Integrase-Defective Lentiviral Vectors for Delivery of Monoclonal Antibodies against Influenza
Abstract
:1. Introduction
2. Material and Methods
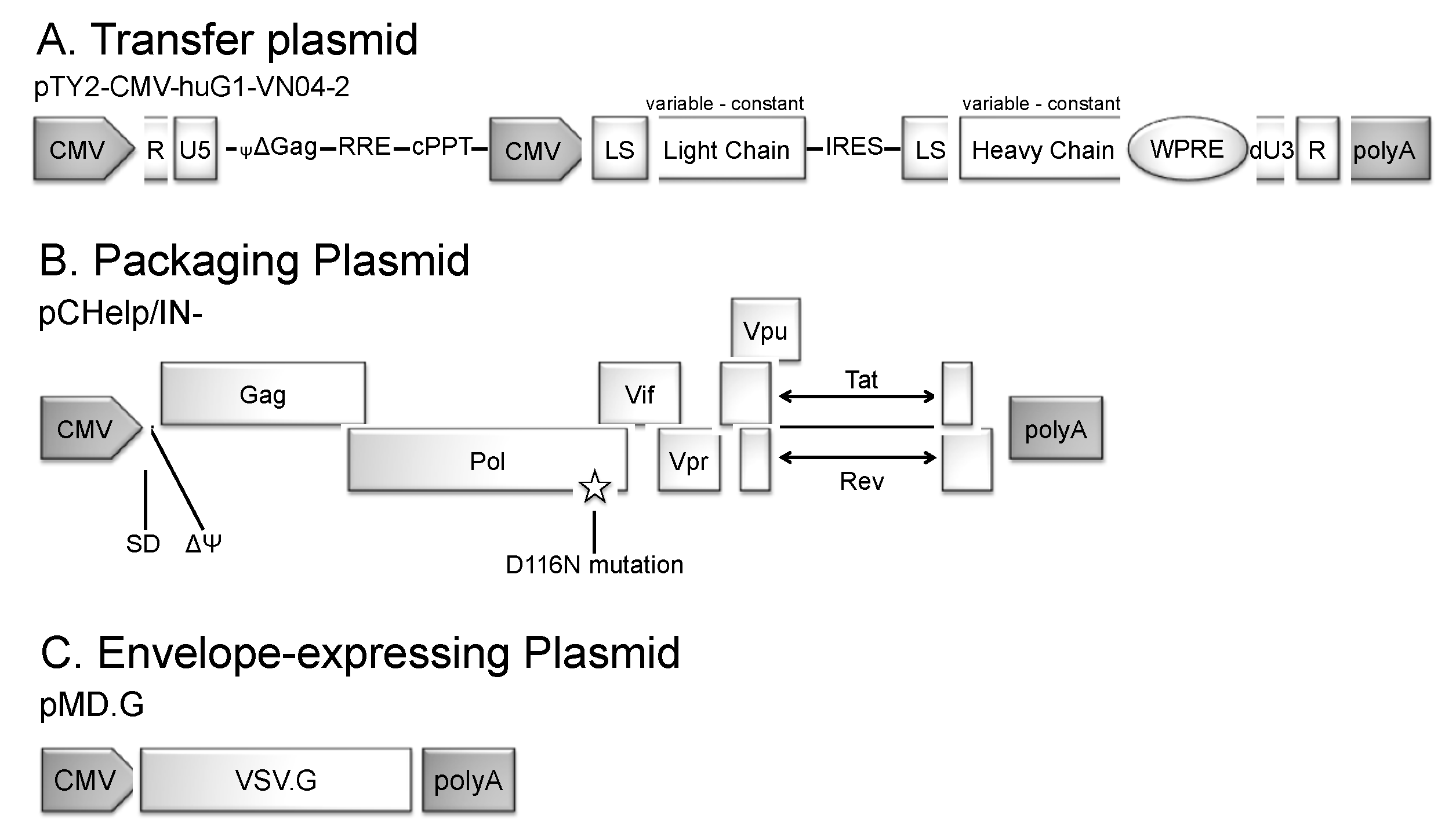
2.1. IDLV Production
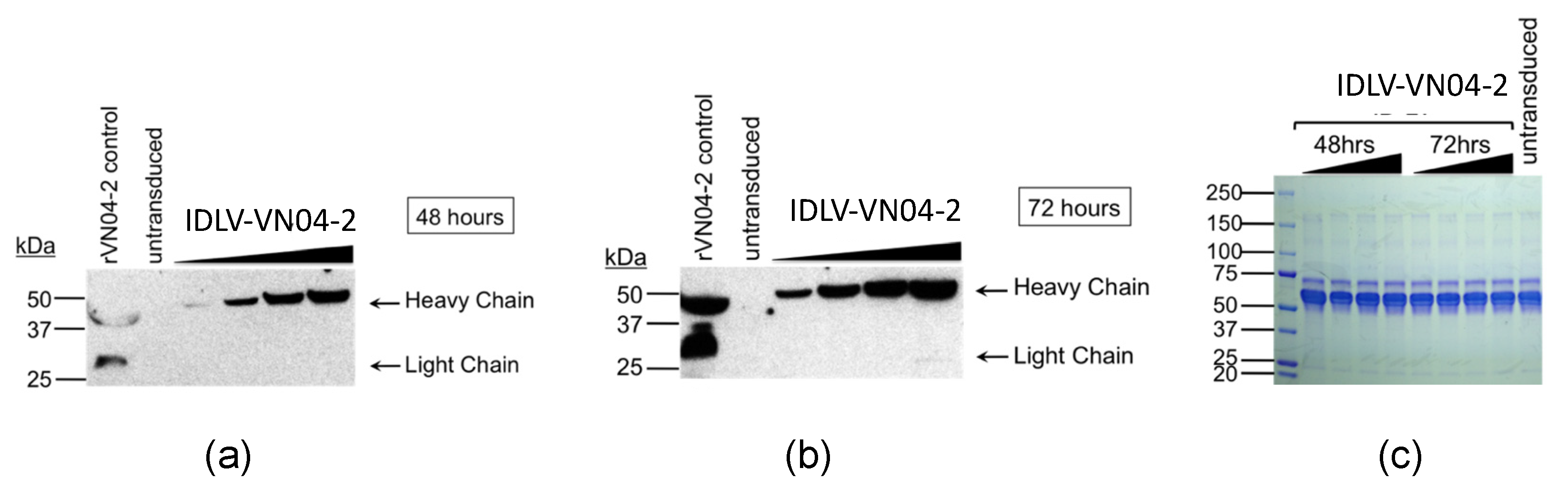
2.2. Western Blot
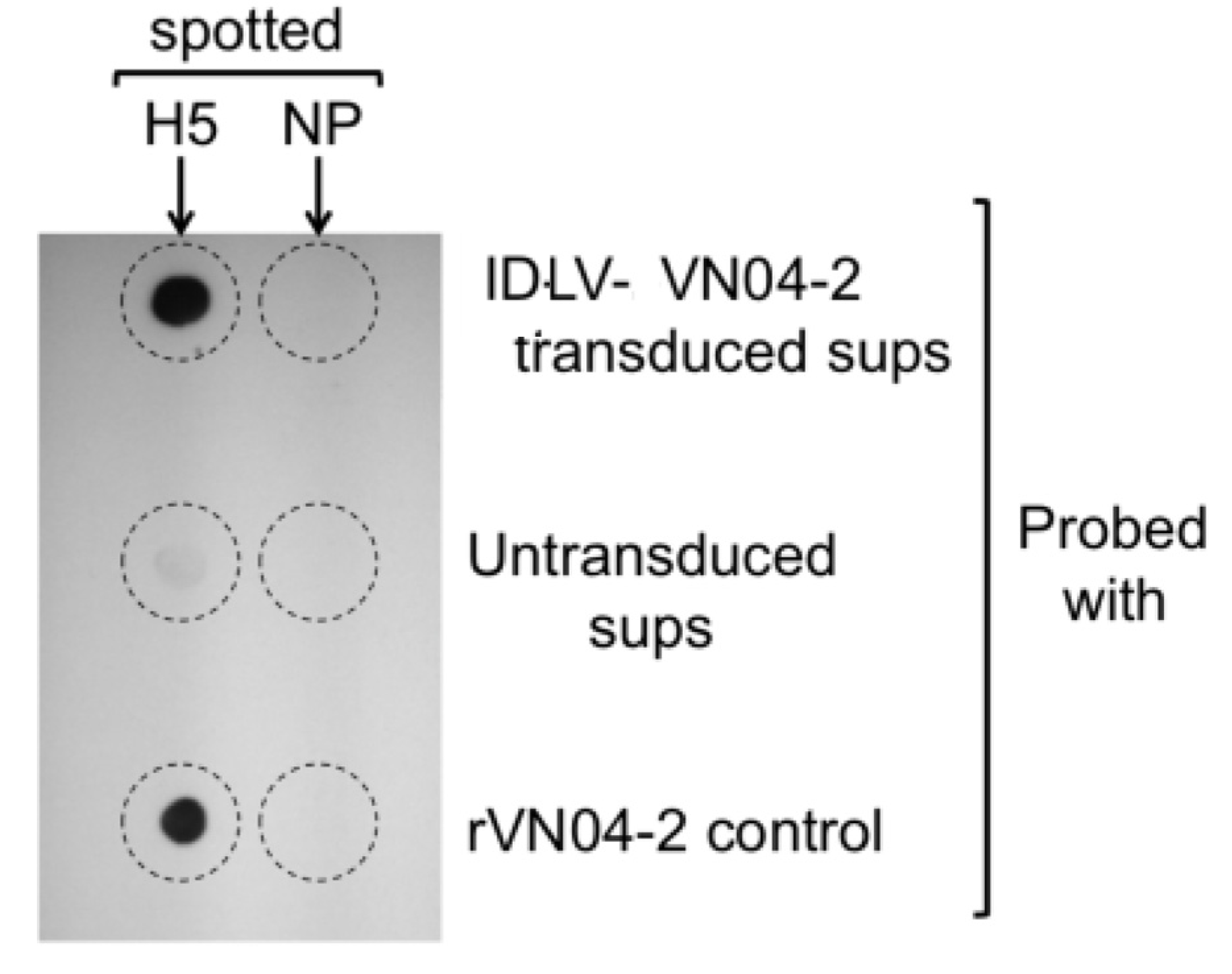
2.3. Dot Blots
2.4. Influenza Viruses
2.5. Mouse Immunizations and Challenge Experiments
2.6. H5 Enzyme-Linked Immunosorbent Assay (ELISA)
2.7. Quantification of IAV RNA
3. Results
3.1. Generation of IDLV Expressing Anti-IAV HA mAbs
3.2. Cells Transduced with IDLV-VN04-2 Produce HA-Binding mAbs in a Time- and Dose-Dependent Fashion
3.3. A Single Administration of IDLV-VN04-2 Induces the Production of mAbs In Vivo
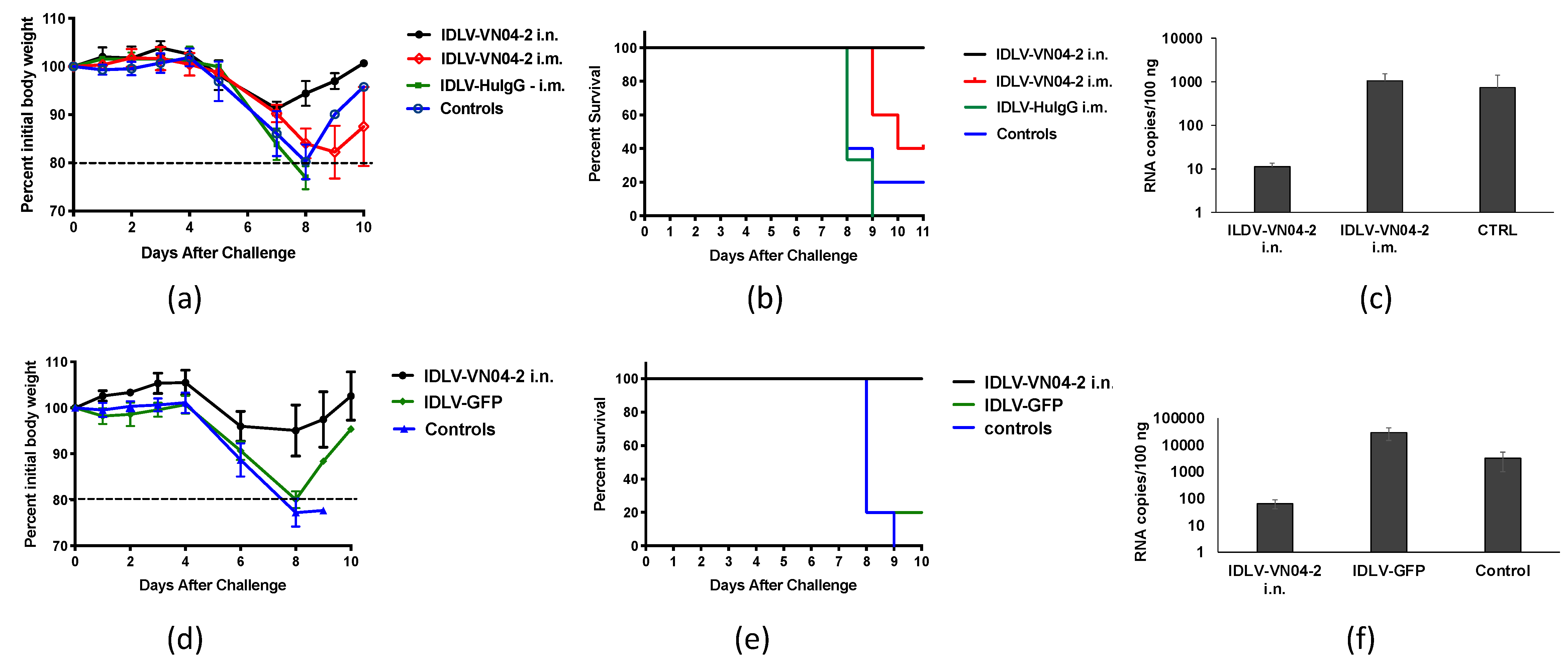
3.4. IDLV-VN04-2 Protects Mice From IAV Challenge
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Luke, T.C.; Casadevall, A.; Watowich, S.J.; Hoffman, S.L.; Beigel, J.H.; Burgess, T.H. Hark back: Passive immunotherapy for influenza and other serious infections. Crit. Care Med. 2010, 38, e66–e73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, D.; Pelegrin, M.; Kramer, S.; Jacquet, C.; Skander, N.; Piechaczyk, M. High in vivo production of a model monoclonal antibody on adenoviral gene transfer. Hum. Gene Ther. 2002, 13, 1483–1493. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Qian, J.J.; Yi, S.; Harding, T.C.; Tu, G.H.; VanRoey, M.; Jooss, K. Stable antibody expression at therapeutic levels using the 2A peptide. Nat. Biotechnol. 2005, 23, 584–590. [Google Scholar] [CrossRef] [PubMed]
- De, B.P.; Hackett, N.R.; Crystal, R.G.; Boyer, J.L. Rapid/sustained anti-anthrax passive immunity mediated by co-administration of Ad/AAV. Mol. Ther. 2008, 16, 203–209. [Google Scholar] [CrossRef] [PubMed]
- BenAmmar-Ceccoli, S.; Humblot, S.; Crouzier, R.; Acres, B.; Kieny, M.P.; Herlyn, D.; Pasquali, J.L.; Martin, T. Recombinant vaccinia viruses expressing immunoglobulin variable regions efficiently and selectively protect mice against tumoral B-cell growth. Cancer Gene Ther. 2001, 8, 815–826. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Shi, W.; Zhang, Q.; Wang, X.; Guo, M.; Cui, Z.; Su, C.; Yang, Q.; Li, Y.; Sham, J.; et al. Gene therapy using adenovirus-mediated full-length anti-HER-2 antibody for HER-2 overexpression cancers. Clin. Cancer Res. 2006, 12, 6179–6185. [Google Scholar] [CrossRef] [Green Version]
- Apolonia, L.; Waddington, S.N.; Fernandes, C.; Ward, N.J.; Bouma, G.; Blundell, M.P.; Thrasher, A.J.; Collins, M.K.; Philpott, N.J. Stable gene transfer to muscle using non-integrating lentiviral vectors. Mol. Ther. 2007, 15, 1947–1954. [Google Scholar] [CrossRef]
- Negri, D.R.; Michelini, Z.; Baroncelli, S.; Spada, M.; Vendetti, S.; Buffa, V.; Bona, R.; Leone, P.; Klotman, M.E.; Cara, A. Successful immunization with a single injection of non-integrating lentiviral vector. Mol. Ther. 2007, 15, 1716–1723. [Google Scholar] [CrossRef]
- Cornu, T.I.; Cathomen, T. Targeted genome modifications using integrase-deficient lentiviral vectors. Mol. Ther. 2007, 15, 2107–2113. [Google Scholar] [CrossRef]
- Moldt, B.; Staunstrup, N.H.; Jakobsen, M.; Yáñez-Muñoz, R.J.; Mikkelsen, J.G. Genomic insertion of lentiviral DNA circles directed by the yeast Flp recombinase. BMC Biotechnol. 2008, 8, 60. [Google Scholar] [CrossRef] [Green Version]
- Nightingale, S.J.; Hollis, R.P.; Pepper, K.A.; Petersen, D.; Yu, X.J.; Yang, C.; Bahner, I.; Kohn, D.B. Transient gene expression by nonintegrating lentiviral vectors. Mol. Ther. 2006, 13, 1121–1132. [Google Scholar] [CrossRef]
- Okada, Y.; Ueshin, Y.; Hasuwa, H.; Takumi, K.; Okabe, M.; Ikawa, M. Targeted gene modification in mouse ES cells using integrase-defective lentiviral vectors. Genesis 2009, 47, 217–223. [Google Scholar] [CrossRef]
- Philippe, S.; Sarkis, C.; Barkats, M.; Mammeri, H.; Ladroue, C.; Petit, C.; Mallet, J.; Serguera, C. Lentiviral vectors with a defective integrase allow efficient and sustained transgene expression in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2006, 103, 17684–17689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staunstrup, N.H.; Moldt, B.; Mátés, L.; Villesen, P.; Jakobsen, M.; Ivics, Z.; Izsvák, Z.; Mikkelsen, J.G. Hybrid lentivirus-transposon vectors with a random integration profile in human cells. Mol. Ther. 2009, 17, 1205–1214. [Google Scholar] [CrossRef] [PubMed]
- Blasi, M.; Negri, D.; LaBranche, C.; Alam, S.M.; Baker, E.J.; Brunner, E.C.; Gladden, M.A.; Michelini, Z.; Vandergrift, N.A.; Wiehe, K.J.; et al. IDLV-HIV-1 Env vaccination in non-human primates induces affinity maturation of antigen-specific memory B cells. Commun. Biol. 2018, 1, 134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michelini, Z.; Negri, D.R.; Baroncelli, S.; Spada, M.; Leone, P.; Bona, R.; Klotman, M.E.; Cara, A. Development and use of SIV-based Integrase defective lentiviral vector for immunization. Vaccine 2009, 27, 4622–4629. [Google Scholar] [CrossRef] [Green Version]
- Cousin, C.; Oberkampf, M.; Felix, T.; Rosenbaum, P.; Weil, R.; Fabrega, S.; Morante, V.; Negri, D.; Cara, A.; Dadaglio, G.; et al. Persistence of Integrase-Deficient Lentiviral Vectors Correlates with the Induction of STING-Independent CD8. Cell Rep. 2019, 26, 1242–1257.e7. [Google Scholar] [CrossRef] [Green Version]
- Negri, D.; Blasi, M.; LaBranche, C.; Parks, R.; Balachandran, H.; Lifton, M.; Shen, X.; Denny, T.; Ferrari, G.; Vescio, M.F.; et al. Immunization with an SIV-based IDLV Expressing HIV-1 Env 1086 Clade C Elicits Durable Humoral and Cellular Responses in Rhesus Macaques. Mol. Ther. 2016, 24, 2021–2032. [Google Scholar] [CrossRef]
- Vasquez, K.M.; Marburger, K.; Intody, Z.; Wilson, J.H. Manipulating the mammalian genome by homologous recombination. Proc. Natl. Acad. Sci. USA 2001, 98, 8403–8410. [Google Scholar] [CrossRef] [Green Version]
- Yáñez, R.J.; Porter, A.C. Influence of DNA delivery method on gene targeting frequencies in human cells. Somat. Cell Mol. Genet. 1999, 25, 27–31. [Google Scholar] [CrossRef]
- Manam, S.; Ledwith, B.J.; Barnum, A.B.; Troilo, P.J.; Pauley, C.J.; Harper, L.B.; Griffiths, T.G.; Niu, Z.; Denisova, L.; Follmer, T.T.; et al. Plasmid DNA vaccines: Tissue distribution and effects of DNA sequence, adjuvants and delivery method on integration into host DNA. Intervirology 2000, 43, 273–281. [Google Scholar] [CrossRef]
- Wang, Z.; Troilo, P.J.; Wang, X.; Griffiths, T.G.; Pacchione, S.J.; Barnum, A.B.; Harper, L.B.; Pauley, C.J.; Niu, Z.; Denisova, L.; et al. Detection of integration of plasmid DNA into host genomic DNA following intramuscular injection and electroporation. Gene Ther. 2004, 11, 711–721. [Google Scholar] [CrossRef]
- Ledwith, B.J.; Manam, S.; Troilo, P.J.; Barnum, A.B.; Pauley, C.J.; Griffiths, T.G.; Harper, L.B.; Beare, C.M.; Bagdon, W.J.; Nichols, W.W. Plasmid DNA vaccines: Investigation of integration into host cellular DNA following intramuscular injection in mice. Intervirology 2000, 43, 258–272. [Google Scholar] [CrossRef]
- Naldini, L.; Blomer, U.; Gallay, P.; Ory, D.; Mulligan, R.; Gage, F.H.; Verma, I.M.; Trono, D. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science 1996, 272, 263–267. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.F.; von Ruden, T.; Kantoff, P.W.; Garber, C.; Seiberg, M.; Ruther, U.; Anderson, W.F.; Wagner, E.F.; Gilboa, E. Self-inactivating retroviral vectors designed for transfer of whole genes into mammalian cells. Proc. Natl. Acad. Sci. USA 1986, 83, 3194–3198. [Google Scholar] [CrossRef] [Green Version]
- Coil, D.A.; Miller, A.D. Phosphatidylserine is not the cell surface receptor for vesicular stomatitis virus. J. Virol. 2004, 78, 10920–10926. [Google Scholar] [CrossRef] [Green Version]
- Breckpot, K.; Aerts, J.L.; Thielemans, K. Lentiviral vectors for cancer immunotherapy: Transforming infectious particles into therapeutics. Gene Ther. 2007, 14, 847–862. [Google Scholar] [CrossRef] [Green Version]
- Kafri, T.; Blomer, U.; Peterson, D.A.; Gage, F.H.; Verma, I.M. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat. Genet. 1997, 17, 314–317. [Google Scholar] [CrossRef]
- Pierson, T.C.; Kieffer, T.L.; Ruff, C.T.; Buck, C.; Gange, S.J.; Siliciano, R.F. Intrinsic stability of episomal circles formed during human immunodeficiency virus type 1 replication. J. Virol. 2002, 76, 4138–4144. [Google Scholar] [CrossRef] [Green Version]
- Butler, S.L.; Johnson, E.P.; Bushman, F.D. Human immunodeficiency virus cDNA metabolism: Notable stability of two-long terminal repeat circles. J. Virol. 2002, 76, 3739–3747. [Google Scholar] [CrossRef] [Green Version]
- Engelman, A.; Englund, G.; Orenstein, J.M.; Martin, M.A.; Craigie, R. Multiple effects of mutations in human immunodeficiency virus type 1 integrase on viral replication. J. Virol. 1995, 69, 2729–2736. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bushman, F. Measuring covert HIV replication during HAART: The abundance of 2-LTR circles is not a reliable marker. Aids 2003, 17, 749–750. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, M.; Haggerty, S.; Lamonica, C.A.; Meier, C.M.; Welch, S.K.; Wasiak, A.J. Integration is not necessary for expression of human immunodeficiency virus type 1 protein products. J. Virol. 1990, 64, 2421–2425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.Y.; Belle, I.; Blasi, M.; Huang, M.N.; Buckley, A.F.; Rountree, W.; Klotman, M.E.; Cara, A.; Negri, D. Skeletal Muscle Is an Antigen Reservoir in Integrase-Defective Lentiviral Vector-Induced Long-Term Immunity. Mol. Ther. Methods Clin. Dev. 2020, 17, 532–544. [Google Scholar] [CrossRef]
- Fontana, J.M.; Christos, P.J.; Michelini, Z.; Negri, D.; Cara, A.; Salvatore, M. Mucosal Immunization with Integrase-Defective Lentiviral Vectors Protects against Influenza Virus Challenge in Mice. PLoS ONE 2014, 9, e97270. [Google Scholar] [CrossRef]
- Hanson, B.J.; Boon, A.C.; Lim, A.P.; Webb, A.; Ooi, E.E.; Webby, R.J. Passive immunoprophylaxis and therapy with humanized monoclonal antibody specific for influenza A H5 hemagglutinin in mice. Respir. Res. 2006, 7, 126. [Google Scholar] [CrossRef] [Green Version]
- Chen, H.; Smith, G.J.; Li, K.S.; Wang, J.; Fan, X.H.; Rayner, J.M.; Vijaykrishna, D.; Zhang, J.X.; Zhang, L.J.; Guo, C.T.; et al. Establishment of multiple sublineages of H5N1 influenza virus in Asia: Implications for pandemic control. Proc. Natl. Acad. Sci. USA 2006, 103, 2845–2850. [Google Scholar] [CrossRef] [Green Version]
- Lim, A.P.; Wong, S.K.; Chan, A.H.; Chan, C.E.; Ooi, E.E.; Hanson, B.J. Epitope characterization of the protective monoclonal antibody VN04-2 shows broadly neutralizing activity against highly pathogenic H5N1. Virol. J. 2008, 5, 80. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, H.; Schwartz, J.P.; Tanaka, K.; Brady, R.O.; Reiser, J. High-titer human immunodeficiency virus type 1-based vector systems for gene delivery into nondividing cells. J. Virol. 1998, 72, 8873–8883. [Google Scholar] [CrossRef] [Green Version]
- Gallinaro, A.; Borghi, M.; Bona, R.; Grasso, F.; Calzoletti, L.; Palladino, L.; Cecchetti, S.; Vescio, M.F.; Macchia, D.; Morante, V.; et al. Integrase Defective Lentiviral Vector as a Vaccine Platform for Delivering Influenza Antigens. Front. Immunol. 2018, 9, 171. [Google Scholar] [CrossRef]
- Sanders, J.W.; Ponzio, T.A. Vectored immunoprophylaxis: An emerging adjunct to traditional vaccination. Trop. Dis. Travel. Med. Vaccines 2017, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rawlins, E.L.; Hogan, B.L. Ciliated epithelial cell lifespan in the mouse trachea and lung. Am. J. Physiol. Lung Cell. Mol. Physiol. 2008, 295, L231–L234. [Google Scholar] [CrossRef] [PubMed]
- Walzl, G.; Tafuro, S.; Moss, P.; Openshaw, P.J.; Hussell, T. Influenza virus lung infection protects from respiratory syncytial virus-induced immunopathology. J. Exp. Med. 2000, 192, 1317–1326. [Google Scholar] [CrossRef] [Green Version]
- Hua, X.; Vijay, R.; Channappanavar, R.; Athmer, J.; Meyerholz, D.K.; Pagedar, N.; Tilley, S.; Perlman, S. Nasal priming by a murine coronavirus provides protective immunity against lethal heterologous virus pneumonia. JCI Insight 2018, 3, e99025. [Google Scholar] [CrossRef] [Green Version]
- Gomi, R.; Sharma, A.; Wu, W.; Worgall, S. Neonatal Genetic Delivery of Anti-Respiratory Syncytial Virus (RSV) Antibody by Non-Human Primate-Based Adenoviral Vector to Provide Protection against RSV. Vaccines 2019, 7, 3. [Google Scholar] [CrossRef] [Green Version]
- Mátrai, J.; Cantore, A.; Bartholomae, C.C.; Annoni, A.; Wang, W.; Acosta-Sanchez, A.; Samara-Kuko, E.; De Waele, L.; Ma, L.; Genovese, P.; et al. Hepatocyte-targeted expression by integrase-defective lentiviral vectors induces antigen-specific tolerance in mice with low genotoxic risk. Hepatology 2011, 53, 1696–1707. [Google Scholar] [CrossRef] [Green Version]
- Copreni, E.; Palmieri, L.; Castellani, S.; Conese, M. A VSV-G Pseudotyped Last Generation Lentiviral Vector Mediates High Level and Persistent Gene Transfer in Models of Airway Epithelium In Vitro and In Vivo. Viruses 2010, 2, 1577–1588. [Google Scholar] [CrossRef] [Green Version]
- Saunders, K.O. Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life. Front. Immunol. 2019, 10, 1296. [Google Scholar] [CrossRef]
- Sridhar, S.; Begom, S.; Bermingham, A.; Hoschler, K.; Adamson, W.; Carman, W.; Bean, T.; Barclay, W.; Deeks, J.J.; Lalvani, A. Cellular immune correlates of protection against symptomatic pandemic influenza. Nat. Med. 2013, 19, 1305–1312. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelini, Z.; Minkoff, J.M.; Yang, J.; Negri, D.; Cara, A.; Hanson, B.J.; Salvatore, M. Integrase-Defective Lentiviral Vectors for Delivery of Monoclonal Antibodies against Influenza. Viruses 2020, 12, 1460. https://doi.org/10.3390/v12121460
Michelini Z, Minkoff JM, Yang J, Negri D, Cara A, Hanson BJ, Salvatore M. Integrase-Defective Lentiviral Vectors for Delivery of Monoclonal Antibodies against Influenza. Viruses. 2020; 12(12):1460. https://doi.org/10.3390/v12121460
Chicago/Turabian StyleMichelini, Zuleika, Judith M. Minkoff, Jianjun Yang, Donatella Negri, Andrea Cara, Brendon J. Hanson, and Mirella Salvatore. 2020. "Integrase-Defective Lentiviral Vectors for Delivery of Monoclonal Antibodies against Influenza" Viruses 12, no. 12: 1460. https://doi.org/10.3390/v12121460
APA StyleMichelini, Z., Minkoff, J. M., Yang, J., Negri, D., Cara, A., Hanson, B. J., & Salvatore, M. (2020). Integrase-Defective Lentiviral Vectors for Delivery of Monoclonal Antibodies against Influenza. Viruses, 12(12), 1460. https://doi.org/10.3390/v12121460





