Comparison of Rapid Antigen Tests for COVID-19
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics and Biosafety Statements
2.2. Cells
2.3. Viruses
2.4. Clinical Samples
2.5. RT-qPCR
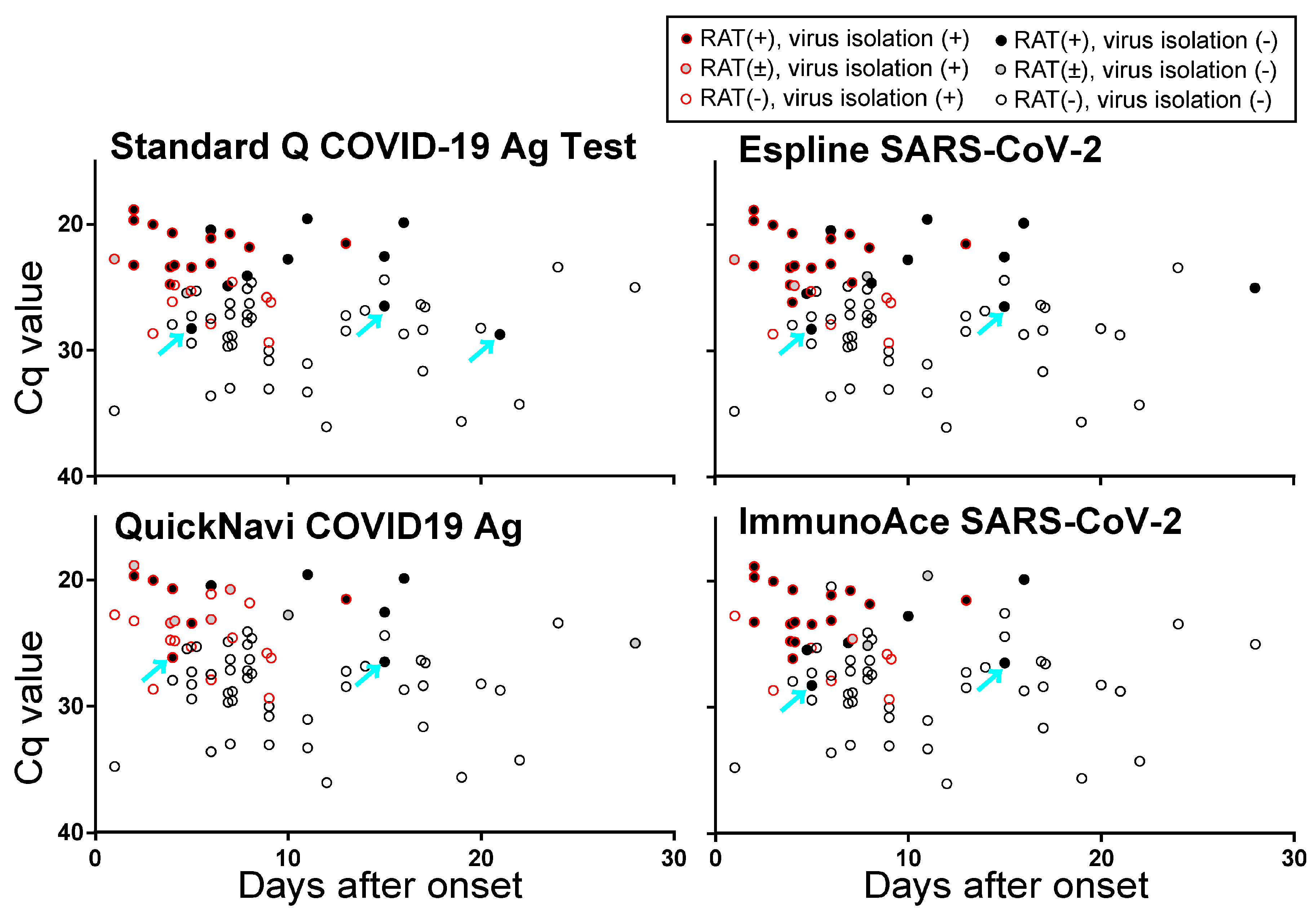
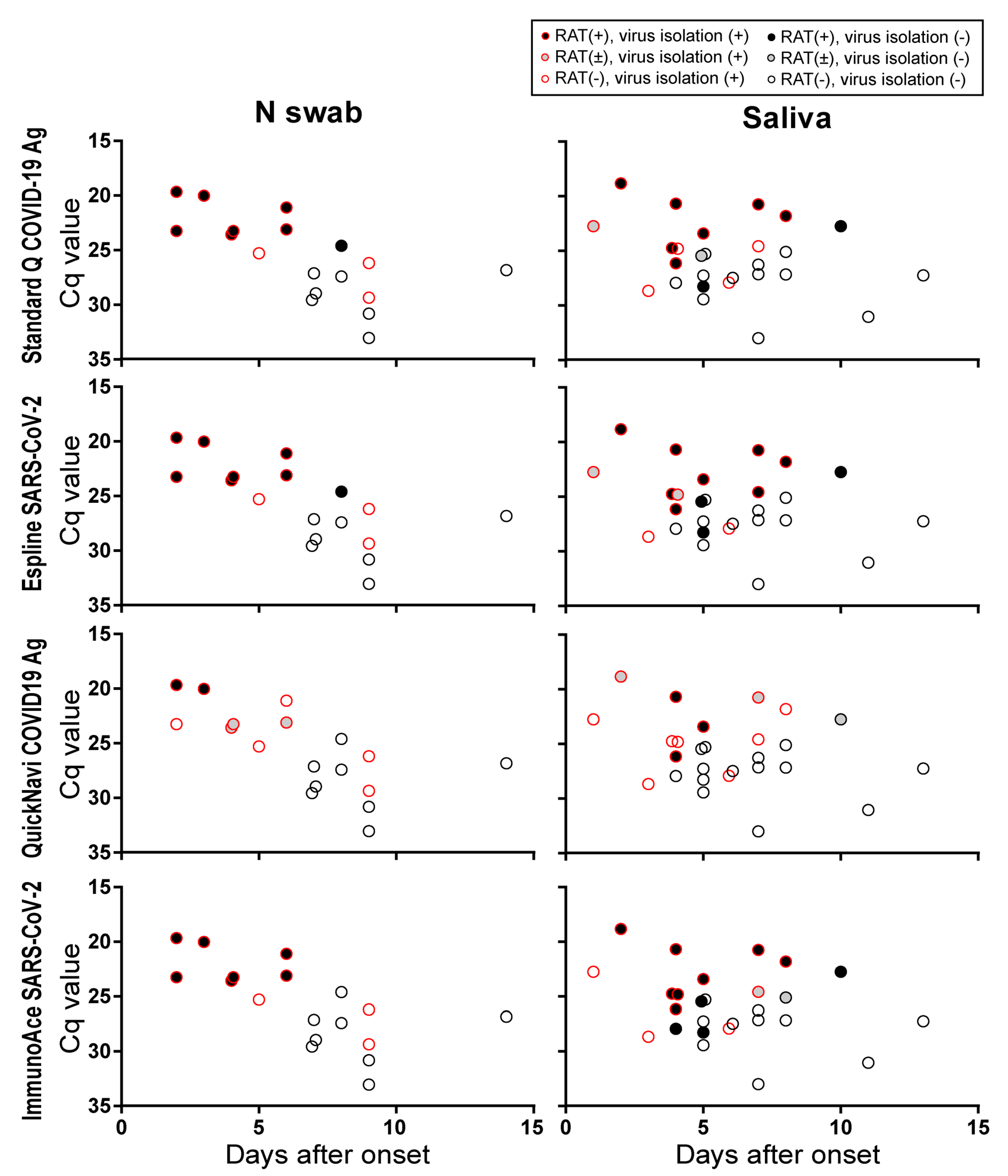
2.6. Rapid Antigen Test (RAT)
2.7. Virus Isolation
3. Results
3.1. Comparison of 4 Rapid Antigen Tests (RATs)
3.2. Sensitivity of RATs for Two Isolated SARS-CoV-2 Strains
3.3. Sensitivity of RATs for Clinical Specimens
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef] [PubMed]
- Nagura-Ikeda, M.; Imai, K.; Tabata, S.; Miyoshi, K.; Murahara, N.; Mizuno, T.; Horiuchi, M.; Kato, K.; Imoto, Y.; Iwata, M.; et al. Clinical Evaluation of Self-Collected Saliva by Quantitative Reverse Transcription-PCR (RT-qPCR), Direct RT-qPCR, Reverse Transcription-Loop-Mediated Isothermal Amplification, and a Rapid Antigen Test to Diagnose COVID-19. J. Clin. Microbiol. 2020, 58, e01438-20. [Google Scholar] [CrossRef] [PubMed]
- Mak, G.C.; Cheng, P.K.; Lau, S.S.; Wong, K.K.; Lau, C.S.; Lam, E.T.; Chan, R.C.; Tsang, D.N. Evaluation of rapid antigen test for detection of SARS-CoV-2 virus. J. Clin. Virol. 2020, 129, 104500. [Google Scholar] [CrossRef] [PubMed]
- Lambert-Niclot, S.; Cuffel, A.; Le Pape, S.; Vauloup-Fellous, C.; Morand-Joubert, L.; Roque-Afonso, A.M.; Le Goff, J.; Delaugerre, C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J. Clin. Microbiol. 2020, 58, e00977-20. [Google Scholar] [CrossRef] [PubMed]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Scohy, A.; Anantharajah, A.; Bodeus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef] [PubMed]
- Mertens, P.; De Vos, N.; Martiny, D.; Jassoy, C.; Mirazimi, A.; Cuypers, L.; Van den Wijngaert, S.; Monteil, V.; Melin, P.; Stoffels, K.; et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front. Med. (Lausanne) 2020, 7, 225. [Google Scholar] [CrossRef] [PubMed]
- Blairon, L.; Wilmet, A.; Beukinga, I.; Tre-Hardy, M. Implementation of rapid SARS-CoV-2 antigenic testing in a laboratory without access to molecular methods: Experiences of a general hospital. J. Clin. Virol. 2020, 129, 104472. [Google Scholar] [CrossRef] [PubMed]
- Matsuyama, S.; Nao, N.; Shirato, K.; Kawase, M.; Saito, S.; Takayama, I.; Nagata, N.; Sekizuka, T.; Katoh, H.; Kato, F.; et al. Enhanced isolation of SARS-CoV-2 by TMPRSS2-expressing cells. Proc. Natl. Acad. Sci. USA 2020, 117, 7001–7003. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imai, M.; Iwatsuki-Horimoto, K.; Hatta, M.; Loeber, S.; Halfmann, P.J.; Nakajima, N.; Watanabe, T.; Ujie, M.; Takahashi, K.; Ito, M.; et al. Syrian hamsters as a small animal model for SARS-CoV-2 infection and countermeasure development. Proc. Natl. Acad. Sci. USA 2020, 117, 16587–16595. [Google Scholar] [CrossRef] [PubMed]
- Shirato, K.; Nao, N.; Katano, H.; Takayama, I.; Saito, S.; Kato, F.; Katoh, H.; Sakata, M.; Nakatsu, Y.; Mori, Y.; et al. Development of Genetic Diagnostic Methods for Detection for Novel Coronavirus 2019(nCoV-2019) in Japan. Jpn. J. Infect. Dis. 2020, 73, 304–307. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Name | Manufacturer | Method for Visualization a | Input Ratio b (%) | Minutes to Assess c | Country of Manufacture |
|---|---|---|---|---|---|
| Standard Q COVID-19 Ag | SD Biosensor | Color particle | 14.3 | 15–30 | Korea |
| Espline SARS-CoV-2 | Fujirebio | Alkaline phosphatase | 10.0 | 30 | Japan |
| QuickNavi -COVID19 Ag | Denka Seiken | Color latex | 12.5 | 15 | Japan |
| ImmunoAce SARS-CoV-2 | Tauns Laboratories | Platinum-gold colloid | 28.6 | 15 | Japan |
| RT-qPCR | Number of Samples a | Standard Q COVID-19 Ag | Espline SARS-CoV-2 | QuickNavi COVID19 Ag | ImmunoAce SARS-CoV-2 | Virus Isolation |
|---|---|---|---|---|---|---|
| (Cq Value) | ||||||
| –20.0 | 4 | 4 b | 4 | 3.5 | 3.5 | 2 c |
| 20.0–22.5 | 7 | 7 | 7 | 4.5 | 6 | 6 |
| 22.5–25.0 | 17 | 10.5 | 12.5 | 5 | 9.5 | 9 |
| 25.0–27.5 | 20 | 2.5 | 3 | 2 | 3.5 | 4 |
| 27.5–30.0 | 17 | 2 | 1 | 0 | 2 | 3 |
| 30.0– | 11 | 0 | 0 | 0 | 0 | 0 |
| Clinical Specimen | Number of Samples | RT-qPCR | Standard Q COVID-19 Ag | Espline SARS-CoV-2 | QuickNavi COVID19 Ag | ImmunoAce SARS-CoV-2 | Virus Isolation |
|---|---|---|---|---|---|---|---|
| (Cq Value) | |||||||
| Gargle lavage | 7 | 26.3–36.0 | 0 a | 0 | 0 | 0 | 0 b |
| Saliva | 27 | 18.8–33.0 | 10 | 12 | 5 | 13 | 12 |
| T Swab | 2 | 25.8c, 33.6 | 0 | 0 | 0 | 0 | 1 |
| Nasal vestibule swab | 1 | 34.8 | 0 | 0 | 0 | 0 | 0 |
| N swab | 18 | 19.7-33.0 | 8 | 8 | 3.5 | 7 | 10 |
| Sputum | 4 | 19.9-34.3 | 1 | 1 | 1 | 1 | 0 |
| Tracheal aspirate | 17 | 19.6-35.6 | 7 | 6.5 | 5.5 | 3.5 | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yamayoshi, S.; Sakai-Tagawa, Y.; Koga, M.; Akasaka, O.; Nakachi, I.; Koh, H.; Maeda, K.; Adachi, E.; Saito, M.; Nagai, H.; et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses 2020, 12, 1420. https://doi.org/10.3390/v12121420
Yamayoshi S, Sakai-Tagawa Y, Koga M, Akasaka O, Nakachi I, Koh H, Maeda K, Adachi E, Saito M, Nagai H, et al. Comparison of Rapid Antigen Tests for COVID-19. Viruses. 2020; 12(12):1420. https://doi.org/10.3390/v12121420
Chicago/Turabian StyleYamayoshi, Seiya, Yuko Sakai-Tagawa, Michiko Koga, Osamu Akasaka, Ichiro Nakachi, Hidefumi Koh, Kenji Maeda, Eisuke Adachi, Makoto Saito, Hiroyuki Nagai, and et al. 2020. "Comparison of Rapid Antigen Tests for COVID-19" Viruses 12, no. 12: 1420. https://doi.org/10.3390/v12121420
APA StyleYamayoshi, S., Sakai-Tagawa, Y., Koga, M., Akasaka, O., Nakachi, I., Koh, H., Maeda, K., Adachi, E., Saito, M., Nagai, H., Ikeuchi, K., Ogura, T., Baba, R., Fujita, K., Fukui, T., Ito, F., Hattori, S.-i., Yamamoto, K., Nakamoto, T., ... Kawaoka, Y. (2020). Comparison of Rapid Antigen Tests for COVID-19. Viruses, 12(12), 1420. https://doi.org/10.3390/v12121420







