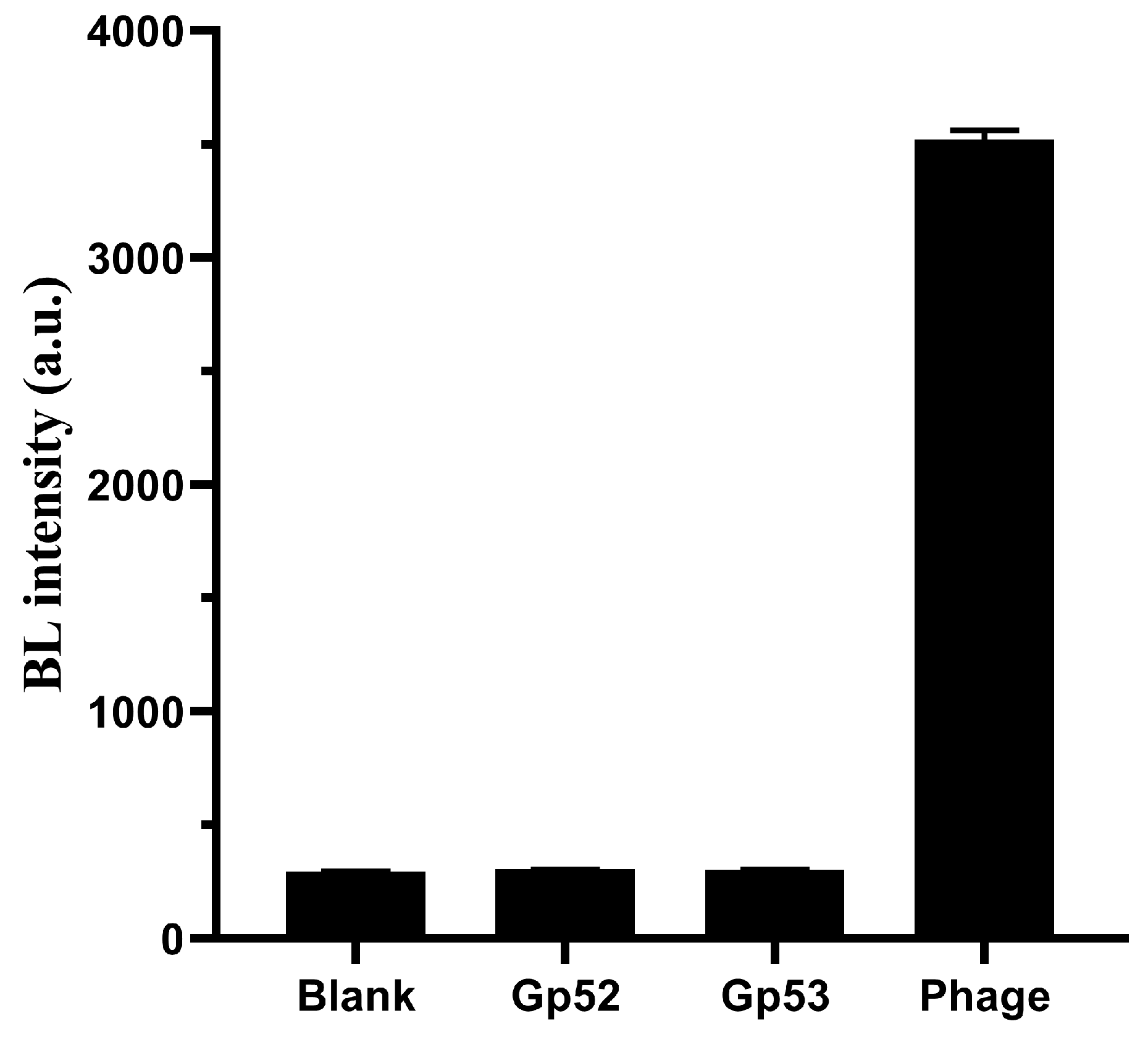
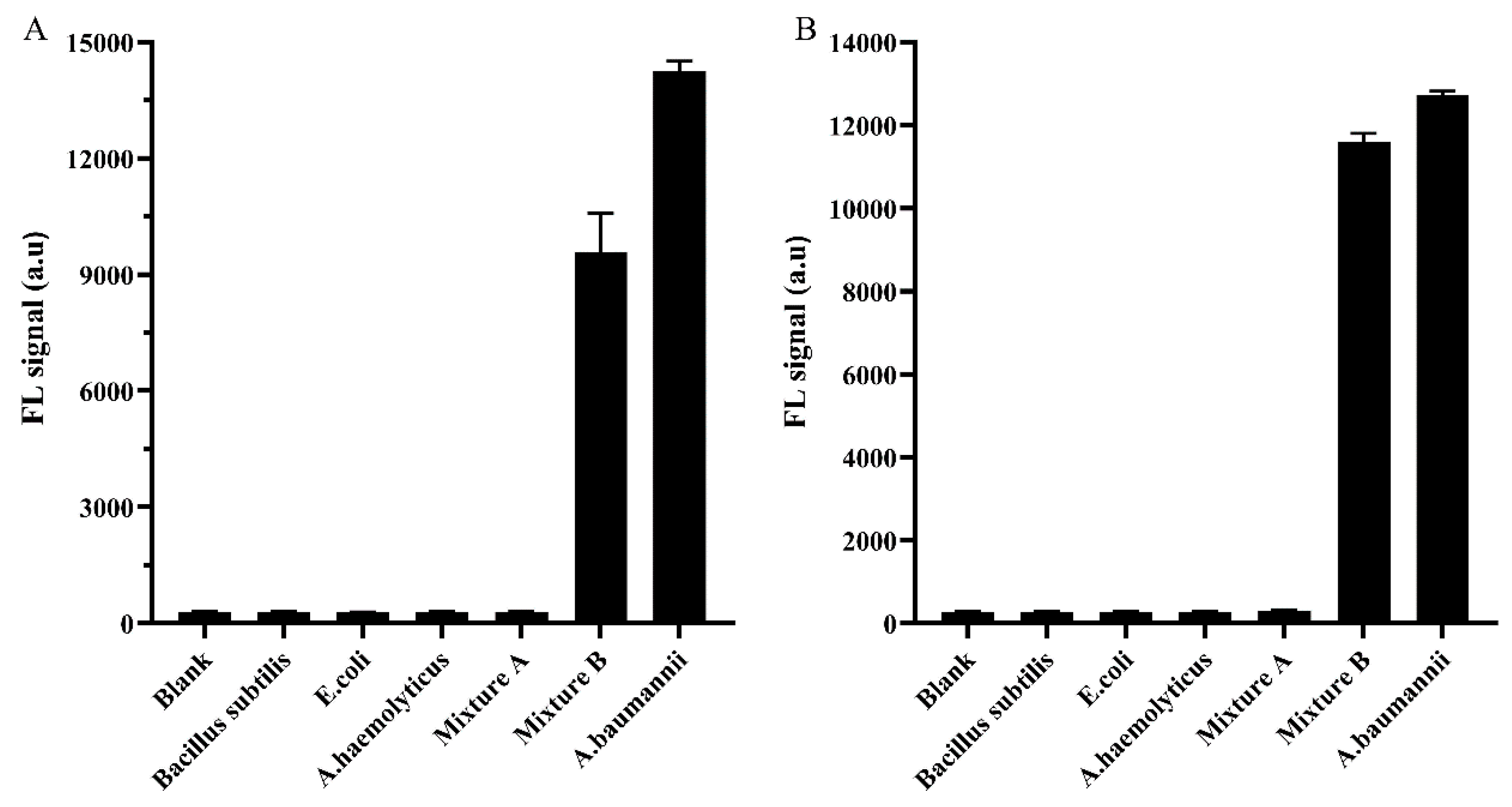
2.10. Bioluminescent and Fluorescent Methods for Functional Identification Using TFPs
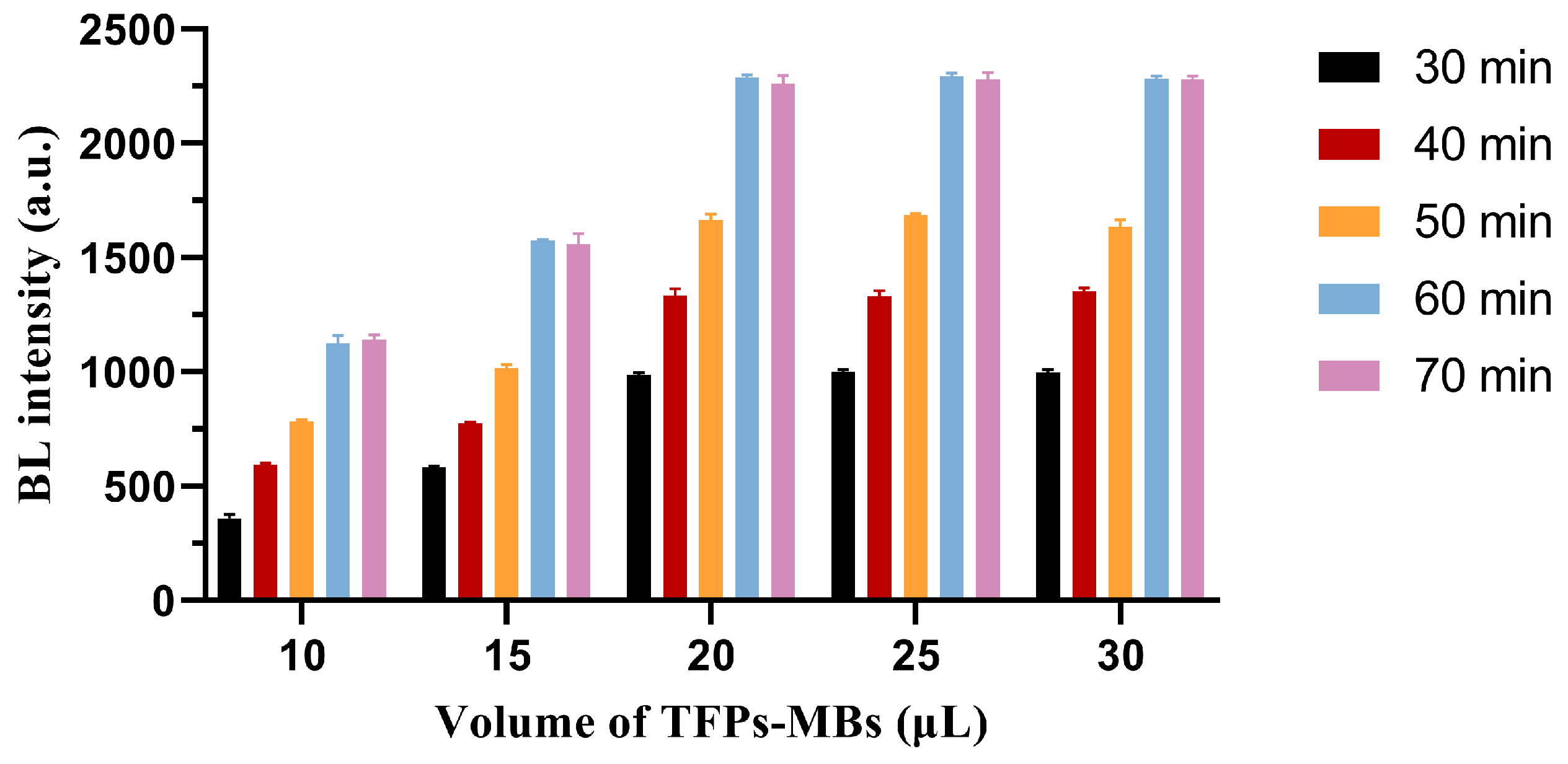
2.11. TFPs-MBs Preparation and Use in A. baumannii Detection
2.12. Nucleotide Sequence Accession Number
3. Results
3.1. Isolation and Morphology of A. baumannii Phages
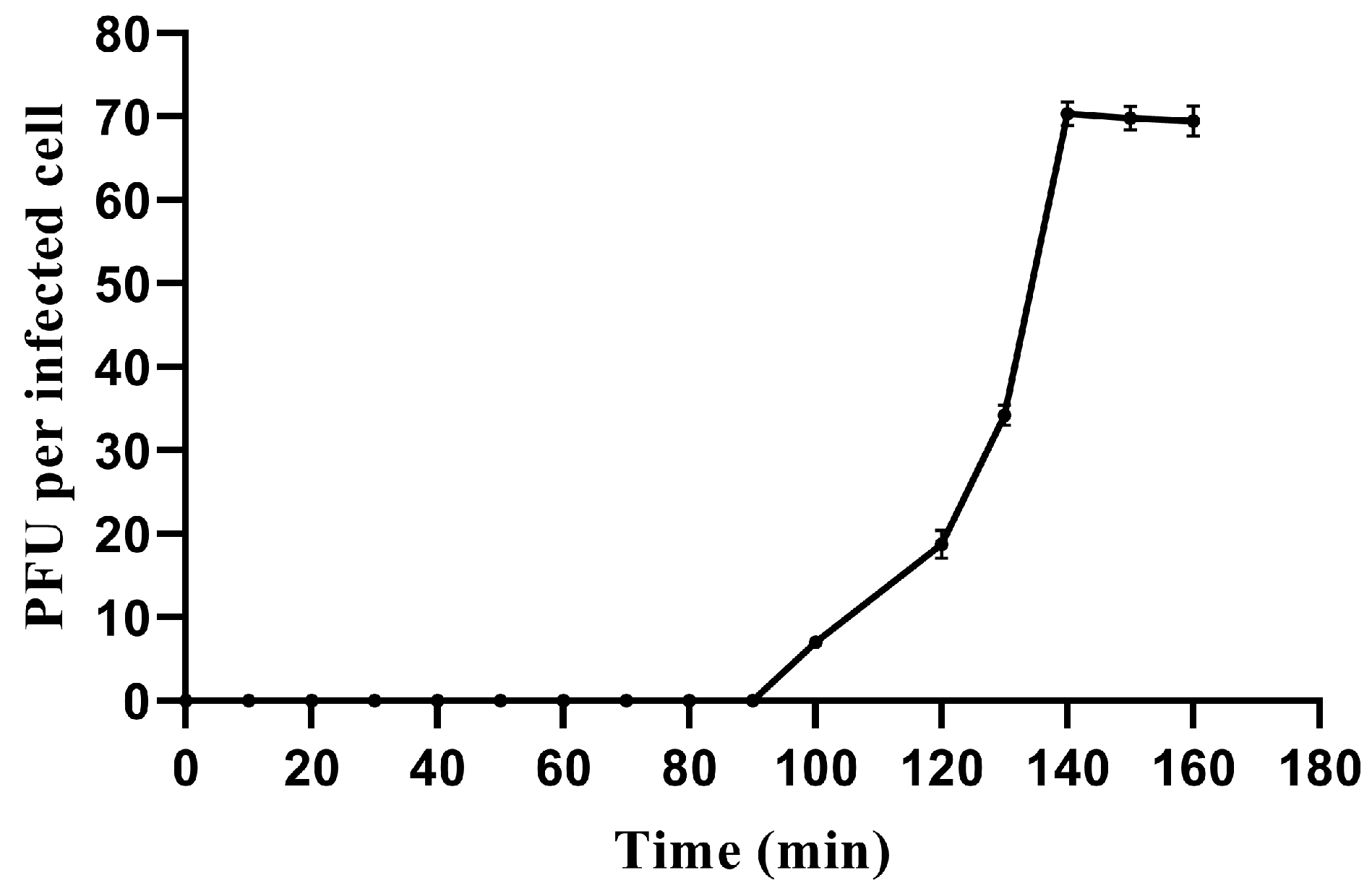
3.2. One-Step Growth Curve and Host range
3.3. Phage Stability Tests
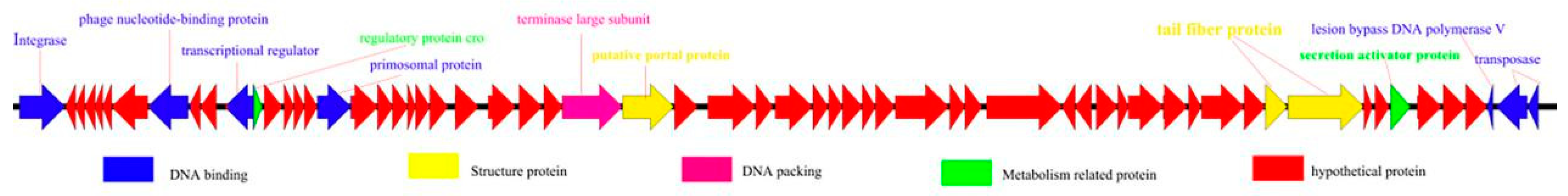
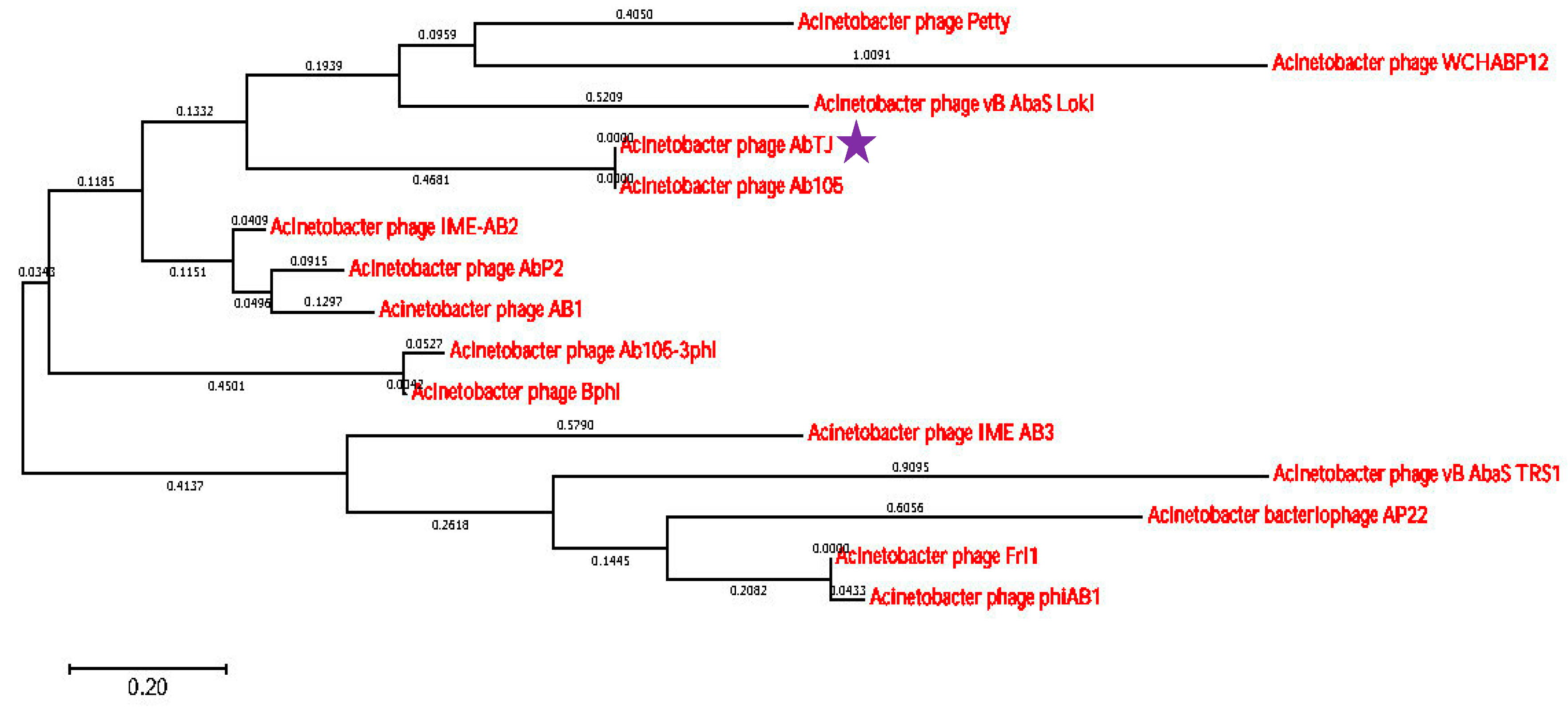
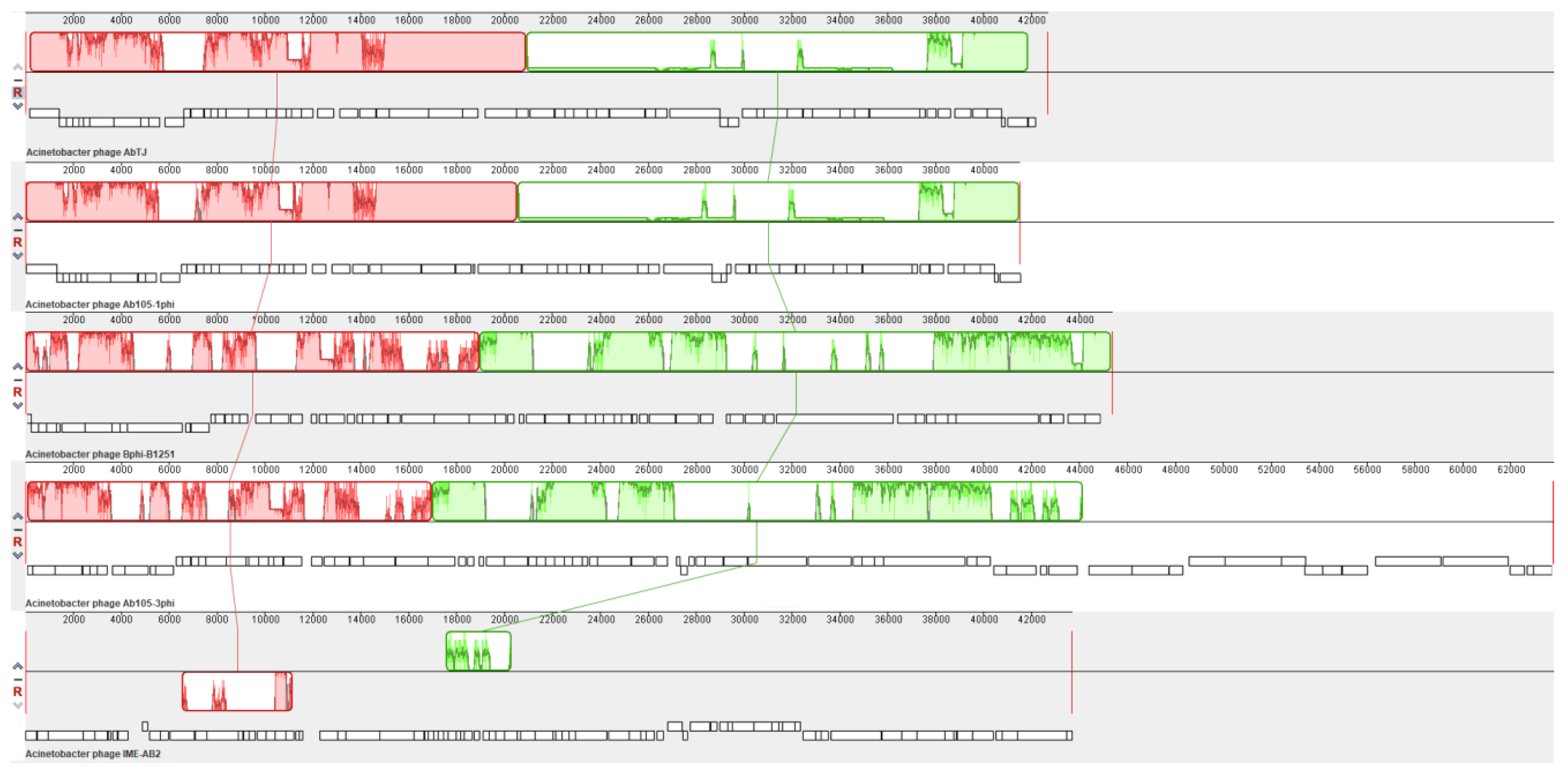
3.4. Whole-Genome Analysis of Acinetobacter Phage AbTJ
3.5. Identification the Function of the Phage Tail Fiber Protein (TFP)
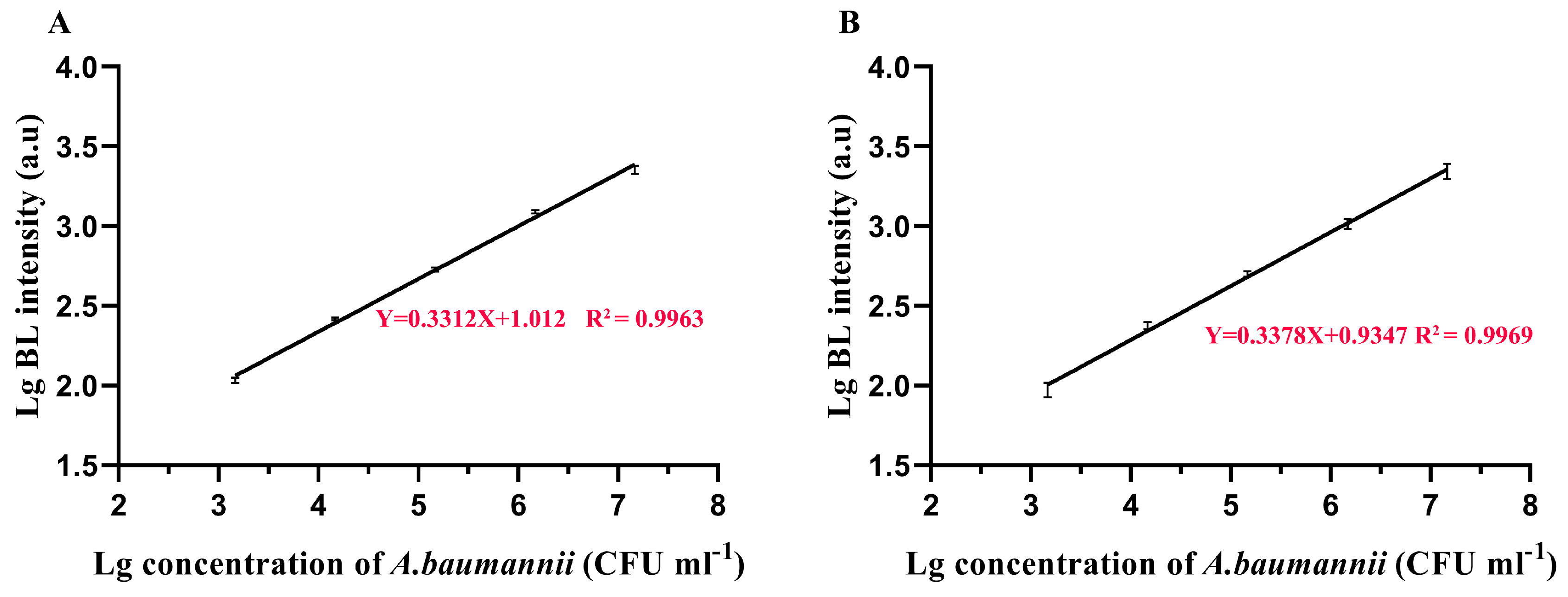
Baumannii Detection Using TFPs and TFPs Use in Diverse Samples
4. Discussion and Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dijkshoorn, L.; Nemec, A.; Seifert, H. An increasing threat in hospitals: Multidrug-resistant Acinetobacter baumannii. Nat. Rev. Microbiol. 2007, 5, 939–951. [Google Scholar] [CrossRef] [PubMed]
- Dubrovin, E.V.; Popova, A.V.; Kraevskiy, S.V.; Ignatov, S.G.; Ignatyuk, T.E.; Yaminsky, I.V.; Volozhantsev, N.V. Atomic force microscopy analysis of the Acinetobacter baumannii bacteriophage AP22 lytic cycle. PLoS ONE 2012, 7, e47348. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, A.C.; Thompson, M.G.; Gebhardt, M.; Corey, B.W.; Yildirim, S.; Shuman, H.A.; Zurawski, D.V. Genetic Manipulation of Acinetobacter baumannii. Curr. Protoc. Microbiol. 2014, 35. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Shi, Y.; Liu, M.; Wang, Y.; Wang, L.; Lu, S.; Fu, Z. Nonlytic Recombinant Phage Tail Fiber Protein for Specific Recognition of Pseudomonas aeruginosa. Anal. Chem. 2018, 90, 14462–14468. [Google Scholar] [CrossRef]
- Kazaks, A.; Dislers, A.; Lipowsky, G.; Nikolajeva, V.; Tars, K. Complete genome sequence of the Enterobacter cancerogenus bacteriophage Enc34. J. Virol. 2012, 86, 11403–11404. [Google Scholar] [CrossRef]
- Cui, Z.; Shen, W.; Wang, Z.; Zhang, H.; Me, R.; Wang, Y.; Zeng, L.; Zhu, Y.; Qin, J.; He, P.; et al. Complete genome sequence of Klebsiella pneumoniae phage JD001. J. Virol. 2012, 86, 13843. [Google Scholar] [CrossRef]
- Krasowska, A.; Biegalska, A.; Augustyniak, D.; Los, M.; Richert, M.; Lukaszewicz, M. Isolation and Characterization of Phages Infecting Bacillus subtilis. Biomed. Res. Int. 2015, 2015, 179597. [Google Scholar] [CrossRef]
- Wiggins, B.A.; Alexander, M. Minimum bacterial density for bacteriophage replication: Implications for significance of bacteriophages in natural ecosystems. Appl. Environ. Microbiol. 1985, 49, 19–23. [Google Scholar] [CrossRef]
- Prevelige, P.E., Jr.; Cortines, J.R. Phage assembly and the special role of the portal protein. Curr. Opin. Virol. 2018, 31, 66–73. [Google Scholar] [CrossRef]
- Nobrega, F.L.; Vlot, M.; de Jonge, P.A.; Dreesens, L.L.; Beaumont, H.J.E.; Lavigne, R.; Dutilh, B.E.; Brouns, S.J.J. Targeting mechanisms of tailed bacteriophages. Nat. Rev. Microbiol. 2018, 16, 760–773. [Google Scholar] [CrossRef]
- Wu, L.; Huang, T.; Yang, L.; Pan, J.; Zhu, S.; Yan, X. Sensitive and selective bacterial detection using tetracysteine-tagged phages in conjunction with biarsenical dye. Angew. Chem. Int. Ed. Engl. 2011, 50, 5873–5877. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Alcaine, S.D.; Jackson, A.A.; Rotello, V.M.; Nugen, S.R. Development of Engineered Bacteriophages for Escherichia coli Detection and High-Throughput Antibiotic Resistance Determination. ACS Sens. 2017, 2, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Yang, Z.L.; Wu, X.M.; Wang, Y.; Liu, Y.J.; Luo, H.; Lv, X.; Gan, Y.R.; Song, S.D.; Gao, F. Complete genome sequence of Acinetobacter baumannii MDR-TJ and insights into its mechanism of antibiotic resistance. J. Antimicrob. Chemother. 2012, 67, 2825–2832. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gao, F.; Wang, Y.; Liu, Y.J.; Wu, X.M.; Lv, X.; Gan, Y.R.; Song, S.D.; Huang, H. Genome sequence of Acinetobacter baumannii MDR-TJ. J. Bacteriol. 2011, 193, 2365–2366. [Google Scholar] [CrossRef]
- Huang, H.; Dong, Y.; Yang, Z.L.; Luo, H.; Zhang, X.; Gao, F. Complete Sequence of pABTJ2, A Plasmid from Acinetobacter baumannii MDR-TJ, Carrying Many Phage-like Elements. Genom. Proteom. Bioinform. 2014, 12, 172–177. [Google Scholar] [CrossRef]
- Luo, J.; Jiang, M.; Xiong, J.; Li, J.; Zhang, X.; Wei, H.; Yu, J. Exploring a phage-based real-time PCR assay for diagnosing Acinetobacter baumannii bloodstream infections with high sensitivity. Anal. Chim. Acta 2018, 1044, 147–153. [Google Scholar] [CrossRef]
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2017, 8, 2659. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.; Mottola, C.; Barbosa, R.; Silva, F.A.; Oliveira, M.; Vilela, C.L.; Melo-Cristino, J.; Gorski, A.; Pimentel, M.; et al. In vitro design of a novel lytic bacteriophage cocktail with therapeutic potential against organisms causing diabetic foot infections. J. Med. Microbiol. 2014, 63, 1055–1065. [Google Scholar] [CrossRef]
- Li, Y.; Wang, M.; Liu, Q.; Song, X.; Wang, D.; Ma, Y.; Shao, H.; Jiang, Y. Complete Genomic Sequence of Bacteriophage H188: A Novel Vibrio kanaloae Phage Isolated from Yellow Sea. Curr. Microbiol. 2016, 72, 628–633. [Google Scholar] [CrossRef]
- Li, E.; Yin, Z.; Ma, Y.; Li, H.; Lin, W.; Wei, X.; Zhao, R.; Jiang, A.; Yuan, J.; Zhao, X. Identification and molecular characterization of bacteriophage phiAxp-2 of Achromobacter xylosoxidans. Sci. Rep. 2016, 6, 34300. [Google Scholar] [CrossRef]
- Jang, S.H.; Yoon, B.H.; Chang, H.I. Complete nucleotide sequence of the temperate bacteriophage LBR48, a new member of the family Myoviridae. Arch. Virol. 2011, 156, 319–322. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, R.A.; Kelly, W.J.; Altermann, E.; Leahy, S.C.; Minchin, C.; Ouwerkerk, D.; Klieve, A.V. Toward Understanding Phage:Host Interactions in the Rumen; Complete Genome Sequences of Lytic Phages Infecting Rumen Bacteria. Front. Microbiol. 2017, 8, 2340. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.H.; Jian, H.H.; Song, C.Y.; Bao, D.P.; Shang, X.D.; Wu, D.Q.; Tan, Q.; Zhang, X.H. Transcriptome analysis of candidate genes and signaling pathways associated with light-induced brown film formation in Lentinula edodes. Appl. Microbiol. Biotechnol. 2013, 97, 4977–4989. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Li, X.; Song, J.; Yang, D.; Liu, W.; Chen, H.; Wu, B.; Qian, P. Isolation and characterization of a novel temperate bacteriophage from gut-associated Escherichia within black soldier fly larvae (Hermetia illucens L. [Diptera: Stratiomyidae]). Arch. Virol. 2019, 164, 2277–2284. [Google Scholar] [CrossRef] [PubMed]
- Carver, T.; Thomson, N.; Bleasby, A.; Berriman, M.; Parkhill, J. DNAPlotter: Circular and linear interactive genome visualization. Bioinformatics 2009, 25, 119–120. [Google Scholar] [CrossRef]
- Khalil, M.A.; Azzazy, H.M.; Attia, A.S.; Hashem, A.G. A sensitive colorimetric assay for identification of Acinetobacter baumannii using unmodified gold nanoparticles. J. Appl. Microbiol. 2014, 117, 465–471. [Google Scholar] [CrossRef]
- Holtappels, M.; Vrancken, K.; Schoofs, H.; Deckers, T.; Remans, T.; Noben, J.P.; Valcke, R. A comparative proteome analysis reveals flagellin, chemotaxis regulated proteins and amylovoran to be involved in virulence differences between Erwinia amylovora strains. J. Proteom. 2015, 123, 54–69. [Google Scholar] [CrossRef]
- Ma, Y.; Li, E.; Qi, Z.; Li, H.; Wei, X.; Lin, W.; Zhao, R.; Jiang, A.; Yang, H.; Yin, Z.; et al. Isolation and molecular characterisation of Achromobacter phage phiAxp-3, an N4-like bacteriophage. Sci. Rep. 2016, 6, 24776. [Google Scholar] [CrossRef]
- Jeon, J.; D’Souza, R.; Pinto, N.; Ryu, C.M.; Park, J.; Yong, D.; Lee, K. Characterization and complete genome sequence analysis of two Myoviral bacteriophages infecting clinical carbapenem-resistant Acinetobacter baumannii isolates. J. Appl. Microbiol. 2016, 121, 68–77. [Google Scholar] [CrossRef]
- Moller-Olsen, C.; Ho, S.F.S.; Shukla, R.D.; Feher, T.; Sagona, A.P. Engineered K1F bacteriophages kill intracellular Escherichia coli K1 in human epithelial cells. Sci. Rep. 2018, 8, 17559. [Google Scholar] [CrossRef]
- World Health Organisation. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery and Development of New Antibiotics; WHO Press: Geneva, Switzerland, 2017; p. 7. [Google Scholar]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a successful pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- Popova, A.V.; Lavysh, D.G.; Klimuk, E.I.; Edelstein, M.V.; Bogun, A.G.; Shneider, M.M.; Goncharov, A.E.; Leonov, S.V.; Severinov, K.V. Novel Fri1-like viruses infecting Acinetobacter baumannii—vB_AbaP_AS11 and vB_AbaP_AS12—characterization, comparative genomic analysis, and host-recognition strategy. Viruses 2017, 9, 188. [Google Scholar] [CrossRef] [PubMed]
- Turner, D.; Ackermann, H.W.; Kropinski, A.M.; Lavigne, R.; Sutton, J.M.; Reynolds, D.M. Comparative analysis of 37 Acinetobacter bacteriophages. Viruses 2018, 10, 5. [Google Scholar] [CrossRef] [PubMed]
- Plotka, M.; Kapusta, M.; Dorawa, S.; Kaczorowska, A.K.; Kaczorowski, T. Ts2631 endolysin from the extremophilic thermus scotoductus bacteriophage vB_Tsc2631 as an antimicrobial agent against gram-negative multidrug-resistant bacteria. Viruses 2019, 11, 657. [Google Scholar] [CrossRef]
- Bai, Y.L.; Shahed-Al-Mahmud, M.; Selvaprakash, K.; Lin, N.T.; Chen, Y.C. Tail Fiber Protein-Immobilized Magnetic Nanoparticle-Based Affinity Approaches for Detection of Acinetobacter baumannii. Anal. Chem. 2019, 91, 10335–10342. [Google Scholar] [CrossRef]
- Lu, T.K.; Bowers, J.; Koeris, M.S. Advancing bacteriophage-based microbial diagnostics with synthetic biology. Trends Biotechnol. 2013, 31, 325–327. [Google Scholar] [CrossRef]
- Mannoor, M.S.; Zhang, S.; Link, A.J.; McAlpine, M.C. Electrical detection of pathogenic bacteria via immobilized antimicrobial peptides. Proc. Natl. Acad. Sci. USA 2010, 107, 19207–19212. [Google Scholar] [CrossRef]
- Miranda, O.R.; Li, X.N.; Garcia-Gonzalez, L.; Zhu, Z.J.; Yan, B.; Bunz, U.H.F.; Rotello, V.M. Colorimetric bacteria sensing using a supramolecular enzyme-nanoparticle biosensor. J. Am. Chem. Soc. 2011, 133, 9650–9653. [Google Scholar] [CrossRef]
- Saussereau, E.; Debarbieux, L. Bacteriophages in the experimental treatment of Pseudomonas aeruginosa infections in mice. Adv. Virus Res. 2012, 83, 123–141. [Google Scholar]
- Jiang, L.; Schlesinger, F.; Davis, C.A.; Zhang, Y.; Li, R.; Salit, M.; Gingeras, T.R.; Oliver, B. Synthetic spike-in standards for RNA-seq experiments. Genome Res. 2011, 21, 1543–1551. [Google Scholar] [CrossRef] [PubMed]
- Mahichi, F.; Synnott, A.J.; Yamamichi, K.; Osada, T.; Tanji, Y. Site-specific recombination of T2 phage using IP008 long tail fiber genes provides a targeted method for expanding host range while retaining lytic activity. FEMS Microbiol. Lett. 2009, 295, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Pires, D.P.; Costa, A.R.; Azeredo, J. Phage Therapy: Going Temperate? Trends Microbiol. 2019, 27, 368–378. [Google Scholar] [CrossRef] [PubMed]
- Hatoum-Aslan, A. Phage Genetic Engineering Using CRISPR(-)Cas Systems. Viruses 2018, 10, 335. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Sao-Jose, C. Enzymes and Mechanisms Employed by Tailed Bacteriophages to Breach the Bacterial Cell Barriers. Viruses 2018, 10, 396. [Google Scholar] [CrossRef]
- Park, J.Y.; Kim, S.; Kim, S.M.; Cha, S.H.; Lim, S.K.; Kim, J. Complete genome sequence of multidrug-resistant Acinetobacter baumannii strain 1656-2, which forms sturdy biofilm. J. Bacteriol. 2011, 193, 6393–6394. [Google Scholar] [CrossRef]
- Higgins, P.G.; Dammhayn, C.; Hackel, M.; Seifert, H. Global spread of carbapenem-resistant Acinetobacter baumannii. J. Antimicrob. Chemother. 2010, 65, 1317. [Google Scholar] [CrossRef]
- Tao, P.; Wu, X.; Tang, W.C.; Zhu, J.; Rao, V. Engineering of Bacteriophage T4 Genome Using CRISPR-Cas9. ACS Synth. Biol. 2017, 6, 1952–1961. [Google Scholar] [CrossRef]






| Species | ID | Infection |
|---|---|---|
| Acinetobacter baumannii | MDR-TJ(CP003500) | + |
| Acinetobacter baumannii | MDR-A | + |
| Acinetobacter baumannii | MDR-B | + |
| Acinetobacter baumannii | MDR-C | + |
| Acinetobacter baumannii | ATCC19606 | + |
| Acinetobacter haemolyticus | TJS01 | − |
| Acinetobacter haemolyticus | TJR01 | − |
| Acinetobacter haemolyticus | H2063 | − |
| Acinetobacter haemolyticus | W65 | − |
| Bacillus subtilis | Sck6 | − |
| Staphylococcus aureus | MRS-TJ | − |
| Escherichia coli | ATCC25922 | − |
| Escherichia coli | MG1655 | − |
| Escherichia coli | BL21 | − |
| Escherichia coli | DH5α | − |
| Escherichia coli | Rosetta | − |
| Escherichia coli | TG1 | − |
| Escherichia coli | TOP10 | − |
| Escherichia coli | TransB | − |
| Pichia pastoris | GS115 | − |
| ORFs | Strand | Start | End | Length (aa) a | aa Identity (%) | Function |
|---|---|---|---|---|---|---|
| Orf01 | + | 179 | 1441 | 420 | 24 | Integrase (Pseudomonas phage vB PaeS PMG1) |
| Orf02 | − | 1447 | 1716 | 89 | 45 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf03 | − | 1717 | 1974 | 85 | 80 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf04 | − | 1978 | 2262 | 94 | 37 | hypothetical protein |
| Orf05 | − | 2259 | 2468 | 69 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf06 | − | 2459 | 2716 | 85 | 92 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf07 | − | 2718 | 3719 | 333 | 51 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf08 | − | 3716 | 4837 | 373 | 94 | phage nucleotide-binding protein (Acinetobacter phage Bphi-B1251) |
| Orf09 | − | 4848 | 5171 | 107 | 93 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf10 | − | 5174 | 5614 | 156 | 96 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf11 | − | 5843 | 6634 | 263 | 26 | putative transcriptional regulator (Acinetobacter phage Ab105-1phi) |
| Orf12 | + | 6637 | 6885 | 82 | 45 | regulatory protein cro (Escherichia phage HK75) |
| Orf13 | + | 6940 | 7440 | 166 | 38 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf14 | + | 7490 | 7762 | 90 | 51 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf15 | + | 7759 | 8055 | 98 | 98 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf16 | + | 8052 | 8408 | 80 | 97 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf17 | + | 8408 | 9337 | 309 | 36 | hypothetical protein BA3 0036[Thalassomonas phage BA3) |
| Orf18 | + | 9330 | 10,079 | 249 | — | hypothetical protein |
| Orf19 | + | 10,076 | 10,498 | 140 | 99 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf20 | + | 10,488 | 10,910 | 140 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf21 | + | 10,903 | 11,127 | 75 | 74 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf22 | + | 11,127 | 11,522 | 131 | 94 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf23 | + | 11,519 | 12,019 | 166 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf24 | + | 12,215 | 12,865 | 216 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf25 | + | 13,127 | 13,885 | 252 | — | hypothetical protein |
| Orf26 | + | 13,980 | 14,627 | 215 | — | hypothetical protein |
| Orf27 | + | 14,675 | 15,184 | 169 | 44 | hypothetical protein (Pectobacterium phage ZF40) |
| Orf28 | + | 15,181 | 16,839 | 552 | 40 | terminase large subunit (Burkholderia phage BcepB1A) |
| Orf29 | + | 16,850 | 18,262 | 470 | 32 | putative portal protein (Vibrio phage CP-T1) |
| Orf30 | − | 18,060 | 17,848 | 70 | 21 | hypothetical protein (Psychrobacter phage pOW20-A) |
| Orf31 | + | 19,209 | 20,525 | 438 | — | hypothetical protein |
| Orf32 | + | 20,529 | 21,005 | 158 | 89 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf33 | + | 21,070 | 22,095 | 341 | — | hypothetical protein |
| Orf34 | + | 22,105 | 22,536 | 143 | — | hypothetical protein |
| Orf35 | + | 22,540 | 22,926 | 128 | 99 | hypothetical protein |
| Orf36 | + | 22,923 | 23,483 | 186 | 30 | hypothetical protein (Klebsiella phage JD001) |
| Orf37 | + | 23,515 | 23,838 | 107 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf38 | + | 23,841 | 24,380 | 179 | 42 | hypothetical protein (Aggregatibacter phage S1249) |
| Orf39 | + | 24,384 | 25,859 | 491 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf40 | + | 25,874 | 26,317 | 147 | 99 | hypothetical protein |
| Orf41 | + | 26,317 | 26,781 | 154 | 66 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf42 | + | 26,911 | 29,001 | 696 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf43 | − | 28,998 | 29,354 | 118 | 98 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf44 | + | 29,586 | 29,774 | 62 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf45 | + | 29,941 | 30,537 | 198 | 27 | hypothetical protein (Psychrobacter phage pOW20-A) |
| Orf46 | + | 30,540 | 30,836 | 98 | 34 | hypothetical protein (Psychrobacter phage pOW20-A) |
| Orf47 | + | 30,833 | 31,792 | 319 | 26 | hypothetical protein (Aggregatibacter phage S1249) |
| Orf48 | + | 31,795 | 32,457 | 220 | 35 | hypothetical protein (Psychrobacter phage pOW20-A) |
| Orf49 | + | 32,492 | 32,845 | 117 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf50 | + | 32,848 | 34,032 | 394 | 99 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf51 | + | 34,032 | 34,622 | 196 | 93 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf52 | + | 34,615 | 35,223 | 202 | 99 | tail fiber protein (Acinetobacter phage Ab105-1phi) |
| Orf53 | + | 35,242 | 37,341 | 699 | 96 | tail fiber protein (Acinetobacter phage Ab105-1phi) |
| Orf54 | + | 37,343 | 37,570 | 75 | 96 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf55 | + | 37,648 | 38,037 | 129 | 100 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf56 | + | 38,080 | 38,625 | 181 | 80 | secretion activator protein (Acinetobacter phage Ab105-1phi) |
| Orf57 | + | 38,814 | 39,494 | 226 | 27 | hypothetical protein (Acinetobacter phage Ab105-1phi) |
| Orf58 | + | 39,505 | 40,152 | 215 | 98 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf59 | + | 40,159 | 40,752 | 197 | 62 | hypothetical protein (Acinetobacter phage Bphi-B1251) |
| Orf60 | − | 40,757 | 40,906 | 50 | 99 | lesion bypass DNA polymerase V (Acinetobacter phage Ab105-1phi) |
| Orf61 | − | 40,998 | 41,852 | 284 | 14 | Transposase (Acinetobacter phage Ab105-1phi) |
| Orf62 | − | 41,864 | 42,163 | 99 | 27 | transposase (Acinetobacter baumannii OIFC099) |
| Sample | Test Bacterial Concentration (CFU mL−1) | Bioluminescence Signal Value (a.u) | Bacteria Captured on the Beads (CFU mL−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Glucose injection | 1.5 × 106 | 1094.1 | 1.34 × 106 | 89.3 | 5.6 |
| 1.5 × 105 | 505.8 | 1.28 × 105 | 85.3 | 1.5 | |
| 1.5 × 104 | 222.3 | 1.11 × 104 | 74.0 | 3.1 | |
| 1.5 × 103 | 95.7 | 8.58 × 102 | 57.2 | 3.8 | |
| Human urine | 1.5 × 106 | 1100.4 | 1.34 × 106 | 89.3 | 5.3 |
| 1.5 × 105 | 495.5 | 1.25 × 105 | 83.3 | 3.1 | |
| 1.5 × 104 | 218.3 | 1.05 × 104 | 70.0 | 2.7 | |
| 1.5 × 103 | 92.9 | 8.44 × 102 | 56.3 | 0.1 | |
| Human feces | 1.5 × 106 | 1076.5 | 1.26 × 106 | 84 | 5.8 |
| 1.5 × 105 | 486.4 | 1.14 × 105 | 76 | 1.9 | |
| 1.5 × 104 | 217.3 | 1.02 × 104 | 68.0 | 3.1 | |
| 1.5 × 103 | 92.3 | 8.77 × 102 | 58.2 | 3.1 | |
| Human sputum | 1.5 × 106 | 1090.6 | 1.31 × 106 | 87.3 | 1.4 |
| 1.5 × 105 | 489.8 | 1.17 × 105 | 78.0 | 2.3 | |
| 1.5 × 104 | 214.3 | 9.80 × 103 | 65.3 | 1.7 | |
| 1.5 × 103 | 91.8 | 8.22 × 102 | 54.8 | 3.8 | |
| Domestic sewage | 1.5 × 106 | 1071.2 | 1.24 × 106 | 82.7 | 1.4 |
| 1.5 × 105 | 483.1 | 1.12 × 105 | 76.7 | 1.9 | |
| 1.5 × 104 | 211.3 | 9.21 × 103 | 61.4 | 3.1 | |
| 1.5 × 103 | 90.3 | 7.67 × 102 | 51.1 | 3.0 |
| Sample | Test Bacterial Concentration (CFU mL−1) | Bioluminescence Signal Value (a.u) | Bacteria Captured on the Beads (CFU mL−1) | Recovery (%) | RSD (%) |
|---|---|---|---|---|---|
| Glucose injection | 1.5 × 106 | 1030.3 | 1.39 × 106 | 92.7 | 0.7 |
| 1.5 × 105 | 465.6 | 1.34 × 105 | 89.3 | 1.8 | |
| 1.5 × 104 | 199.9 | 1.11 × 104 | 74 | 4.0 | |
| 1.5 × 103 | 83.8 | 8.43 × 102 | 56.3 | 1.2 | |
| Human urine | 1.5 × 106 | 1025.7 | 1.39 × 106 | 92.7 | 1.0 |
| 1.5 × 105 | 459.2 | 1.28 × 105 | 85.3 | 1.8 | |
| 1.5 × 104 | 197.2 | 1.06 × 104 | 70.7 | 2.4 | |
| 1.5 × 103 | 80.4 | 7.83 × 102 | 52.2 | 3.6 | |
| Human feces | 1.5 × 106 | 1020.9 | 1.37 × 106 | 91.3 | 0.9 |
| 1.5 × 105 | 454.9 | 1.28 × 105 | 85.3 | 2.5 | |
| 1.5 × 104 | 195.9 | 1.04 × 104 | 69.3 | 2.4 | |
| 1.5 × 103 | 80.1 | 7.74 × 102 | 51.6 | 3.6 | |
| Human sputum | 1.5 × 106 | 1018.7 | 1.33 × 106 | 90.6 | 1.5 |
| 1.5 × 105 | 456.0 | 1.27 × 105 | 84.7 | 1.5 | |
| 1.5 × 104 | 195.4 | 1.04 × 104 | 69.3 | 2.8 | |
| 1.5 × 103 | 80.1 | 7.74 × 102 | 51.6 | 4.4 | |
| Domestic sewage | 1.5 × 106 | 1016.2 | 1.32 × 106 | 88.0 | 1.5 |
| 1.5 × 105 | 450.8 | 1.22 × 105 | 81.3 | 1.4 | |
| 1.5 × 104 | 192.3 | 9.94 × 103 | 66.7 | 2.9 | |
| 1.5 × 103 | 79.3 | 7.57 × 102 | 50.5 | 4.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Li, X.; Kang, G.; Bai, L.; Wang, P.; Huang, H. Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules. Viruses 2020, 12, 205. https://doi.org/10.3390/v12020205
Xu J, Li X, Kang G, Bai L, Wang P, Huang H. Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules. Viruses. 2020; 12(2):205. https://doi.org/10.3390/v12020205
Chicago/Turabian StyleXu, Jingzhi, Xiaobo Li, Guangbo Kang, Liang Bai, Ping Wang, and He Huang. 2020. "Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules" Viruses 12, no. 2: 205. https://doi.org/10.3390/v12020205
APA StyleXu, J., Li, X., Kang, G., Bai, L., Wang, P., & Huang, H. (2020). Isolation and Characterization of AbTJ, an Acinetobacter baumannii Phage, and Functional Identification of Its Receptor-Binding Modules. Viruses, 12(2), 205. https://doi.org/10.3390/v12020205






