Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viral Isolation
2.2. RNA Extraction
2.3. Sequencing and Analysis of PEDV’s Complete Genome
2.4. Distribution of Two Novel PEDV Strains in the Farms of Taiwan
2.5. Growth Curves of Two Novel PEDV Strains in the Various Concentration of Trypsin
2.6. Characterization of the Virulence In Vivo
2.7. Detection of PEDV Loads by RRT-PCR
2.8. Detection of Anti-PEDV Antibody
2.9. Virus-Neutralizing Assay for PEDV
2.10. Cross-Neutralization in Serum between the 5-17-O and 5-17-V Strains
2.11. Statistical Analysis
3. Results
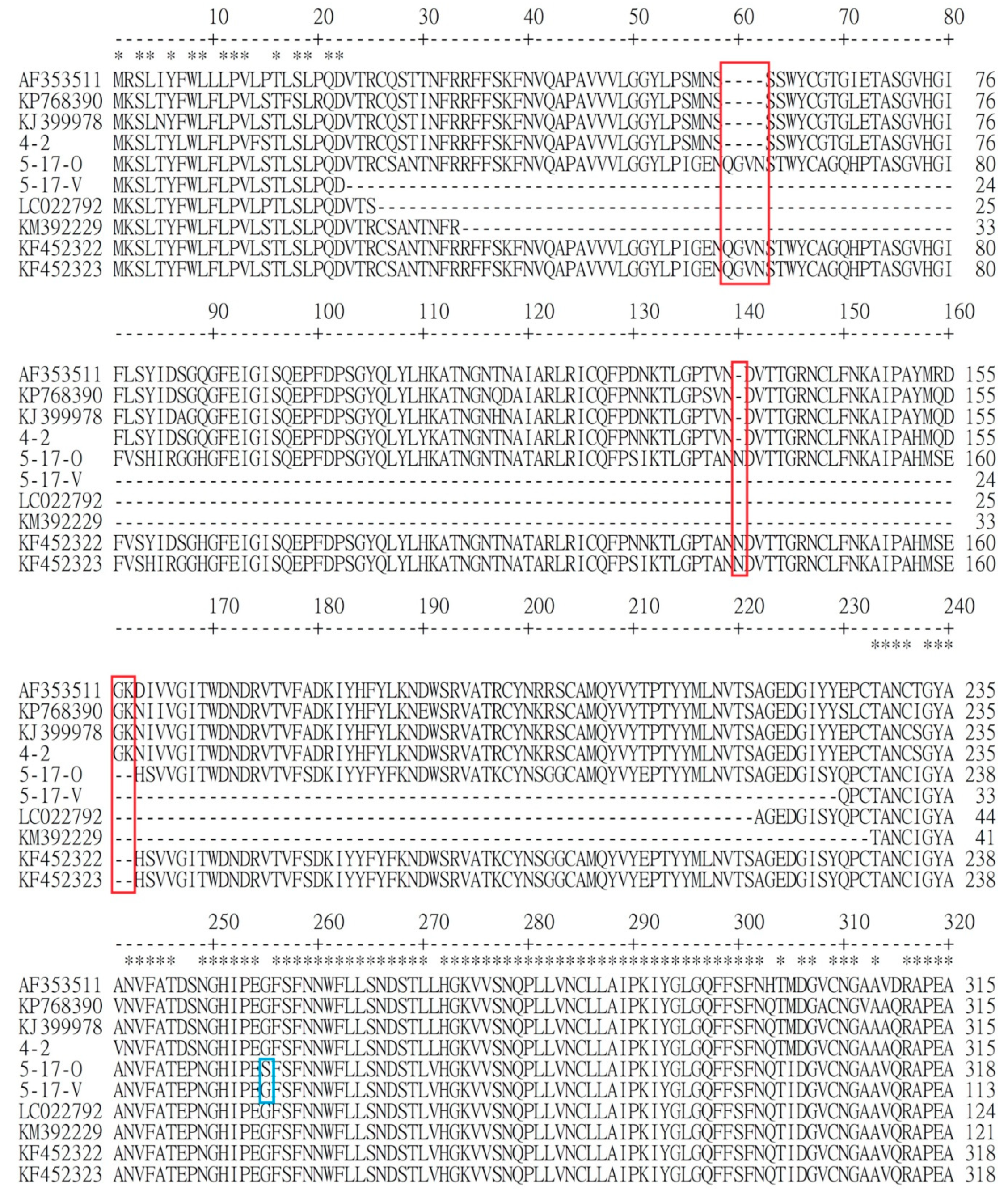
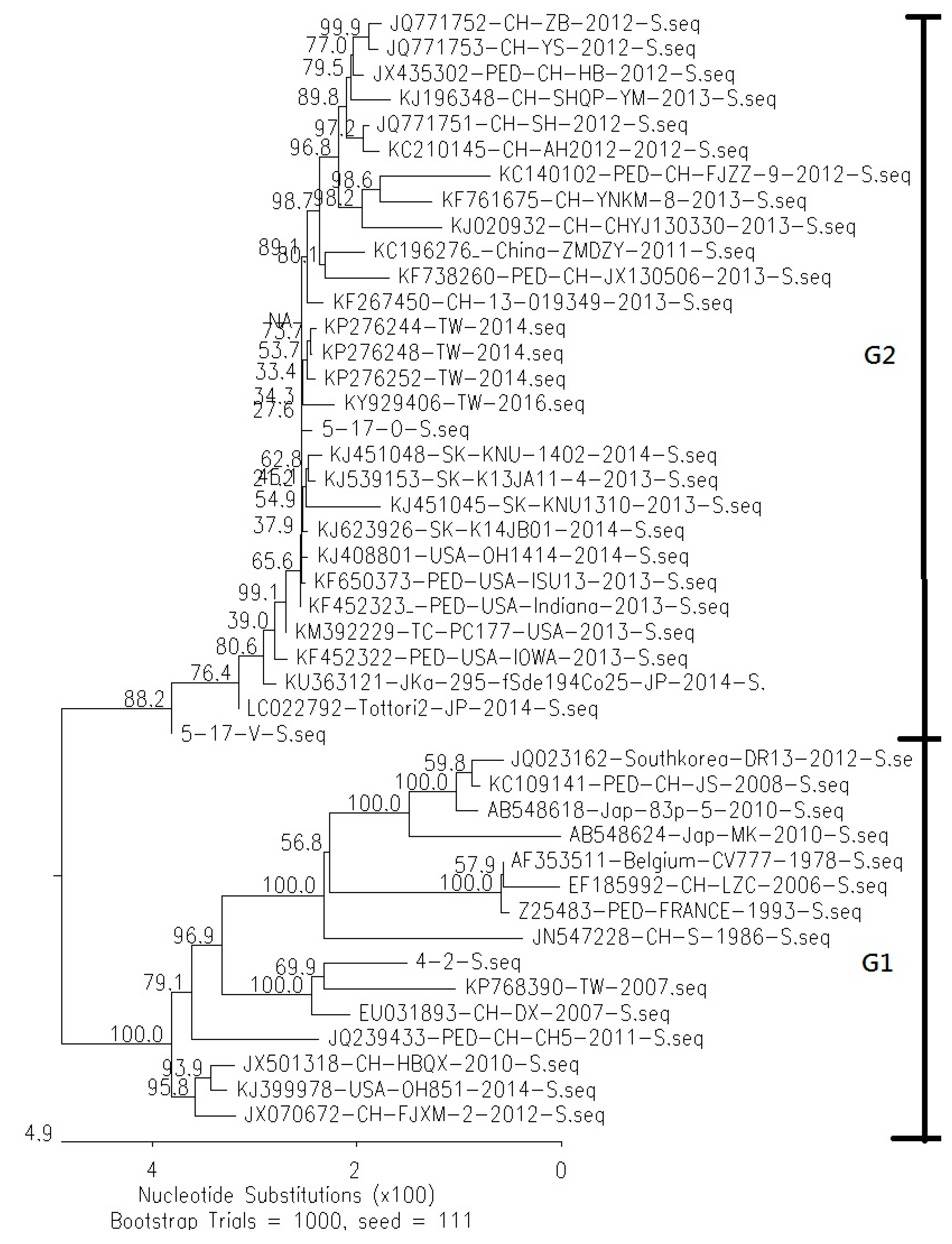
3.1. Comparison of Genetic Similarity between 5-17-O and 5-17-V Strains of PEDV
3.2. Comparison of Difference of Various Genes between Reference PEDV and Novel Taiwanese PEDV
3.3. Population Distribution of the 5-17-O and 5-17-V Strains in Taiwan
3.4. Comparison of Replication Kinetics between the 5-17-O and 5-17-V Strains in an In Vitro Experiment
3.5. Comparison of Virulence between the 5-17-O and 5-17-V Strains In Vivo
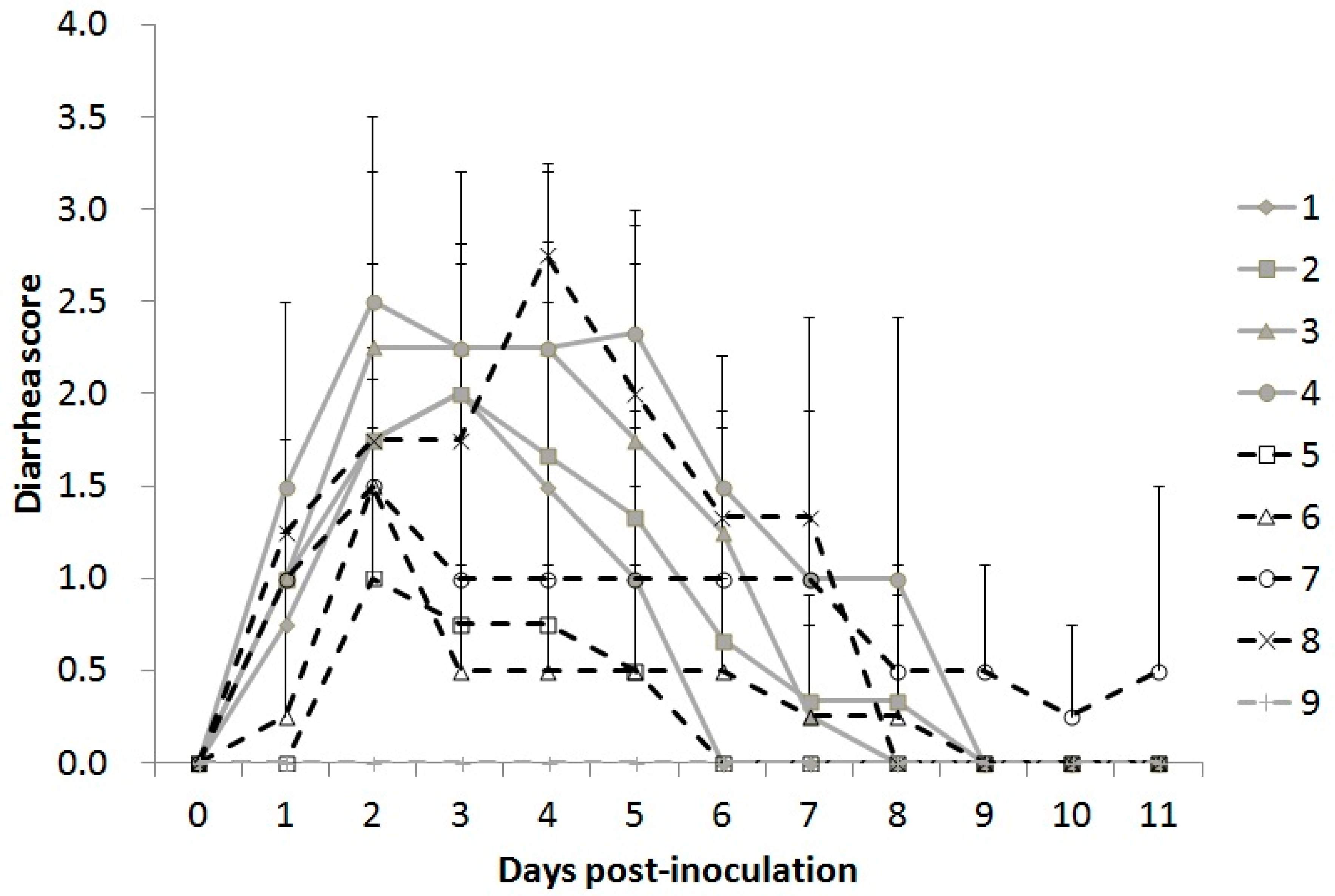
3.5.1. Clinical Signs
3.5.2. Viral Shedding in Feces
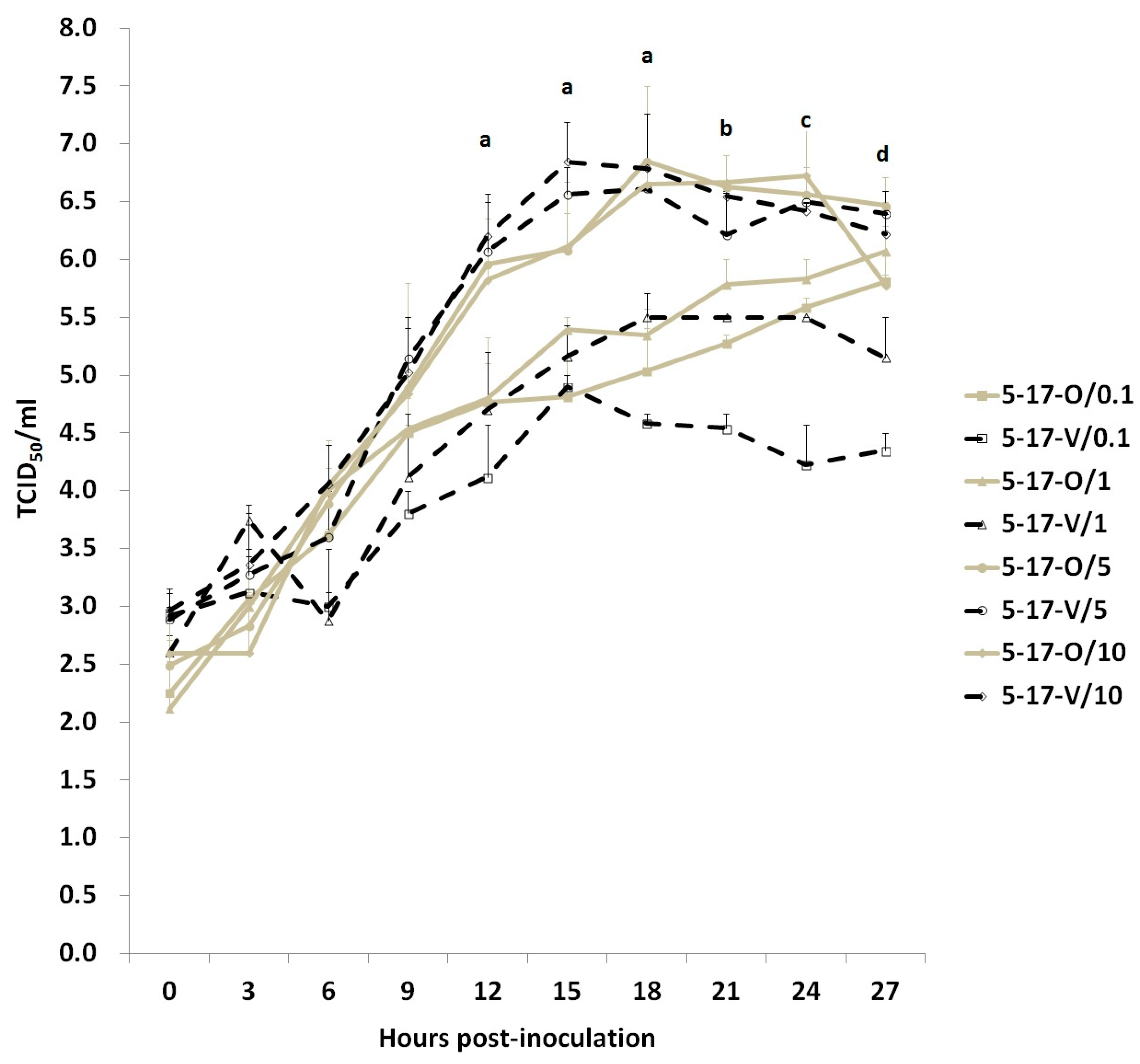
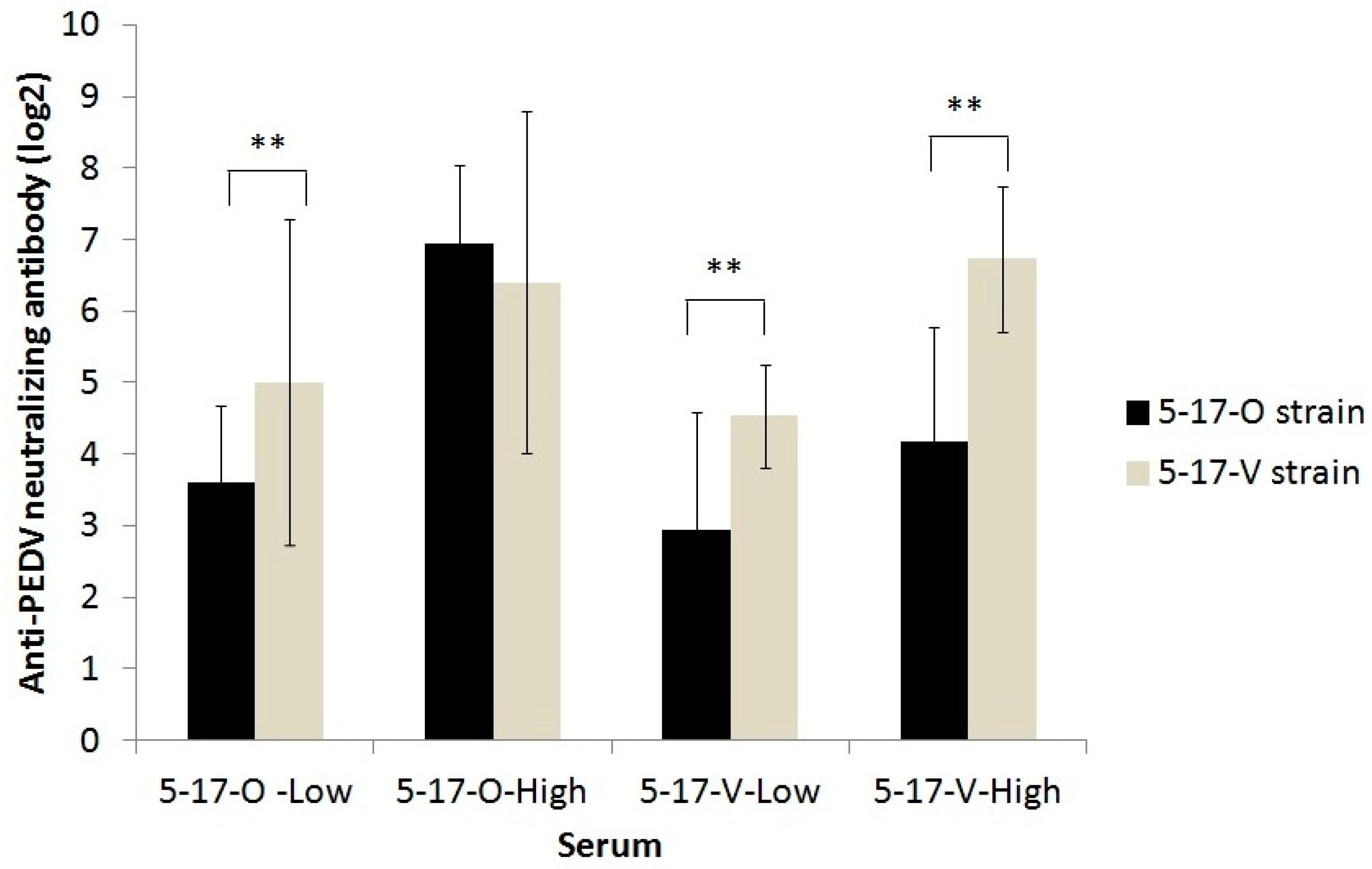
3.5.3. The Titer of Anti-PEDV Serum Antibody Detected by IFA and Neutralizing Assay in Experimentally Infected Pigs
3.6. Cross-Neutralization between the 5-17-O and 5-17-V Strains
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Jung, K.; Annamalai, T.; Lu, Z.; Saif, L.J. Comparative pathogenesis of US porcine epidemic diarrhea virus (PEDV) strain PC21A in conventional 9-day-oldnursing piglets vs. 26-day-old weaned pigs. Vet. Microbiol. 2015, 178, 31–40. [Google Scholar] [CrossRef]
- Chen, Y.M.; Helm, E.T.; Gabler, N.; Hostetter, J.M.; Burrough, E.R. Alterations in Intestinal Innate Mucosal Immunity of Weaned Pigs During Porcine Epidemic Diarrhea Virus Infection. Vet. Pathol. 2020, 57, 642–652. [Google Scholar] [CrossRef]
- Vlasova, A.N.; Halpin, R.; Wang, S.; Ghedin, E.; Spiro, D.J.; Saif, L.J. Molecular characterization of a new species in the genus Alphacoronavirus associated with mink epizootic catarrhal gastroenteritis. J. Gen. Virol. 2011, 92, 1369–1379. [Google Scholar] [CrossRef]
- Woo, P.C.; Lau, S.K.; Lam, C.S.; Lau, C.C.; Tsang, A.K.; Lau, J.H.; Bai, R.; Teng, J.L.; Tsang, C.C.; Wang, M.; et al. Discovery of seven novel mammalian and avian coronaviruses in the genus deltacoronavirus supports batcoronaviruses as the gene source of alphacoronavirus and betacoronavirus and avian coronaviruses as the gene source of gammacoronavirus and deltacoronavirus. J. Virol. 2012, 86, 3995–4008. [Google Scholar]
- Song, D.S.; Yang, J.S.; Oh, J.S.; Han, J.H.; Park, B.K. Differentiation of a Vero cell adapted porcine epidemic diarrhea virus from Korean field strains by restriction fragment length polymorphism analysis of ORF 3. Vaccine 2003, 21, 1833–1842. [Google Scholar] [CrossRef]
- Chang, S.H.; Bae, J.L.; Kang, T.J.; Kim, J.; Chung, G.H.; Lim, C.W.; Laude, H.; Yang, M.S.; Jang, Y.S. Identification of the epitope region capable of inducing neutralizing antibodies against the porcine epidemic diarrhea virus. Mol. Cells 2002, 14, 295–299. [Google Scholar]
- Lee, D.K.; Cha, S.Y.; Lee, C. The N-terminal region of the porcine epidemic diarrhea virus spike protein is important for the receptor binding. Korean J. Microbiol. Biotechnol. 2011, 39, 140–145. [Google Scholar]
- Sun, D.B.; Feng, L.; Shi, H.Y.; Chen, J.F.; Liu, S.W.; Chen, H.Y.; Wang, Y.F. Spike protein region (aa 636–789) of porcine epidemic diarrhea virus is essential for induction of neutralizing antibodies. Acta. Virol. 2007, 51, 149–156. [Google Scholar]
- Nam, E.; Lee, C. Contribution of the porcine aminopeptidase N (CD13) receptor density to porcine epidemic diarrhea virus infection. Vet. Microbiol. 2010, 144, 41–50. [Google Scholar] [CrossRef]
- Laude, H.; Gelfi, J.; Lavenant, L.; Charley, B. Single amino acid changes in the viral glycoprotein M affect induction of alpha interferon by the coronavirus transmissible gastroenteritis virus. J. Virol. 1992, 66, 743–749. [Google Scholar] [CrossRef] [Green Version]
- Saif, L.J. Coronavirus immunogens. Vet. Microbiol. 1993, 37, 285–297. [Google Scholar] [CrossRef]
- Wood, E.N. An apparently new syndrome of porcine epidemic diarrhoea. Vet. Rec. 1977, 100, 243–244. [Google Scholar] [CrossRef]
- Debouck, P.; Pensaert, M. Experimental infection of pigs with a new porcine enteric coronavirus, CV 777. Am. J. Vet. Res. 1980, 41, 219–223. [Google Scholar] [PubMed]
- Kawashima, T. Porcine Epidemic Diarrhea (PED) in Japan. In International Conference on Novel Swine Enteric Coronavirus Disease Viruses (nSECDv); Animal and Plant Health Inspection Service: Chicago, IL, USA, 2014; p. 19. [Google Scholar]
- Romero-Gonzalez, L.J. Porcine Epidemic Diarrhea in Spain. In International Conference on Novel Swine Enteric Coronavirus Disease Viruses (nSECDv); Animal and Plant Health Inspection Service: Chicago, IL, USA, 2014; p. 29. [Google Scholar]
- Park, S.J.; Kim, H.K.; Song, D.S.; Moon, H.J.; Park, B.K. Mocleular characterization and phylogenetic analysis of porcine epidemic diarrhea virus (PEDV) field isolates in Korea. Arch. Virol. 2011, 156, 577–585. [Google Scholar] [CrossRef]
- Fan, J.H.; Li, Y.J. Cloning and sequence analysis of the M gene of porcine epidemic diarrhea virus LJB/03. Virus Genes 2005, 30, 69–73. [Google Scholar]
- Cheun-Arom, T.; Temeeyasen, G.; Tripipat, T.; Kaewprommal, P.; Piriyapongsa, J.; Sukrong, S.; Chongcharoen, W.; Tantituvanont, A.; Nilubo, D. Full-length genome analysis of two genetically distinct variants of porcine epidemic diarrhea virus in Thailand. Infect. Genet. Evol. 2016, 44, 114–121. [Google Scholar] [CrossRef]
- Chiou, H.Y.; Huang, Y.L.; Deng, M.C.; Chang, C.Y.; Jeng, C.R.; Tsai, P.S.; Yang, C.; Pang, V.F.; Chang, H.W. Phylogenetic Analysis of the spike (S) gene of the new variants of porcine epidemic diarrhoea virus in Taiwan. Transbound. Emerg. Dis. 2017, 64, 157–166. [Google Scholar] [CrossRef] [Green Version]
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.W.; Dickerman, A.W.; Piñeyro, P.; Li, L.; Fang, L.; Kiehne, R.; Opriessnig, T.; Meng, X.J. Origin, evolution, and genotyping of emmergent porcine epidemic diarrhea virus strains in the United States. mBio 2013, 4, e00737. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Murakami, S.; Takahashi, O.; Kodera, A.; Masuda, T.; Itoh, S.; Miyazaki, A.; Ohashi, S.; Tsutsui, T. Molecular characterization of pig epidemic diarrhoea viruses isolated in Japan from 2013 to 2014. Infect. Genet. Evol. 2015, 36, 363–368. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lim, S.I.; Lim, J.A.; Cho, I.S.; Park, E.H.; Le, V.P.; Hien, N.B.; Thach, P.N.; Quynh do, H.; Vui, T.Q.; et al. A novel strain of porcine epidemic diarrhea virus in Vietnamese pigs. Arch. Virol. 2015, 160, 1573–1577. [Google Scholar] [CrossRef]
- Lee, S.; Lee, C. Outbreak-related porcine epidemic diarrhea virus strains similar to US strains, South Korea. Emerg. Infect. Dis. 2014, 20, 1223–1226. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.; Saif, L.J. Porcine epidemic diarrhea virus infection: Etiology, epidemiology, pathogenesis and immunoprophylaxis. Vet. J. 2015, 204, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Gao, X.; Yao, Y.; Zhang, Y.; Lv, C.; Sun, Z.; Wang, Y.; Jia, X.; Zhuang, J.; Xiao, Y.; et al. The dynamics of Chinese of Chinese variant porcine epidemic diarrhea virus production in Vero cells and intestines of 2-day old piglets. Virus Res. 2015, 208, 82–88. [Google Scholar] [CrossRef]
- Chen, Q.; Li, G.; Stasko, J.; Thomas, J.T.; Stensland, W.R.; Pillatzki, A.E.; Gauger, P.C.; Schwartz, K.J.; Madson, D.; Yoon, K.J.; et al. Isolation and characterization of porcine epidemic diarrhea viruses associated with the 2013 disease outbreak among swine in the United States. J. Clin. Microbiol. 2014, 52, 234–243. [Google Scholar] [CrossRef] [Green Version]
- Masuda, T.; Murakami, S.; Takahashi, O.; Miyazaki, A.; Ohashi, S.; Yamasato, H.; Suzuki, T. New porcine epidemic diarrhoea virus variant with a large deletion in the spike gene identified in domestic pigs. Arch. Virol. 2015, 160, 2565–2568. [Google Scholar] [CrossRef]
- Su, Y.F.; Hou, Y.; Prarat, M.; Zhang, Y.; Wang, Q.H. New variants of porcine epidemic diarrhea virus with large deletions in the spike protein, identified in the United States, 2016–2017. Arch. Virol. 2018, 163, 2485–2489. [Google Scholar] [CrossRef]
- Nguyen, V.D.; Junzo, N.; Masuo, S.; Nguyen, T.L.; Ryoji, Y. Novel Porcine epidemic diarrhea virus (PEDV) variants with large deletions in the spike (S) gene coexist with pedv strains possessing an intact s gene in domestic pigs in Japan: A new disease situation. PLoS ONE 2017, 12, e0170126. [Google Scholar]
- Kim, L.; Hayes, J.; Lewis, P.; Parwani, A.V.; Chang, K.O.; Saif, L.J. Molecular characterization and pathogenesis of transmissible gastroenteritis coronavirus (TGEV) and porcine respiratory coronavirus (PRCV) field isolates co-circulating in a swine herd. Arch. Virol. 2000, 145, 1133–1147. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.; Rowe, L.A.; Frace, M.; Stevens, J.; Abedi, G.R.; Elnile, O.; Banassir, T.; Al-Masri, M.; Watson, J.T.; Assiri, A.; et al. Spike gene deletion quasispecies in serum of patient with acute MERS-CoV infection. J. Med. Virol. 2017, 89, 542–545. [Google Scholar] [CrossRef]
- Park, J.E.; Cruz, D.J.M.; Shin, H.J. Receptor-bound porcine epidemic diarrhea virus spike protein cleaved by trypsin induces membrane fusion. Arch. Virol. 2011, 156, 1749–1756. [Google Scholar] [CrossRef]
- Wicht, O.; Li, W.; Willems, L.; Meuleman, T.J.; Wubbolts, R.W.; van Kuppeveld, F.J.M.; Rottier, P.J.M.; Bosch, B.J. Proteolytic activation of the porcine epidemic diarrhea coronavirus spike fusion protein by trypsin in cell culture. J. Virol. 2014, 88, 7952–7961. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Terada, Y.; Enjuanes, L.; Ohashi, S.; Kamitani, W. S1 Subunit of spike protein from a current highly virulent porcine epidemic diarrhea virus is an important determinant of virulence in piglets. Viruses 2018, 10, 467. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, X.; Lin, C.M.; Annamalai, T.; Gao, X.; Lu, Z.; Esseili, M.A.; Jung, K.; El-Tholoth, M.; Saif, L.J.; Wang, Q.H. Determination of the infectious titer and virulence of an original US porcine epidemic diarrhea virus PC22A strain. Vet. Res. 2015, 46, 109. [Google Scholar] [CrossRef] [Green Version]
- Thomas, J.T.; Chen, Q.; Gauger, P.C.; Giménez-Lirola, L.G.; Sinha, A.; Harmon, K.M.; Madson, D.M.; Burrough, E.R.; Magstadt, D.R.; Salzbrenner, H.M.; et al. Effect of porcine epidemic diarrhea virus infectious doses on infection outcomes in naïve conventional neonatal and weaned pigs. PLoS ONE 2015, 10, e0139266. [Google Scholar] [CrossRef] [Green Version]
- Shan, Z.; Yin, J.; Wang, Z.; Chen, P.; Li, Y.; Tang, L. Identification of the functional domain of the porcine epidemic diarrhoea virus receptor. J. Gen. Virol. 2015, 96, 2656–2660. [Google Scholar] [CrossRef]
- Deng, F.; Ye, G.; Liu, Q.; Navid, M.T.; Zhong, X.; Li, Y.; Wan, C.; Xiao, S.; He, Q.; Fu, Z.F. Identification and comparison of receptor binding characteristics of the spike protein of two porcine epidemic diarrhea virus strains. Viruses 2016, 8, 55. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; van Kuppeveld, F.J.; He, Q.; Rottier, P.J.; Bosch, B. Cellular entry of the porcine epidemic diarrhea virus. Virus Res. 2016, 226, 117–127. [Google Scholar] [CrossRef] [Green Version]
- Li, C.; Li, W.; de Esesarte, E.L.; Guo, H.; van den Elzen, P.; Aarts, E.; van den Born, E.; Rottier, P.J.M.; Bosch, B.J. Cell Attachment domains of the porcine epidemic diarrhea virus spike protein are key targets of neutralizing antibodies. J. Virol. 2017, 91, e00273. [Google Scholar] [CrossRef] [Green Version]
| Strains | Gene | 4-2 c (MW165329) | CV777 (AF353511) | USA/Indiana/17846/2013 (KF452323) | |||
|---|---|---|---|---|---|---|---|
| Nucleotide | Amino Acids | Nucleotide | Amino Acids | Nucleotide | Amino Acids | ||
| 5-17-O (5-17-V) | 5′-UTR | 98.9 a | - | 98.9 | - | 100 | - |
| ORF1a | 98.6 b | 98.7 | 97.7 | 97.7 | 99.9 | 99.9 | |
| ORF1b | 98.7 | 98.4 | 97.8 | 99.3 | 100 | 100 | |
| ORF3 | 96.7 | 96.4 | 96.7 | 96.0 | 99.6 | 99.1 | |
| S | 94.9 (96.9) | 95.2 (97.6) | 94.0 (94.7) | 93.6 (95.6) | 99.9 (99.0) | 99.8 (99.7) | |
| E | 97.4 | 98.7 | 97.0 | 98.7 | 100 | 100 | |
| M | 98.1 | 99.6 | 98.2 | 98.7 | 100 | 100 | |
| N | 98.6 | 99.3 | 96.0 | 97.1 | 100 | 100 | |
| 3′-UTR | 99.4 | - | 97.9 | - | 100 | - | |
| Group | Strain/Inoculated Load (TCID50) | Diarrhea%/Shedding% | Average Diarrhea Days | Average Shedding Days | Mortality (%) |
|---|---|---|---|---|---|
| 1 | 5-17-O/103 | 100/100 | 4.5 ± 0.9 c,d | 7.8 ± 1.5 b | 0 |
| 2 | 5-17-O/104 | 100/100 | 5.0 ± 1.9 c,d | 8.3 ± 1.1 b | 0 |
| 3 | 5-17-O/105 | 100/100 | 6.0 ± 0.7 c,d | 8.3 ± 1.1 b | 0 |
| 4 | 5-17-O/106 | 100/100 | 6.0 ± 1.4 c,d | 8.5 ± 2.9 b | 50 (2/4) * |
| 5 | 5-17-V/104 | 100/100 | 1.5 ± 0.9 a,b | 6.3 ± 0.8 b | 0 |
| 6 | 5-17-V/105 | 100/100 | 3.5 ± 2.6 b,c | 7.5 ± 0.5 b | 0 |
| 7 | 5-17-V/106 | 100/100 | 9.0 ± 2.4 e | 7.3 ± 0.4 b | 0 |
| 8 | 5-17-V/107 | 100/100 | 7.3 ± 2.0 d,e | 6.3 ± 1.4 b | 50 (2/4) ** |
| 9 | MEM | 0 | 0.0 ± 0.0 a | 0.0 ± 0.0 a | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, K.-J.; Deng, M.-C.; Wang, F.-I.; Tsai, S.-H.; Chang, C.; Chang, C.-Y.; Huang, Y.-L. Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced. Viruses 2020, 12, 1378. https://doi.org/10.3390/v12121378
Tsai K-J, Deng M-C, Wang F-I, Tsai S-H, Chang C, Chang C-Y, Huang Y-L. Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced. Viruses. 2020; 12(12):1378. https://doi.org/10.3390/v12121378
Chicago/Turabian StyleTsai, Kuo-Jung, Ming-Chung Deng, Fun-In Wang, Shu-Hui Tsai, Chieh Chang, Chia-Yi Chang, and Yu-Liang Huang. 2020. "Deletion in the S1 Region of Porcine Epidemic Diarrhea Virus Reduces the Virulence and Influences the Virus-Neutralizing Activity of the Antibody Induced" Viruses 12, no. 12: 1378. https://doi.org/10.3390/v12121378






