Identification of the Cleavage Domain within Glycoprotein G of Herpes Simplex Virus Type 2
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Plasmids
2.3. Transfection of DNA into Eukaryotic Cells
2.4. Western Blot
2.5. Liquid Chromatography–Mass Spectrometry (LC–MS)
2.6. Furin Inhibition
3. Results
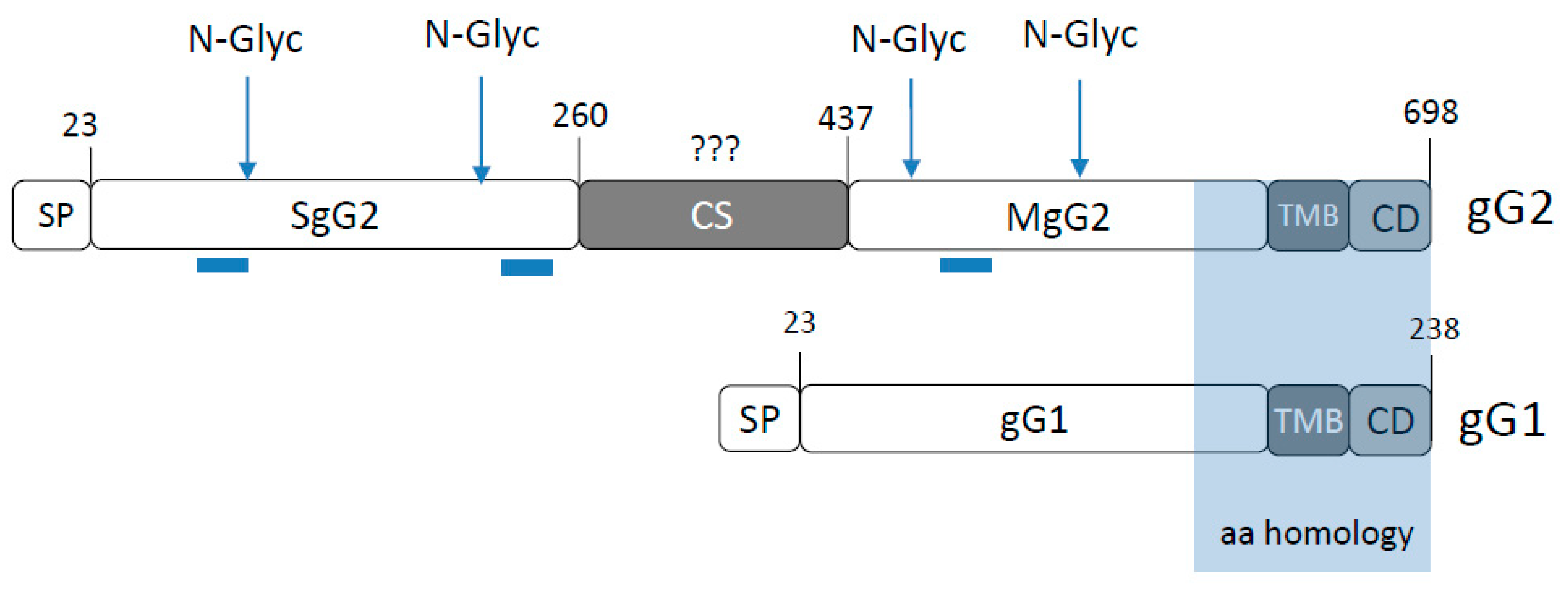
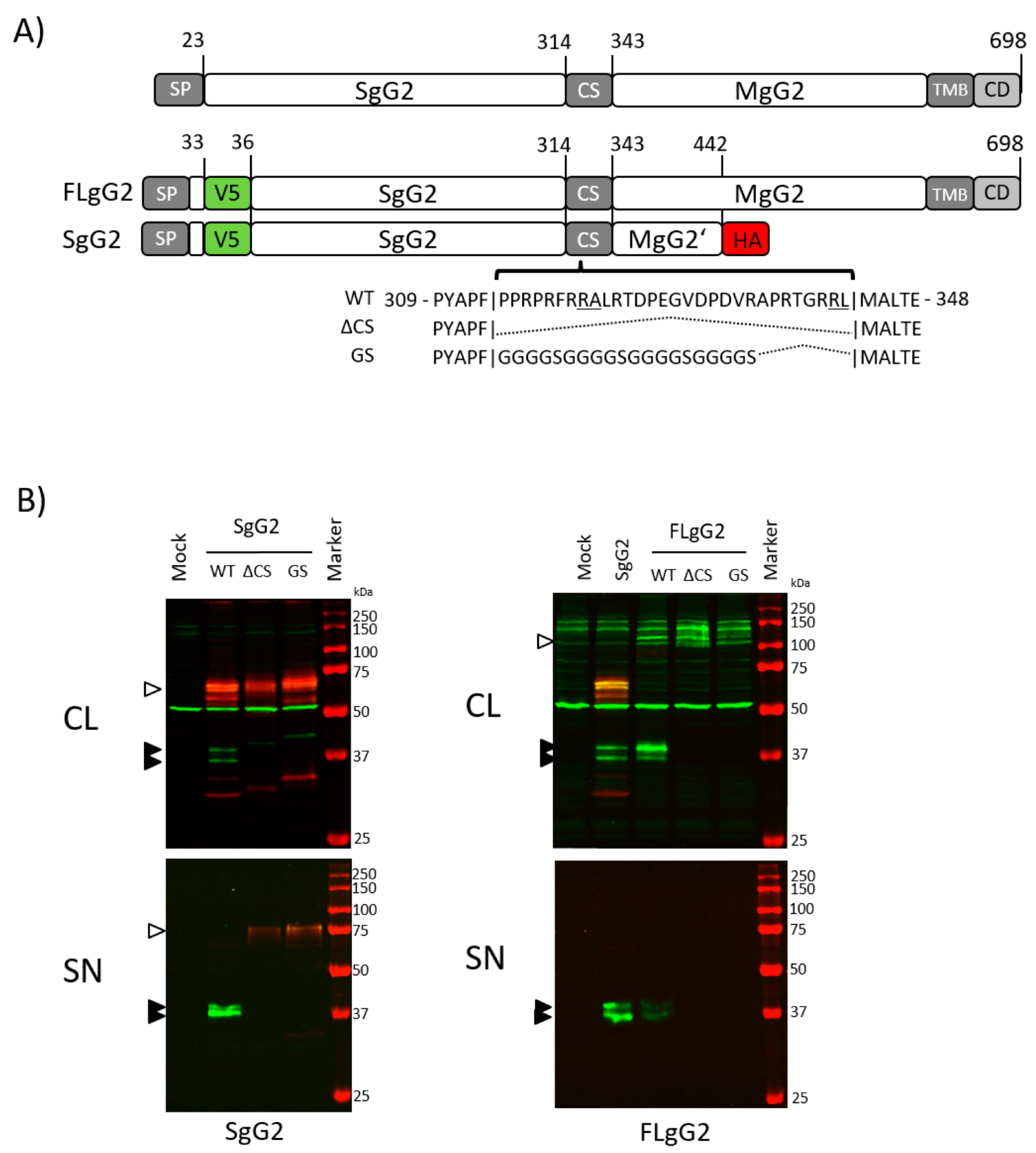
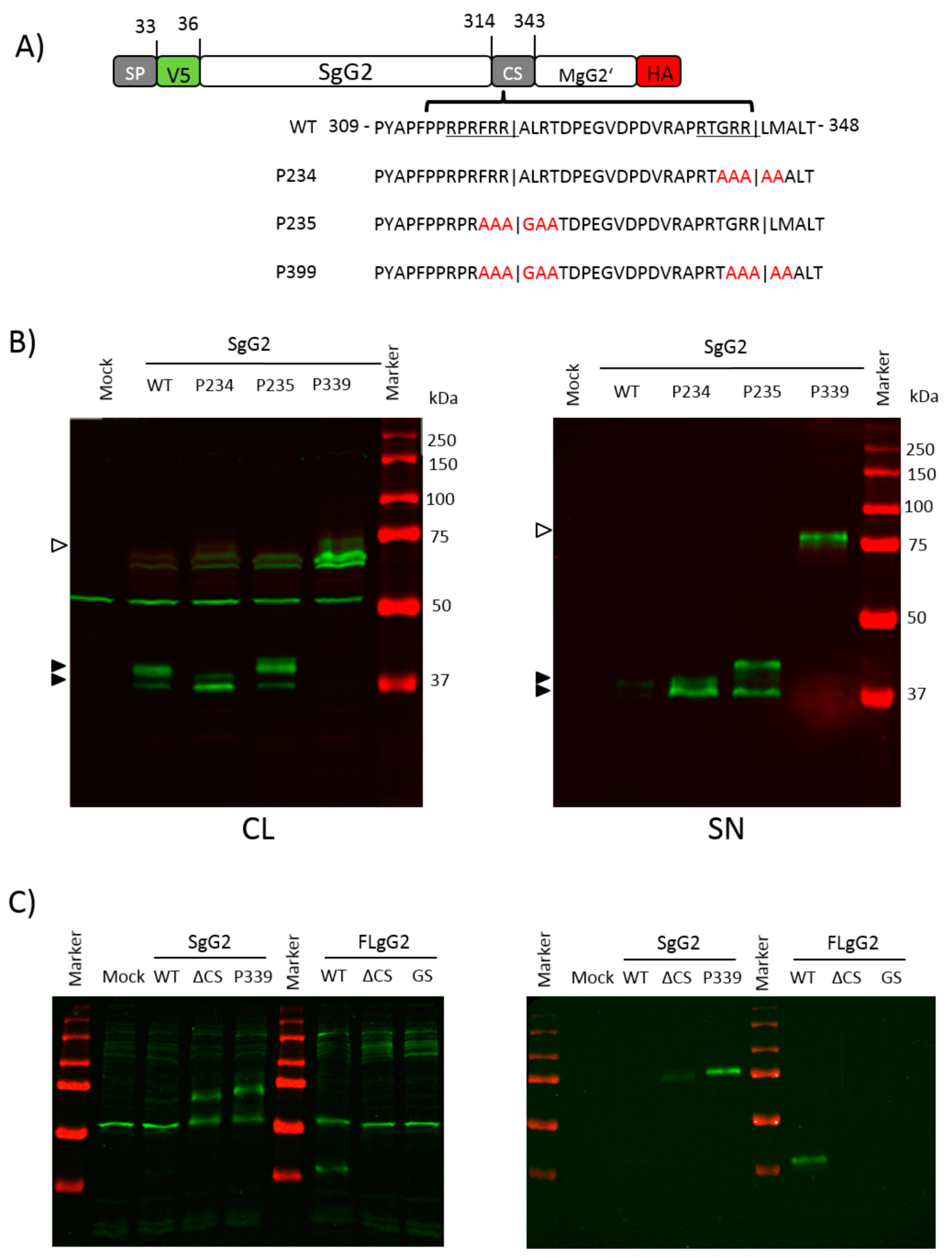
3.1. The Region Encompassing Residues 314–343 is Responsible for gG2 Cleavage
3.2. Transfer of the CS Sequence to Reporter Proteins Results in Cleavage
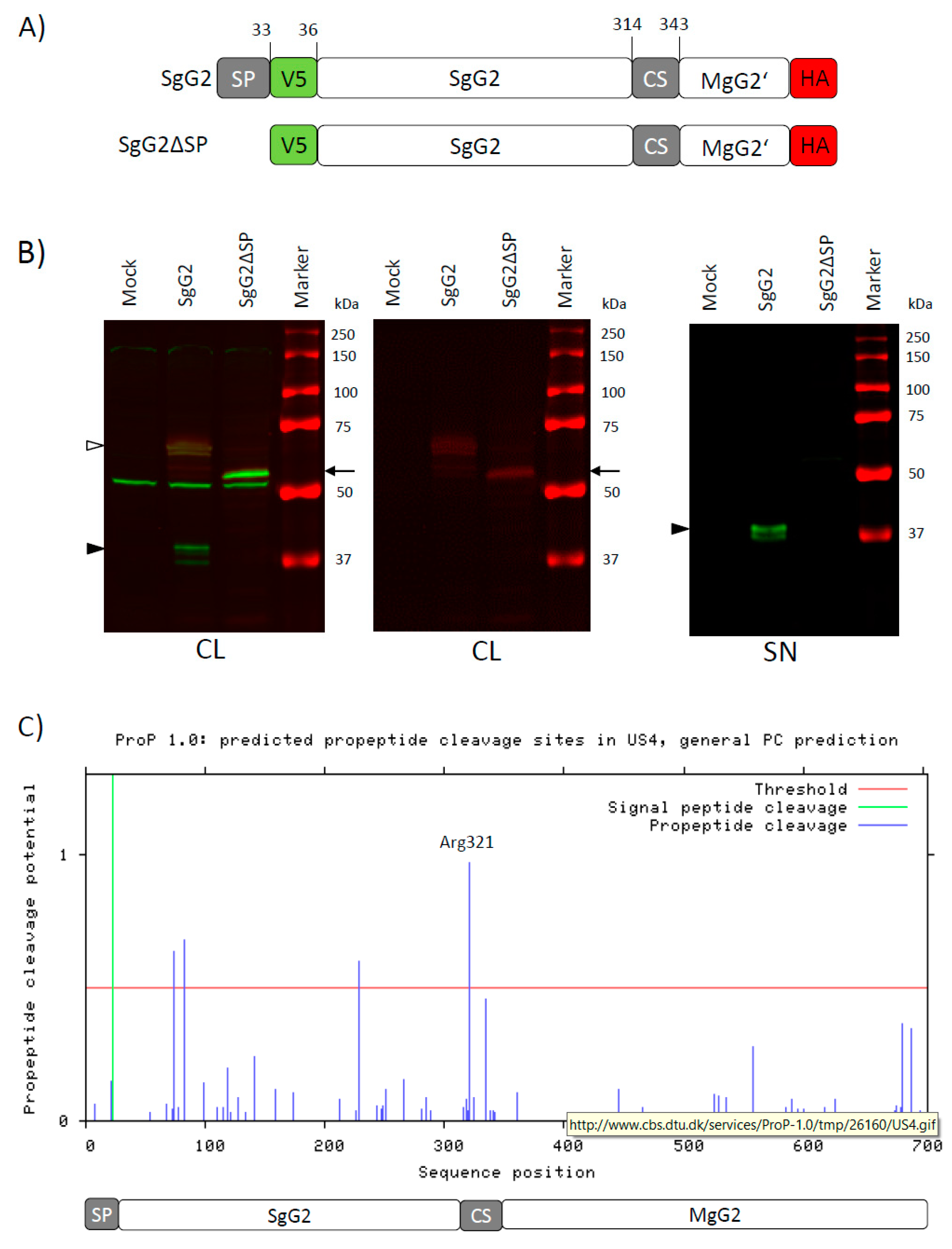
3.3. The CS Sequence Contains a Perfect Furin Consensus Motif
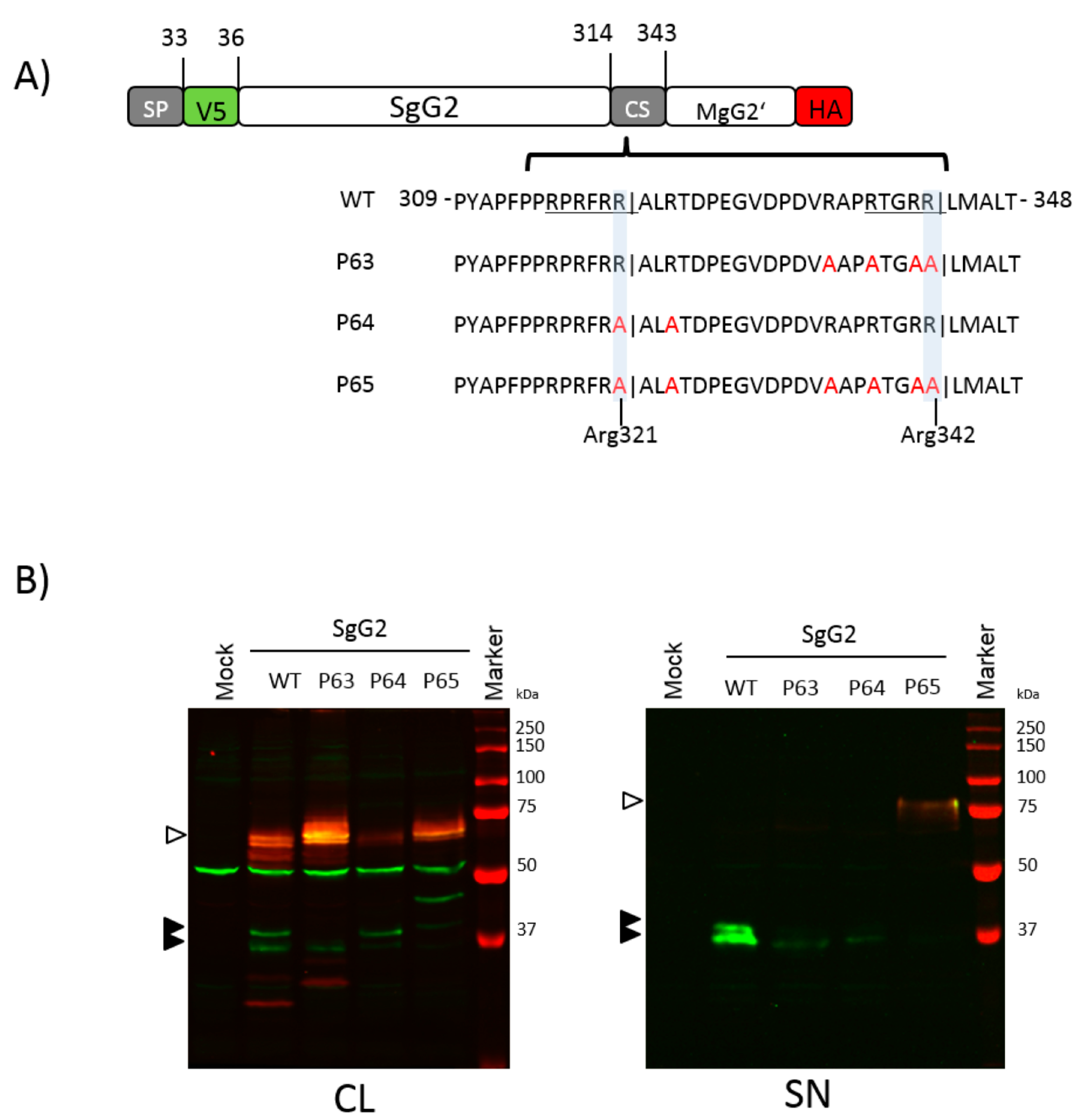
3.4. The Amino Acid Region 314 to 343 Contains Two Functional Cleavage Motifs
3.5. Imperfect Cleavage Motifs within the CS Region Are also Employed
3.6. Furin is not Essential for SgG2 Processing
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.E.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and Regional Estimates of Prevalent and Incident Herpes Simplex Virus Type 1 Infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef]
- Looker, K.J.; Magaret, A.S.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global estimates of prevalent and incident herpes simplex virus type 2 infections in 2012. PLoS ONE 2015, 10, e114989. [Google Scholar] [CrossRef]
- Richman, D.D.; Buckmaster, A.; Bell, S.; Hodgman, C.; Minson, A.C. Identification of a new glycoprotein of herpes simplex virus type 1 and genetic mapping of the gene that codes for it. J. Virol. 1986, 57, 647–655. [Google Scholar] [CrossRef]
- Balachandran, N.; Hutt-Fletcher, L.M. Synthesis and processing of glycoprotein gG of herpes simplex virus type 2. J. Virol. 1985, 54, 825–832. [Google Scholar] [CrossRef]
- Su, H.K.; Eberle, R.; Courtney, R.J. Processing of the herpes simplex virus type 2 glycoprotein gG-2 results in secretion of a 34,000-Mr cleavage product. J. Virol. 1987, 61, 1735–1737. [Google Scholar] [CrossRef]
- Su, H.K.; Fetherston, J.D.; Smith, M.E.; Courtney, R.J. Orientation of the cleavage site of the herpes simplex virus glycoprotein G-2. J. Virol. 1993, 67, 2954–2959. [Google Scholar] [CrossRef]
- Liljeqvist, J.A.; Svennerholm, B.; Bergstrom, T. Herpes simplex virus type 2 glycoprotein G-negative clinical isolates are generated by single frameshift mutations. J. Virol. 1999, 73, 9796–9802. [Google Scholar] [CrossRef]
- Viejo-Borbolla, A.; Martinez-Martín, N.; Nel, H.J.; Rueda, P.; Martín, R.; Blanco, S.; Arenzana-Seisdedos, F.; Thelen, M.; Fallon, P.G.; Alcamí, A. Enhancement of Chemokine Function as an Immunomodulatory Strategy Employed by Human Herpesviruses. PLoS Pathog. 2012, 8, e1002497. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Viejo-Borbolla, A.; Martín, R.; Blanco, S.; Benovic, J.L.; Thelen, M.; Alcamí, A. Herpes simplex virus enhances chemokine function through modulation of receptor trafficking and oligomerization. Nat. Commun. 2015, 6, 61–63. [Google Scholar] [CrossRef]
- Martínez-Martín, N.; Viejo-Borbolla, A.; Alcamí, A. Herpes simplex virus particles interact with chemokines and enhance cell migration. J. Gen. Virol. 2016, 97, 3007–3016. [Google Scholar] [CrossRef]
- Cabrera, J.R.; Viejo-Borbolla, A.; Martinez-Martin, N.; Blanco, S.; Wandosell, F.; Alcami, A. Secreted herpes simplex virus-2 glycoprotein G modifies NGF-TrkA signaling to attract free nerve endings to the site of infection. PLoS Pathog. 2015, 11, e1004571. [Google Scholar] [CrossRef]
- Kropp, K.A.; López-Muñoz, A.D.; Ritter, B.; Martín, R.; Rastrojo, A.; Srivaratharajan, S.; Döhner, K.; Dhingra, A.; Czechowicz, J.S.; Nagel, C.-H.; et al. Herpes Simplex Virus Type 2 Counteracts Neurite Outgrowth Repulsion During Infection In A Nerve Growth Factor-Dependent Manner. J. Virol. 2020, 94, JVI.01370-20. [Google Scholar] [CrossRef]
- Izaguirre, G. The Proteolytic Regulation of Virus Cell Entry by Furin and Other Proprotein Convertases. Viruses 2019, 11, 837. [Google Scholar] [CrossRef]
- Seidah, N.G.; Chrétien, M. Eukaryotic protein processing: Endoproteolysis of precursor proteins. Curr. Opin. Biotechnol. 1997, 8, 602–607. [Google Scholar] [CrossRef]
- Meseda, C.A.; Schmeisser, F.; Pedersen, R.; Woerner, A.; Weir, J.P. DNA immunization with a herpes simplex virus 2 bacterial artificial chromosome. Virology 2004, 318, 420–428. [Google Scholar] [CrossRef]
- Lodermeyer, V.; Suhr, K.; Schrott, N.; Kolbe, C.; Stuerzel, C.M.; Krnavek, D.; Münch, J.; Dietz, C.; Waldmann, T.; Kirchhoff, F.; et al. 90K, an interferon-stimulated gene product, reduces the infectivity of HIV-1. Retrovirology 2013, 10, 111. [Google Scholar] [CrossRef]
- Bohne, J.; Wodrich, H.; Kräusslich, H.-G. Splicing of human immunodeficiency virus RNA is position-dependent suggesting sequential removal of introns from the 5′ end. Nucleic Acids Res. 2005, 33, 825–837. [Google Scholar] [CrossRef]
- Jochim, N.; Gerhard, R.; Just, I.; Pich, A. Impact of clostridial glucosylating toxins on the proteome of colonic cells determined by isotope-coded protein labeling and LC-MALDI. Proteome Sci. 2011, 9, 48. [Google Scholar] [CrossRef]
- Dall’Olio, F.; Malagolini, N.; Campadelli-Fiume, G.; Serafini-Cessi, F. Glycosylation pattern of herpes simplex virus type 2 glycoprotein G from precursor species to the mature form. Arch. Virol. 1987, 97, 237–249. [Google Scholar] [CrossRef]
- Wurdinger, T.; E Badr, C.; Pike, L.; De Kleine, R.; Weissleder, R.; Breakefield, X.O.; Tannous, B.A. A secreted luciferase for ex vivo monitoring of in vivo processes. Nat. Methods 2008, 5, 171–173. [Google Scholar] [CrossRef]
- Luft, C.; Freeman, J.; Elliott, D.; Al-Tamimi, N.; Kriston-Vizi, J.; Heintze, J.; Lindenschmidt, I.; Seed, B.; Ketteler, R. Application of Gaussia luciferase in bicistronic and non-conventional secretion reporter constructs. BMC Biochem. 2014, 15, 14. [Google Scholar] [CrossRef]
- Atkins, J.F.; Wills, N.M.; Loughran, G.; Wu, C.-Y.; Parsawar, K.; Ryan, M.D.; Wang, C.-H.; Nelson, C.C. A case for “StopGo”: Reprogramming translation to augment codon meaning of GGN by promoting unconventional termination (Stop) after addition of glycine and then allowing continued translation (Go). RNA 2007, 13, 803–810. [Google Scholar] [CrossRef]
- Donnelly, M.L.L.; Luke, G.; Mehrotra, A.; Li, X.; Hughes, L.E.; Gani, D.; Ryan, M.D. Analysis of the aphthovirus 2A/2B polyprotein ‘cleavage’ mechanism indicates not a proteolytic reaction, but a novel translational effect: A putative ribosomal ‘skip’. J. Gen. Virol. 2001, 82, 1013–1025. [Google Scholar] [CrossRef]
- Weldon, S.K.; Su, H.K.; Fetherston, J.D.; Courtney, R.J. In vitro synthesis and processing of herpes simplex virus type 2 gG-2, using cell-free transcription and translation systems. J. Virol. 1990, 64, 1357–1359. [Google Scholar] [CrossRef]
- Duckert, P.; Brunak, S.; Blom, N. Prediction of proprotein convertase cleavage sites. Protein Eng. Des. Sel. 2004, 17, 107–112. [Google Scholar] [CrossRef]
- Takahashi, S.; Kasai, K.; Hatsuzawa, K.; Kitamura, N.; Misumi, Y.; Ikehara, Y.; Murakami, K.; Nakayama, K. A Mutation of Furin Causes the Lack of Precursor-Processing Activity in Human Colon Carcinoma LoVo Cells. Biochem. Biophys. Res. Commun. 1993, 195, 1019–1026. [Google Scholar] [CrossRef]
- Adamiak, B.; Ekblad, M.; Bergström, T.; Ferro, V.; Trybala, E. Herpes Simplex Virus Type 2 Glycoprotein G Is Targeted by the Sulfated Oligo- and Polysaccharide Inhibitors of Virus Attachment to Cells. J. Virol. 2007, 81, 13424–13434. [Google Scholar] [CrossRef]
- Ryan, M.D.; King, A.M.Q.; Thomas, G.P. Cleavage of foot-and-mouth disease virus polyprotein is mediated by residues located within a 19 amino acid sequence. J. Gen. Virol. 1991, 72, 2727–2732. [Google Scholar] [CrossRef]
- Couture, F.; D’Anjou, F.; Day, R. On the cutting edge of proprotein convertase pharmacology: From molecular concepts to clinical applications. Biomol. Concepts 2011, 2, 421–438. [Google Scholar] [CrossRef]
- Basak, A. Inhibitors of proprotein convertases. J. Mol. Med. 2005, 83, 844–855. [Google Scholar] [CrossRef]
- Bessonnard, S.; Mesnard, D.; Constam, D.B. PC7 and the related proteases Furin and Pace4 regulate E-cadherin function during blastocyst formation. J. Cell Biol. 2015, 210, 1185–1197. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Shirato, K.; Kawase, M.; Terada, Y.; Kawachi, K.; Fukushi, S.; Kamitani, W. Middle East Respiratory Syndrome Coronavirus Spike Protein Is Not Activated Directly by Cellular Furin during Viral Entry into Target Cells. J. Virol. 2018, 92, 19. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kropp, K.A.; Srivaratharajan, S.; Ritter, B.; Yu, P.; Krooss, S.; Polten, F.; Pich, A.; Alcami, A.; Viejo-Borbolla, A. Identification of the Cleavage Domain within Glycoprotein G of Herpes Simplex Virus Type 2. Viruses 2020, 12, 1428. https://doi.org/10.3390/v12121428
Kropp KA, Srivaratharajan S, Ritter B, Yu P, Krooss S, Polten F, Pich A, Alcami A, Viejo-Borbolla A. Identification of the Cleavage Domain within Glycoprotein G of Herpes Simplex Virus Type 2. Viruses. 2020; 12(12):1428. https://doi.org/10.3390/v12121428
Chicago/Turabian StyleKropp, Kai A., Sangar Srivaratharajan, Birgit Ritter, Pengfei Yu, Simon Krooss, Felix Polten, Andreas Pich, Antonio Alcami, and Abel Viejo-Borbolla. 2020. "Identification of the Cleavage Domain within Glycoprotein G of Herpes Simplex Virus Type 2" Viruses 12, no. 12: 1428. https://doi.org/10.3390/v12121428
APA StyleKropp, K. A., Srivaratharajan, S., Ritter, B., Yu, P., Krooss, S., Polten, F., Pich, A., Alcami, A., & Viejo-Borbolla, A. (2020). Identification of the Cleavage Domain within Glycoprotein G of Herpes Simplex Virus Type 2. Viruses, 12(12), 1428. https://doi.org/10.3390/v12121428





