Discovery of Lanama Virus, a Distinct Member of Species Kunsagivirus C (Picornavirales: Picornaviridae), in Wild Vervet Monkeys (Chlorocebus pygerythrus)
Abstract
:1. Introduction
2. Materials and Methods
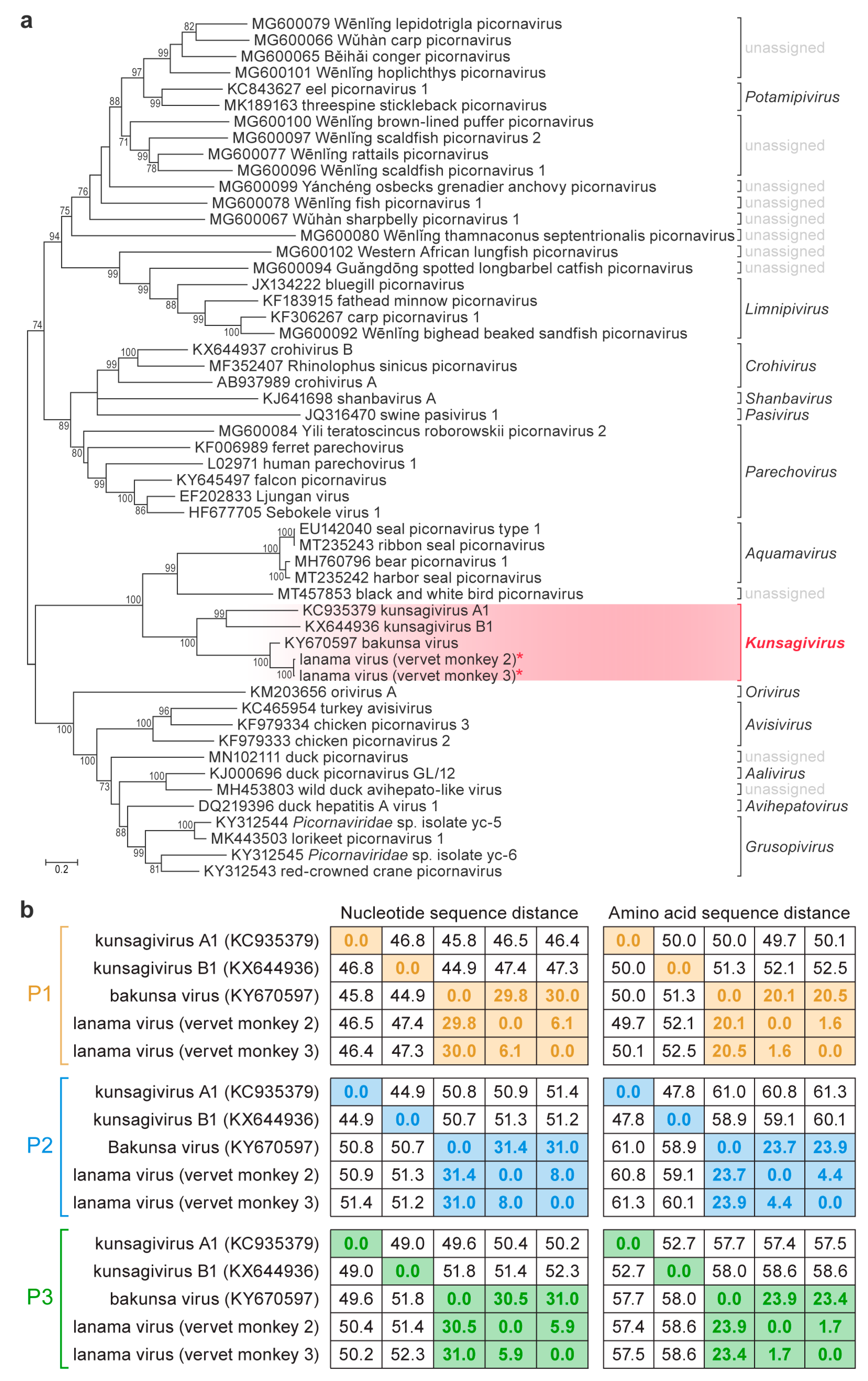
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Koonin, E.V.; Dolja, V.V.; Krupovic, M.; Arvind, V.; Wolf, Y.I.; Yutin, N.; Zerbini, F.M.; Kuhn, J.H. Global organization and proposed megataxonomy of the virus world. Microbiol. Mol. Biol. Rev. 2020, 84, e00061-19. [Google Scholar] [CrossRef] [PubMed]
- Zell, R.; Delwart, E.; Gorbalenya, A.E.; Hovi, T.; King, A.M.Q.; Knowles, N.J.; Lindberg, A.M.; Pallansch, M.A.; Palmenberg, A.C.; Reuter, G.; et al. ICTV virus taxonomy profile: Picornaviridae. J. Gen. Virol. 2017, 98, 2421–2422. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Zell, R. Picornaviridae—The ever-growing virus family. Arch. Virol. 2018, 163, 299–317. [Google Scholar] [CrossRef] [PubMed]
- Yinda, C.K.; Zell, R.; Deboutte, W.; Zeller, M.; Conceição-Neto, N.; Heylen, E.; Maes, P.; Knowles, N.J.; Ghogomu, S.M.; van Ranst, M.; et al. Highly diverse population of Picornaviridae and other members of the Picornavirales, in Cameroonian fruit bats. BMC Genom. 2017, 18, 249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boros, Á.; Kiss, T.; Kiss, O.; Pankovics, P.; Kapusinszky, B.; Delwart, E.; Reuter, G. Genetic characterization of a novel picornavirus distantly related to the marine mammal-infecting aquamaviruses in a long-distance migrant bird species, European roller (Coracias garrulus). J. Gen. Virol. 2013, 94, 2029–2035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buechler, C.R.; Bailey, A.L.; Lauck, M.; Heffron, A.; Johnson, J.C.; Campos Lawson, C.; Rogers, J.; Kuhn, J.H.; O’Connor, D.H. Genome sequence of a novel kunsagivirus (Picornaviridae: Kunsagivirus) from a wild baboon (Papio cynocephalus). Genome Announc. 2017, 5, e00261-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valenta, K.; Twinomugisha, D.; Godfrey, K.; Liu, C.; Schoof, V.A.M.; Goldberg, T.L.; Chapman, C.A. Comparison of gastrointestinal parasite communities in vervet monkeys. Integr. Zool. 2017, 12, 512–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Svardal, H.; Jasinska, A.J.; Apetrei, C.; Coppola, G.; Huang, Y.; Schmitt, C.A.; Jacquelin, B.; Ramensky, V.; Müller-Trutwin, M.; Antonio, M.; et al. Ancient hybridization and strong adaptation to viruses across African vervet monkey populations. Nat. Genet. 2017, 49, 1705–1713. [Google Scholar] [CrossRef] [Green Version]
- Butynski, T.M.; de Jong, Y.A. Chlorocebus pygerythrus. IUCN Red List Threat. Species 2019, e.T136271A17957823. [Google Scholar] [CrossRef]
- Lauck, M.; Sibley, S.D.; Hyeroba, D.; Tumukunde, A.; Weny, G.; Chapman, C.A.; Ting, N.; Switzer, W.M.; Kuhn, J.H.; Friedrich, T.C.; et al. Exceptional simian hemorrhagic fever virus diversity in a wild African primate community. J. Virol. 2013, 87, 688–691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lauck, M.; Hyeroba, D.; Tumukunde, A.; Weny, G.; Lank, S.M.; Chapman, C.A.; O’Connor, D.H.; Friedrich, T.C.; Goldberg, T.L. Novel, divergent simian hemorrhagic fever viruses in a wild Ugandan red colobus monkey discovered using direct pyrosequencing. PLoS ONE 2011, 6, e19056. [Google Scholar] [CrossRef] [PubMed]
- Scully, E.J.; Basnet, S.; Wrangham, R.W.; Muller, M.N.; Otali, E.; Hyeroba, D.; Grindle, K.A.; Pappas, T.E.; Thompson, M.E.; Machanda, Z.; et al. Lethal respiratory disease associated with human rhinovirus C in wild chimpanzees, Uganda, 2013. Emerg. Infect. Dis. 2018, 24, 267–274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J.; Clustal, W. Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user friendly biological sequence alignment editor and analyses program for windows 95/98/NT. Nucl. Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Le, S.Q.; Gascuel, O. An improved general amino acid replacement matrix. Mol. Biol. Evol. 2008, 25, 1307–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donnelly, M.L.L.; Luke, G.; Mehrotra, A.; Li, X.; Hughes, L.E.; Gani, D.; Ryan, M.D. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: A putative ribosomal ‘skip’. J. Gen. Virol. 2001, 82, 1013–1025. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhn, J.H.; Sibley, S.D.; Chapman, C.A.; Knowles, N.J.; Lauck, M.; Johnson, J.C.; Lawson, C.C.; Lackemeyer, M.G.; Valenta, K.; Omeja, P.; et al. Discovery of Lanama Virus, a Distinct Member of Species Kunsagivirus C (Picornavirales: Picornaviridae), in Wild Vervet Monkeys (Chlorocebus pygerythrus). Viruses 2020, 12, 1436. https://doi.org/10.3390/v12121436
Kuhn JH, Sibley SD, Chapman CA, Knowles NJ, Lauck M, Johnson JC, Lawson CC, Lackemeyer MG, Valenta K, Omeja P, et al. Discovery of Lanama Virus, a Distinct Member of Species Kunsagivirus C (Picornavirales: Picornaviridae), in Wild Vervet Monkeys (Chlorocebus pygerythrus). Viruses. 2020; 12(12):1436. https://doi.org/10.3390/v12121436
Chicago/Turabian StyleKuhn, Jens H., Samuel D. Sibley, Colin A. Chapman, Nick J. Knowles, Michael Lauck, Joshua C. Johnson, Cristine Campos Lawson, Matthew G. Lackemeyer, Kim Valenta, Patrick Omeja, and et al. 2020. "Discovery of Lanama Virus, a Distinct Member of Species Kunsagivirus C (Picornavirales: Picornaviridae), in Wild Vervet Monkeys (Chlorocebus pygerythrus)" Viruses 12, no. 12: 1436. https://doi.org/10.3390/v12121436
APA StyleKuhn, J. H., Sibley, S. D., Chapman, C. A., Knowles, N. J., Lauck, M., Johnson, J. C., Lawson, C. C., Lackemeyer, M. G., Valenta, K., Omeja, P., Jahrling, P. B., O’Connor, D. H., & Goldberg, T. L. (2020). Discovery of Lanama Virus, a Distinct Member of Species Kunsagivirus C (Picornavirales: Picornaviridae), in Wild Vervet Monkeys (Chlorocebus pygerythrus). Viruses, 12(12), 1436. https://doi.org/10.3390/v12121436






