Bioprospecting Staphylococcus Phages with Therapeutic and Bio-Control Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains, Phages and Media
2.2. Phage Isolation and Purification
2.3. Transmission Electron Microscopy (TEM)
2.4. Experiments on Physical and Chemical Properties
2.5. Adsorption Rate Experiment
2.6. One Step Growth Curves
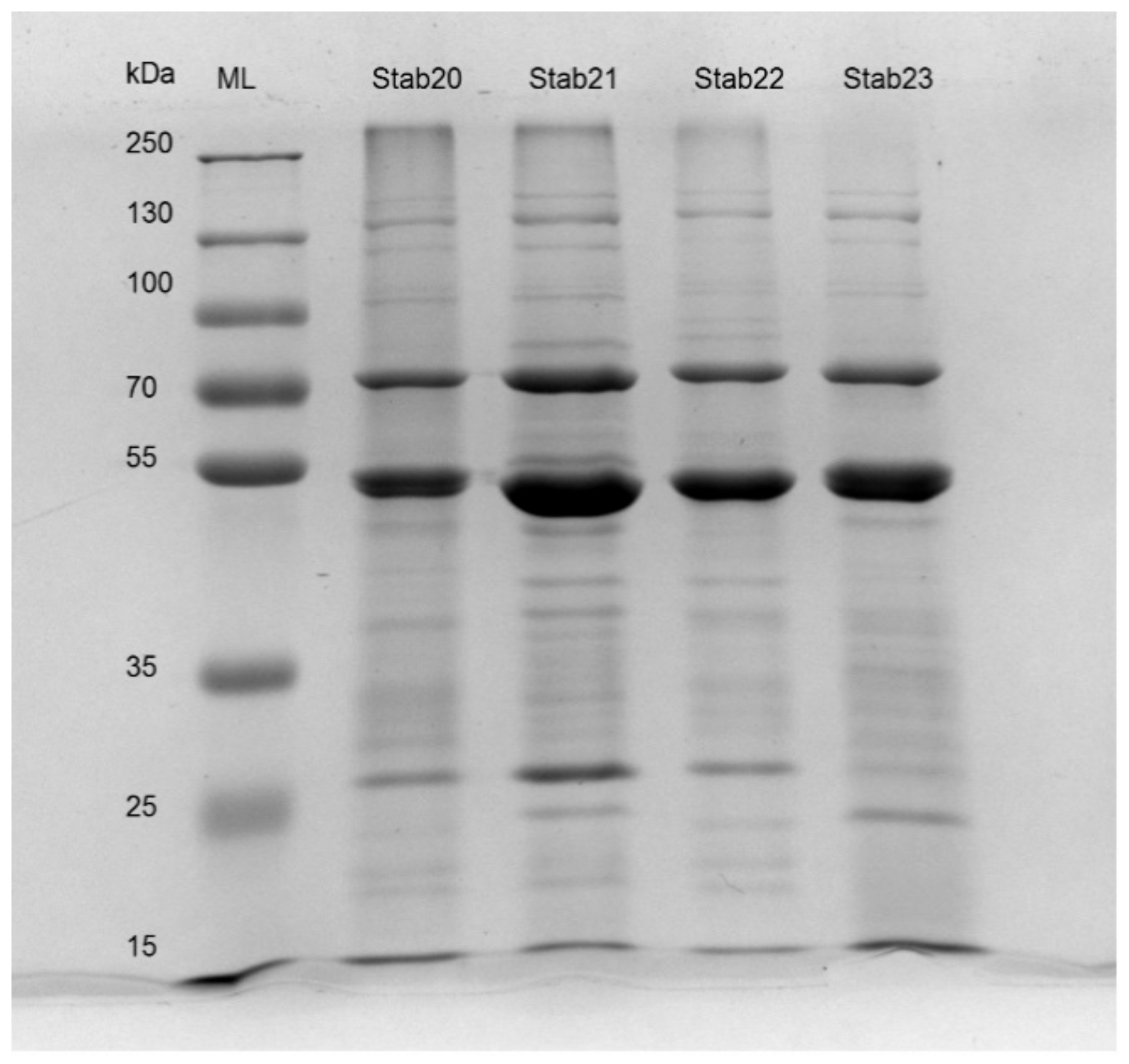
2.7. SDS-PAGE and LC/MS-MS Analysis
2.8. Host Range Testing
2.9. Statistical Analysis
3. Results
3.1. Morphology
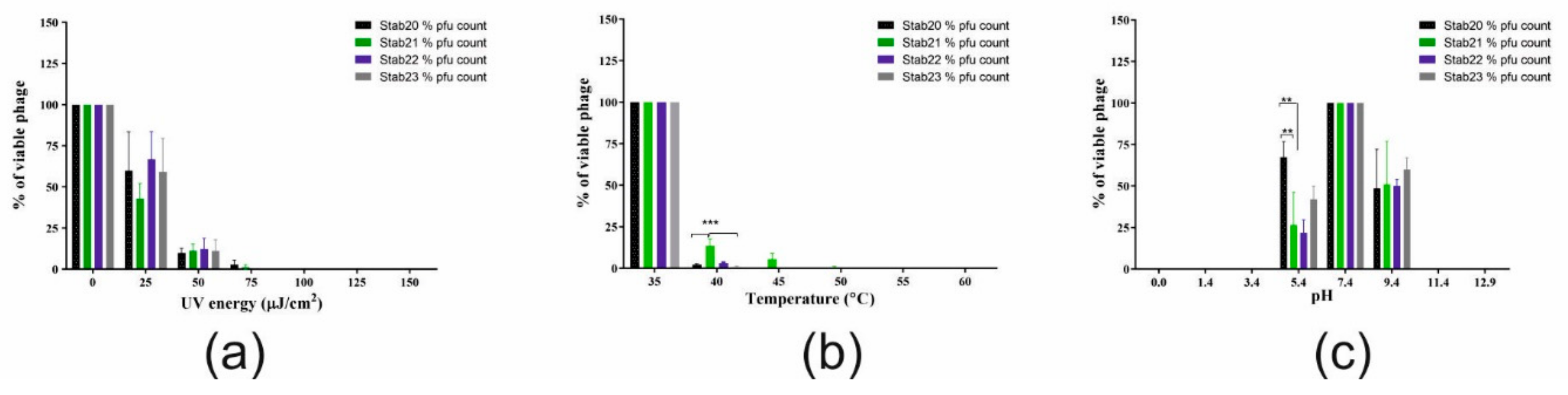
3.2. Physico-Chemical Stability
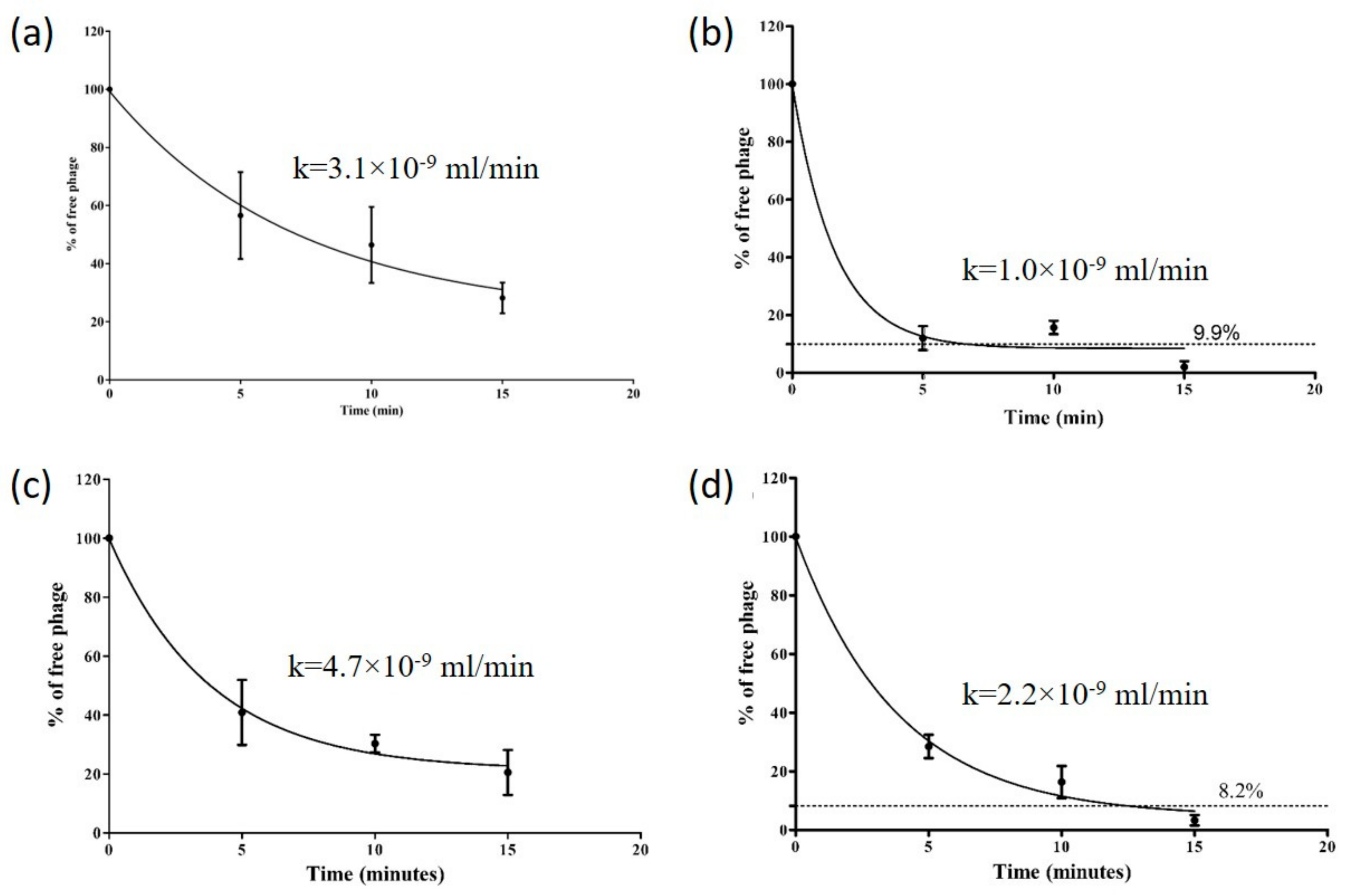
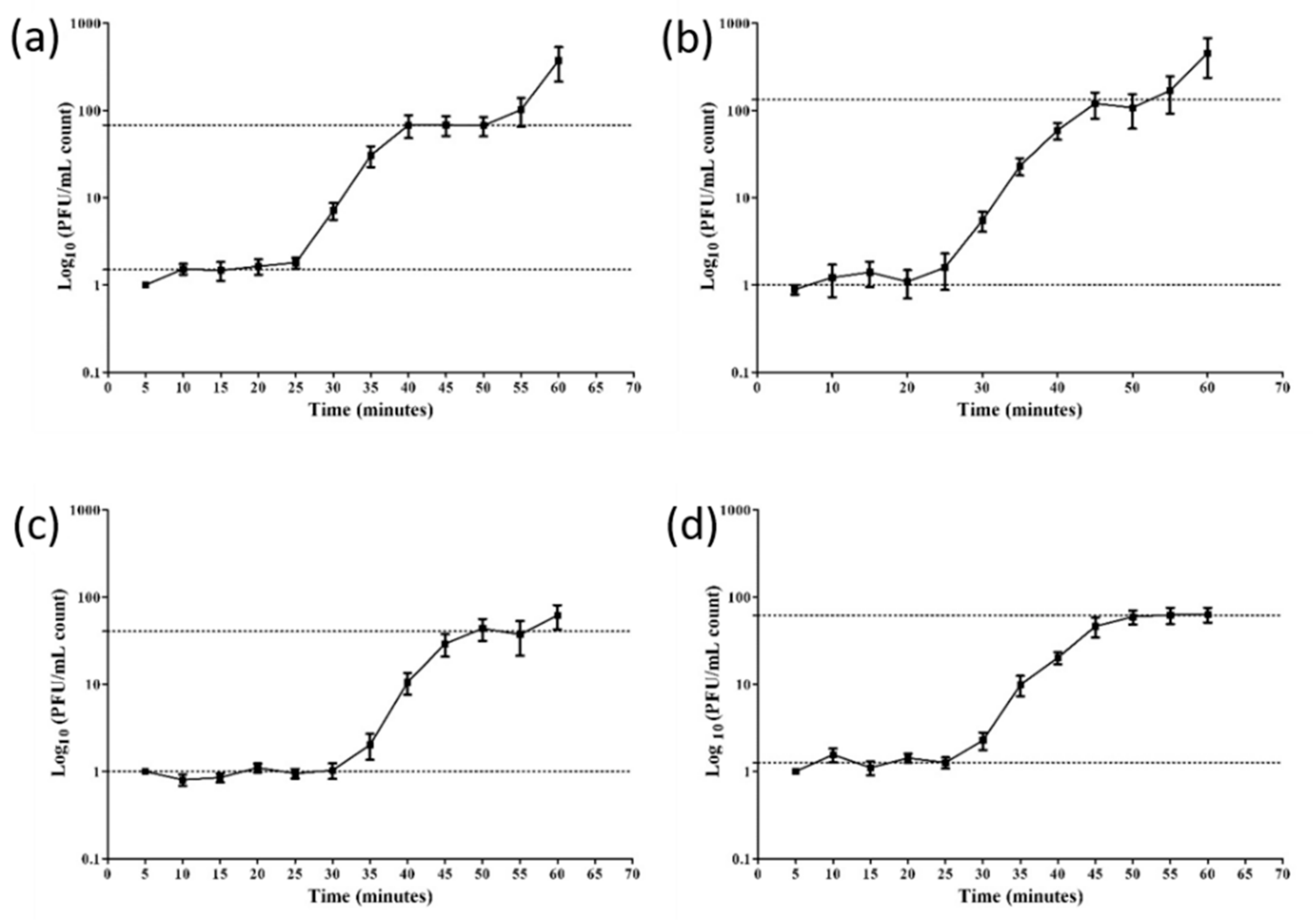
3.3. Adsorption Rate and Growth Curves
3.4. Analysis of Stab Phage Particle Proteomes
3.5. Host Range Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization Antibiotic Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antibiotic-resistance (accessed on 27 November 2019).
- O’Neill, J. Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. Available online: https://amr-review.org/ (accessed on 24 October 2019).
- Adeyi, O.O.; Baris, E.; Jonas, O.B.; Irwin, A.; Berthe, F.C.J.; Le Gall, F.G.; Marquez, P.V.; Nikolic, I.A.; Plante, C.A.; Schneidman, M.; et al. Final Report Drug-Resistant Infections: A Threat to Our Economic Future; The World Bank: Washington, DC, USA, 2017; pp. 1–172. [Google Scholar]
- David, M.Z.; Daum, R.S. Community-Associated Methicillin-Resistant Staphylococcus aureus: Epidemiology and Clinical Consequences of an Emerging Epidemic. Clin. Microbiol. Rev. 2010, 23, 616–687. [Google Scholar] [CrossRef] [PubMed]
- Becker, K.; Heilmann, C.; Peters, G. Coagulase-Negative Staphylococci. Clin. Microbiol. Rev. 2014, 27, 870–926. [Google Scholar] [CrossRef] [PubMed]
- Foster, A.P. Staphylococcal skin disease in livestock. Vet. Dermatol. 2012, 23, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lowder, B.V.; Guinane, C.M.; Ben Zakour, N.L.; Weinert, L.A.; Conway-Morris, A.; Cartwright, R.A.; Simpson, A.J.; Rambaut, A.; Nübel, U.; Fitzgerald, J.R. Recent human-to-poultry host jump, adaptation, and pandemic spread of Staphylococcus aureus. Proc. Natl. Acad. Sci. USA 2009, 106, 19545–19550. [Google Scholar] [CrossRef] [PubMed]
- Bhullar, K.; Waglechner, N.; Pawlowski, A.; Koteva, K.; Banks, E.D.; Johnston, M.D.; Barton, H.A.; Wright, G.D. Antibiotic resistance is prevalent in an isolated cave microbiome. PLoS ONE 2012, 7, e34953. [Google Scholar] [CrossRef] [PubMed]
- Nawrocki, K.L.; Crispell, E.K.; McBride, S.M. Antimicrobial Peptide Resistance Mechanisms of Gram-Positive Bacteria. Antibiotics 2014, 3, 461–492. [Google Scholar] [CrossRef]
- Joo, H.-S.; Otto, M. Mechanisms of resistance to antimicrobial peptides in staphylococci. Biochim. Biophys. Acta 2015, 1848, 3055–3061. [Google Scholar] [CrossRef]
- Foster, T.J. Antibiotic resistance in Staphylococcus aureus. Current status and future prospects. FEMS Microbiol. Rev. 2017, 41, 430–449. [Google Scholar] [CrossRef]
- Czekaj, T.; Ciszewski, M.; Szewczyk, E.M. Staphylococcus haemolyticus—An emerging threat in the twilight of the antibiotics age. Microbiol. Read. Engl. 2015, 161, 2061–2068. [Google Scholar] [CrossRef]
- Livermore, D.M.; Blaser, M.; Carrs, O.; Cassell, G.; Fishman, N.; Guidos, R.; Levy, S.; Powers, J.; Norrby, R.; Tillotson, G.; et al. Discovery research: The scientific challenge of finding new antibiotics. J. Antimicrob. Chemother. 2011, 66, 1941–1944. [Google Scholar] [CrossRef]
- Simpkin, V.L.; Renwick, M.J.; Kelly, R.; Mossialos, E. Incentivising innovation in antibiotic drug discovery and development: Progress, challenges and next steps. J. Antibiot. (Tokyo) 2017, 70, 1087–1096. [Google Scholar] [CrossRef] [PubMed]
- Renwick, M.; Mossialos, E. What are the economic barriers of antibiotic R&D and how can we overcome them? Expert Opin. Drug Discov. 2018, 13, 889–892. [Google Scholar] [PubMed]
- Knafl, D.; Tobudic, S.; Cheng, S.C.; Bellamy, D.R.; Thalhammer, F. Dalbavancin reduces biofilms of methicillin-resistant Staphylococcus aureus (MRSA) and methicillin-resistant Staphylococcus epidermidis (MRSE). Eur. J. Clin. Microbiol. Infect. Dis. 2017, 36, 677–680. [Google Scholar] [CrossRef] [PubMed]
- Jurczak-Kurek, A.; Gąsior, T.; Nejman-Faleńczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Rohde, C.; Resch, G.; Pirnay, J.-P.; Blasdel, B.G.; Debarbieux, L.; Gelman, D.; Górski, A.; Hazan, R.; Huys, I.; Kakabadze, E.; et al. Expert Opinion on Three Phage Therapy Related Topics: Bacterial Phage Resistance, Phage Training and Prophages in Bacterial Production Strains. Viruses 2018, 10, 178. [Google Scholar] [CrossRef]
- Zhvania, P.; Hoyle, N.S.; Nadareishvili, L.; Nizharadze, D.; Kutateladze, M. Phage Therapy in a 16-Year-Old Boy with Netherton Syndrome. Front. Med. 2017, 4, 94. [Google Scholar] [CrossRef]
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage Applications for Food Production and Processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef]
- Oduor, J.M.O.; Onkoba, N.; Maloba, F.; Nyachieo, A. Experimental phage therapy against hematogenous multi-drug resistant Staphylococcus aureus pneumonia in mice. Afr. J. Lab. Med. 2016, 5, 1–7. [Google Scholar]
- Hua, Y.; Luo, T.; Yang, Y.; Dong, D.; Wang, R.; Wang, Y.; Xu, M.; Guo, X.; Hu, F.; He, P. Phage Therapy as a Promising New Treatment for Lung Infection Caused by Carbapenem-Resistant Acinetobacter baumannii in Mice. Front. Microbiol. 2018, 8, 2659. [Google Scholar] [CrossRef]
- Hanlon, G.W. Bacteriophages: An appraisal of their role in the treatment of bacterial infections. Int. J. Antimicrob. Agents 2007, 30, 118–128. [Google Scholar] [CrossRef]
- Oechslin, F. Resistance Development to Bacteriophages Occurring during Bacteriophage Therapy. Viruses 2018, 10, 351. [Google Scholar] [CrossRef] [PubMed]
- Kronheim, S.; Daniel-Ivad, M.; Duan, Z.; Hwang, S.; Wong, A.I.; Mantel, I.; Nodwell, J.R.; Maxwell, K.L. A chemical defence against phage infection. Nature 2018, 564, 283–286. [Google Scholar] [CrossRef] [PubMed]
- Scanlan, P.D.; Hall, A.R.; Lopez-Pascua, L.D.C.; Buckling, A. Genetic basis of infectivity evolution in a bacteriophage. Mol. Ecol. 2011, 20, 981–989. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, K.L. Phages Fight Back: Inactivation of the CRISPR-Cas Bacterial Immune System by Anti-CRISPR Proteins. PLOS Pathog. 2016, 12, e1005282. [Google Scholar] [CrossRef] [PubMed]
- Tao, P.; Wu, X.; Rao, V. Unexpected evolutionary benefit to phages imparted by bacterial CRISPR-Cas9. Sci. Adv. 2018, 4, eaar4134. [Google Scholar] [CrossRef]
- Samson, J.E.; Magadán, A.H.; Sabri, M.; Moineau, S. Revenge of the phages: Defeating bacterial defences. Nat. Rev. Microbiol. 2013, 11, 675–687. [Google Scholar] [CrossRef]
- Leskinen, K.; Tuomala, H.; Wicklund, A.; Horsma-Heikkinen, J.; Kuusela, P.; Skurnik, M.; Kiljunen, S. Characterization of vB_SauM-fRuSau02, a Twort-Like Bacteriophage Isolated from a Therapeutic Phage Cocktail. Viruses 2017, 9, 258. [Google Scholar] [CrossRef]
- Oduor, J.M.O.; Kiljunen, S.; Kadija, E.; Mureithi, M.W.; Nyachieo, A.; Skurnik, M. Genomic characterization of four novel Staphylococcus myoviruses. Arch. Virol. 2019, 164, 2171–2173. [Google Scholar] [CrossRef]
- Moller, J.K.; Hinrichsen, L.L.; Andersen, H.J. Formation of amino acid (L-leucine, L-phenylalanine) derived volatile flavour compounds by Moraxella phenylpyruvica and Staphylococcus xylosus in cured meat model systems. Int J Food Microbiol 1998, 42, 101–117. [Google Scholar] [CrossRef]
- Sambrook, J.; Russell, D.W.; Laboratory, C.S.H. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory: New York, NY, USA, 2001; ISBN 978-0-87969-576-7. [Google Scholar]
- Fischer, S.; Kittler, S.; Klein, G.; Glünder, G. Microplate-Test for the Rapid Determination of Bacteriophage-Susceptibility of Campylobacter Isolates—Development and Validation. PLoS ONE 2013, 8. [Google Scholar] [CrossRef]
- AJ US Analytik Jena AG. Available online: http://us.analytik-jena.com/ (accessed on 11 February 2019).
- Vandersteegen, K.; Kropinski, A.M.; Nash, J.H.E.; Noben, J.-P.; Hermans, K.; Lavigne, R. Romulus and Remus, Two Phage Isolates Representing a Distinct Clade within the Twortlikevirus Genus, Display Suitable Properties for Phage Therapy Applications. J. Virol. 2013, 87, 3237–3247. [Google Scholar] [CrossRef] [PubMed]
- Kropinski, A.M. Practical Advice on the One-Step Growth Curve. Methods Mol. Biol. Clifton N. J. 2018, 1681, 41–47. [Google Scholar]
- Sartorius Vivaspin 20, 100,000 MWCO PES, 48pc. Sartorius. Available online: https://www.sartorius.com/shop/ww/en/usd/master-products/centrifugal-ultrafiltration-devices/vivaspin-20%2c-100%2c000-mwco-pes%2c-48pc/p/VS2042 (accessed on 2 February 2019).
- Sambrook, J.; Russell, D.W. Purification of Bacteriophage λ Particles by Centrifugation through a Glycerol Step Gradient. Cold Spring Harb. Protoc. 2006, 2006, pdb-prot3969. [Google Scholar] [CrossRef] [PubMed]
- Varjosalo, M.; Keskitalo, S.; Van Drogen, A.; Nurkkala, H.; Vichalkovski, A.; Aebersold, R.; Gstaiger, M. The Protein Interaction Landscape of the Human CMGC Kinase Group. Cell Rep. 2013, 3, 1306–1320. [Google Scholar] [CrossRef] [PubMed]
- Prism 8. Available online: https://www.graphpad.com/scientific-software/prism/ (accessed on 25 July 2019).
- Cui, Z.; Guo, X.; Dong, K.; Zhang, Y.; Li, Q.; Zhu, Y.; Zeng, L.; Tang, R.; Li, L. Safety assessment of Staphylococcus phages of the family Myoviridae based on complete genome sequences. Sci. Rep. 2017, 7, 41259. [Google Scholar] [CrossRef] [PubMed]
- Takemura-Uchiyama, I.; Uchiyama, J.; Kato, S.; Inoue, T.; Ujihara, T.; Ohara, N.; Daibata, M.; Matsuzaki, S. Evaluating efficacy of bacteriophage therapy against Staphylococcus aureus infections using a silkworm larval infection model. FEMS Microbiol. Lett. 2013, 347, 52–60. [Google Scholar] [CrossRef]
- Storms, Z.J.; Sauvageau, D. Modeling tailed bacteriophage adsorption: Insight into mechanisms. Virology 2015, 485, 355–362. [Google Scholar] [CrossRef]
- Cornelissen, A.; Sadovskaya, I.; Vinogradov, E.; Blangy, S.; Spinelli, S.; Casey, E.; Mahony, J.; Noben, J.-P.; Dal Bello, F.; Cambillau, C.; et al. The Baseplate of Lactobacillus delbrueckii Bacteriophage Ld17 Harbors a Glycerophosphodiesterase. J. Biol. Chem. 2016, 291, 16816–16827. [Google Scholar] [CrossRef]
- Myers, C.L.; Ireland, R.G.; Garrett, T.A.; Brown, E.D. Characterization of Wall Teichoic Acid Degradation by the Bacteriophage ϕ29 Appendage Protein GP12 Using Synthetic Substrate Analogs. J. Biol. Chem. 2015, 290, 19133–19145. [Google Scholar] [CrossRef]
- Lee, J.Y.; Li, Z.; Miller, E.S. Vibrio Phage KVP40 Encodes a Functional NAD+ Salvage Pathway. J. Bacteriol. 2017, 199. [Google Scholar] [CrossRef]
- Harrison, A.O.; Moore, R.M.; Polson, S.W.; Wommack, K.E. Reannotation of the Ribonucleotide Reductase in a Cyanophage Reveals Life History Strategies within the Virioplankton. Front. Microbiol. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Mitsunobu, H.; Zhu, B.; Lee, S.-J.; Tabor, S.; Richardson, C.C. Flap endonuclease of bacteriophage T7. Bacteriophage 2014, 4, 134. [Google Scholar] [CrossRef] [PubMed]
- Lionnet, T.; Spiering, M.M.; Benkovic, S.J.; Bensimon, D.; Croquette, V. Real-time observation of bacteriophage T4 gp41 helicase reveals an unwinding mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 19790–19795. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.; Li, K.; Fridlund, J.; Šulčius, S.; Bunse, C.; Karlsson, C.M.G.; Lindh, M.; Lundin, D.; Pinhassi, J.; Holmfeldt, K. Genomic and Seasonal Variations among Aquatic Phages Infecting the Baltic Sea Gammaproteobacterium Rheinheimera sp. Strain BAL341. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.K.; Shuman, S. Bacteriophage T4 RNA ligase 2 (gp24.1) exemplifies a family of RNA ligases found in all phylogenetic domains. Proc. Natl. Acad. Sci. USA 2002, 99, 12709–12714. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, J.; Yu, Z.; Jin, J.; Liu, X.; Wang, G. Novel groups and unique distribution of phage phoH genes in paddy waters in northeast China. Sci. Rep. 2016, 6, 38428. [Google Scholar] [CrossRef]
- Wagemans, J.; Blasdel, B.G.; den Bossche, A.V.; Uytterhoeven, B.; Smet, J.D.; Paeshuyse, J.; Cenens, W.; Aertsen, A.; Uetz, P.; Delattre, A.-S.; et al. Functional elucidation of antibacterial phage ORFans targeting Pseudomonas aeruginosa. Cell. Microbiol. 2014, 16, 1822–1835. [Google Scholar] [CrossRef]
- Fernández-Ruiz, I.; Coutinho, F.H.; Rodriguez-Valera, F. Thousands of Novel Endolysins Discovered in Uncultured Phage Genomes. Front. Microbiol. 2018, 9, 1033. [Google Scholar] [CrossRef]
- El Haddad, L.; Ben Abdallah, N.; Plante, P.-L.; Dumaresq, J.; Katsarava, R.; Labrie, S.; Corbeil, J.; St-Gelais, D.; Moineau, S. Improving the Safety of Staphylococcus aureus Polyvalent Phages by Their Production on a Staphylococcus xylosus Strain. PLoS ONE 2014, 9, e102600. [Google Scholar] [CrossRef]
- Sergueev, K.V.; Filippov, A.A.; Farlow, J.; Su, W.; Kvachadze, L.; Balarjishvili, N.; Kutateladze, M.; Nikolich, M.P. Correlation of Host Range Expansion of Therapeutic Bacteriophage Sb-1 with Allele State at a Hypervariable Repeat Locus. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef]
- Ajuebor, J.; Buttimer, C.; Arroyo-Moreno, S.; Chanishvili, N.; Gabriel, E.M.; O’Mahony, J.; McAuliffe, O.; Neve, H.; Franz, C.; Coffey, A. Comparison of Staphylococcus Phage K with Close Phage Relatives Commonly Employed in Phage Therapeutics. Antibiotics 2018, 7, 37. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, S.; Uchiyama, J.; Takemura-Uchiyama, I.; Daibata, M. Perspective: The age of the phage. Nature 2014, 509, S9. [Google Scholar] [CrossRef] [PubMed]
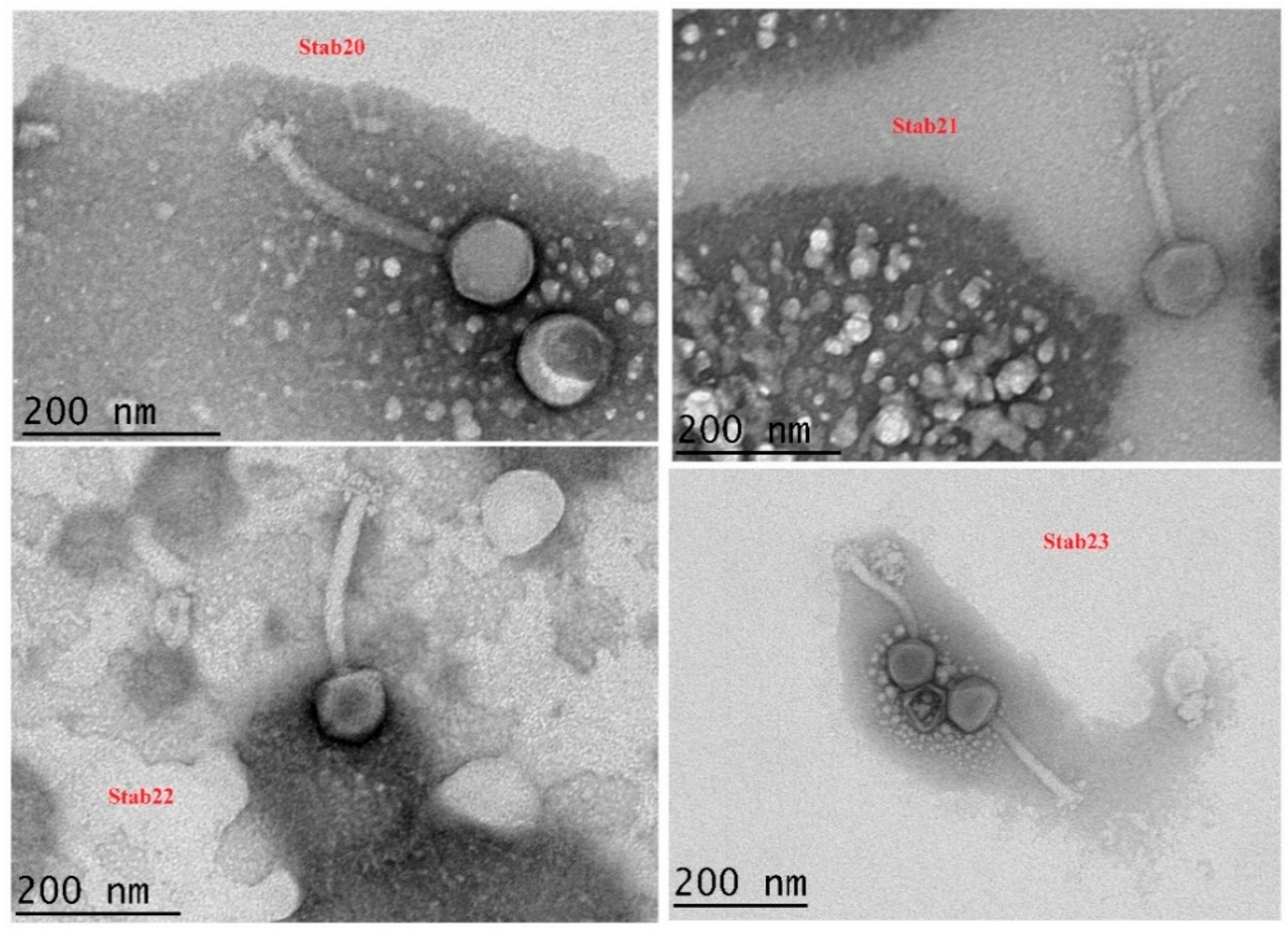

| Phage | Structural Dimensions of the Stab Phages | |||
|---|---|---|---|---|
| Capsid Head | Tail Length | Tail Width | Baseplate Width | |
| Stab20 | 84.0 ± 3.1 nm (n = 5) | 163.2 ± 11.4 nm (n = 5) | 21.1 ± 0.7 nm (n = 5) | 48.1 ± 1.2 nm (n = 5) |
| Stab21 | 91.3 ± 0.25 nm (n = 8) | 196.5 ± 3.1 nm (n = 8) | 23.4 ± 0.6 nm (n = 5) | 44.9 ± 1.5 nm (n = 7) |
| Stab22 | 94.3 ± 0.5 nm (n = 10) | 201.6 ± 0.6 nm (n = 5) | 21.3 ± 0.4 nm (n = 5) | 41.8 ± 0.7 nm (n = 5) |
| Stab23 | 92.5 ± 2.6 nm (n = 10) | 198.9 ± 2.9 nm (n = 9) | 20.3 ± 0.3 nm (n = 9) | 42.3 ± 0.8 nm (n = 5) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oduor, J.M.O.; Kadija, E.; Nyachieo, A.; Mureithi, M.W.; Skurnik, M. Bioprospecting Staphylococcus Phages with Therapeutic and Bio-Control Potential. Viruses 2020, 12, 133. https://doi.org/10.3390/v12020133
Oduor JMO, Kadija E, Nyachieo A, Mureithi MW, Skurnik M. Bioprospecting Staphylococcus Phages with Therapeutic and Bio-Control Potential. Viruses. 2020; 12(2):133. https://doi.org/10.3390/v12020133
Chicago/Turabian StyleOduor, Joseph M. Ochieng’, Ermir Kadija, Atunga Nyachieo, Marianne W. Mureithi, and Mikael Skurnik. 2020. "Bioprospecting Staphylococcus Phages with Therapeutic and Bio-Control Potential" Viruses 12, no. 2: 133. https://doi.org/10.3390/v12020133
APA StyleOduor, J. M. O., Kadija, E., Nyachieo, A., Mureithi, M. W., & Skurnik, M. (2020). Bioprospecting Staphylococcus Phages with Therapeutic and Bio-Control Potential. Viruses, 12(2), 133. https://doi.org/10.3390/v12020133







