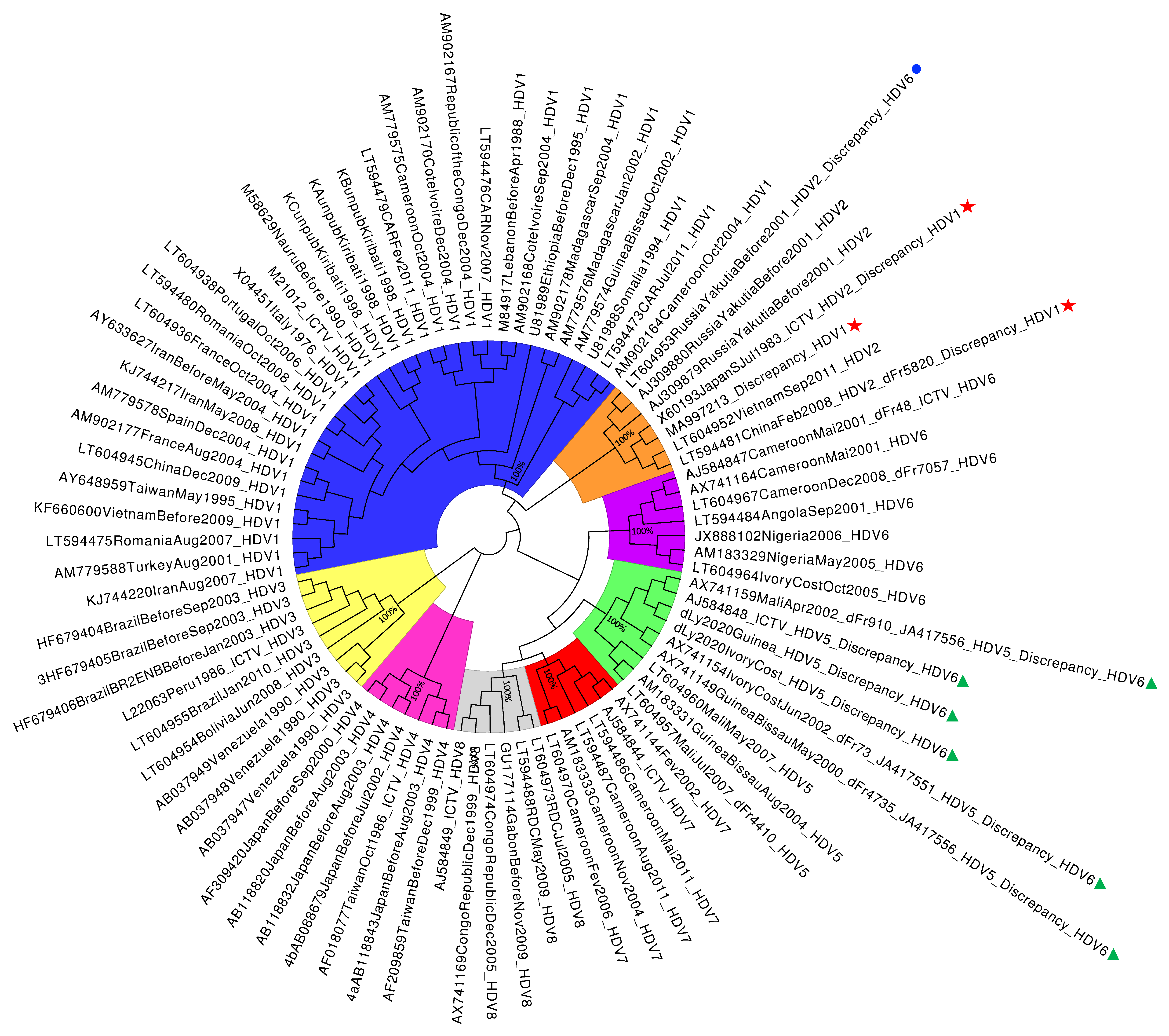
Mis-Genotyping of Some Hepatitis D Virus Genotype 2 and 5 Sequences Using HDVdb
Abstract
Author Contributions
Funding
Conflicts of Interest
References
- Usman, Z.; Velkov, S.; Protzer, U.; Roggendorf, M.; Frishman, D.; Karimzadeh, H. HDVdb: A Comprehensive Hepatitis D Virus Database. Viruses 2020, 12, 538. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Radjef, N.; Gordien, E.; Ivaniushina, V.; Gault, E.; Anaïs, P.; Drugan, T.; Trinchet, J.-C.; Roulot, D.; Tamby, M.; Milinkovitch, M.C.; et al. Molecular Phylogenetic Analyses Indicate a Wide and Ancient Radiation of African Hepatitis Delta Virus, Suggesting a Deltavirus Genus of at Least Seven Major Clades. J. Virol. 2004, 78, 2537–2544. [Google Scholar] [CrossRef] [PubMed]
- Gal, F.L.; Brichler, S.; Drugan, T.; Alloui, C.; Roulot, D.; Pawlotsky, J.-M.; Dény, P.; Gordien, E. Genetic diversity and worldwide distribution of the deltavirus genus: A study of 2,152 clinical strains. Hepatology 2017, 66, 1826–1841. [Google Scholar] [CrossRef] [PubMed]
- Ivaniushina, V.; Radjef, N.; Alexeeva, M.; Gault, E.; Semenov, S.; Salhi, M.; Kiselev, O.; Dény, P. Hepatitis delta virus genotypes I and II cocirculate in an endemic area of Yakutia, Russia. J. Gen. Virol. 2001, 82, 2709–2718. [Google Scholar] [CrossRef] [PubMed]
- Imazeki, F.; Omata, M.; Ohto, M. Heterogeneity and evolution rates of delta virus RNA sequences. J. Virol. 1990, 64, 5594–5599. [Google Scholar] [CrossRef] [PubMed]
- Dény, P.; Drugan, C.; Gatherer, D.; Kay, A.C.; Le Gal, F.; Drugan, T. Hepatitis D Virus clades and genotypes: A practical approach in Rizzetto M & Smedile A, Il Pensiero Scientifico Editore. Viruses 2019, 4, 49–80. [Google Scholar]
- Kumar, S.; Stecher, G.; Tamura, K. MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Mol. Biol. Evol. 2016, 33, 1870–1874. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Saitou, N.; Nei, M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987, 4, 406–425. [Google Scholar] [CrossRef] [PubMed]
- Kimura, M. A simple method for estimating evolutionary rates of base substitutions through comparative studies of nucleotide sequences. J. Mol. Evol. 1980, 16, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.L.; Brown, T.L.; Colan, E.J.; Wignall, F.S.; Gerin, J.L. A genotype of hepatitis D virus that occurs in northern South America. Proc. Natl. Acad. Sci. USA 1993, 90, 9016–9020. [Google Scholar] [CrossRef] [PubMed]
- Casey, J.L.; Niro, G.A.; Engle, R.E.; Vega, A.; Gomez, H.; McCarthy, M.; Watts, D.M.; Hyams, K.C.; Gerin, J.L. Hepatitis B virus (HBV)/hepatitis D virus (HDV) coinfection in outbreaks of acute hepatitis in the Peruvian Amazon basin: The roles of HDV genotype III and HBV genotype F. J. Infect. Dis. 1996, 174, 920–926. [Google Scholar] [CrossRef] [PubMed]
- Spaan, M.; Carey, I.; Bruce, M.; Shang, D.; Horner, M.; Dusheiko, G.; Agarwal, K. Hepatitis delta genotype 5 is associated with favourable disease outcome and better response to treatment compared to genotype 1. J. Hepatol. 2020, 72, 1097–1104. [Google Scholar] [CrossRef] [PubMed]
- Roulot, D.; Brichler, S.; Layese, R.; BenAbdesselam, Z.; Zoulim, F.; Thibault, V.; Scholtes, C.; Roche, B.; Castelnau, C.; Poynard, T.; et al. Origin, HDV genotype and persistent viremia determine outcome and treatment response in patients with chronic hepatitis Delta. J. Hepatol. 2020. [Google Scholar] [CrossRef] [PubMed]
- Mentha, N.; Clément, S.; Negro, F.; Alfaiate, D. A review on hepatitis D: From virology to new therapies. J. Adv. Res. 2019, 17, 3–15. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Charre, C.; le Gal, F.; Dény, P.; Scholtès, C. Mis-Genotyping of Some Hepatitis D Virus Genotype 2 and 5 Sequences Using HDVdb. Viruses 2020, 12, 1066. https://doi.org/10.3390/v12101066
Charre C, le Gal F, Dény P, Scholtès C. Mis-Genotyping of Some Hepatitis D Virus Genotype 2 and 5 Sequences Using HDVdb. Viruses. 2020; 12(10):1066. https://doi.org/10.3390/v12101066
Chicago/Turabian StyleCharre, Caroline, Frédéric le Gal, Paul Dény, and Caroline Scholtès. 2020. "Mis-Genotyping of Some Hepatitis D Virus Genotype 2 and 5 Sequences Using HDVdb" Viruses 12, no. 10: 1066. https://doi.org/10.3390/v12101066
APA StyleCharre, C., le Gal, F., Dény, P., & Scholtès, C. (2020). Mis-Genotyping of Some Hepatitis D Virus Genotype 2 and 5 Sequences Using HDVdb. Viruses, 12(10), 1066. https://doi.org/10.3390/v12101066





