Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Challenge
2.2. Ethics Statement
2.3. RNA Isolation
2.4. RT-qPCR
2.5. Data Analysis
2.6. Western Blotting
2.7. In-Situ Hybridization (ISH)
2.8. Duplex In-Situ Hybridization
3. Results
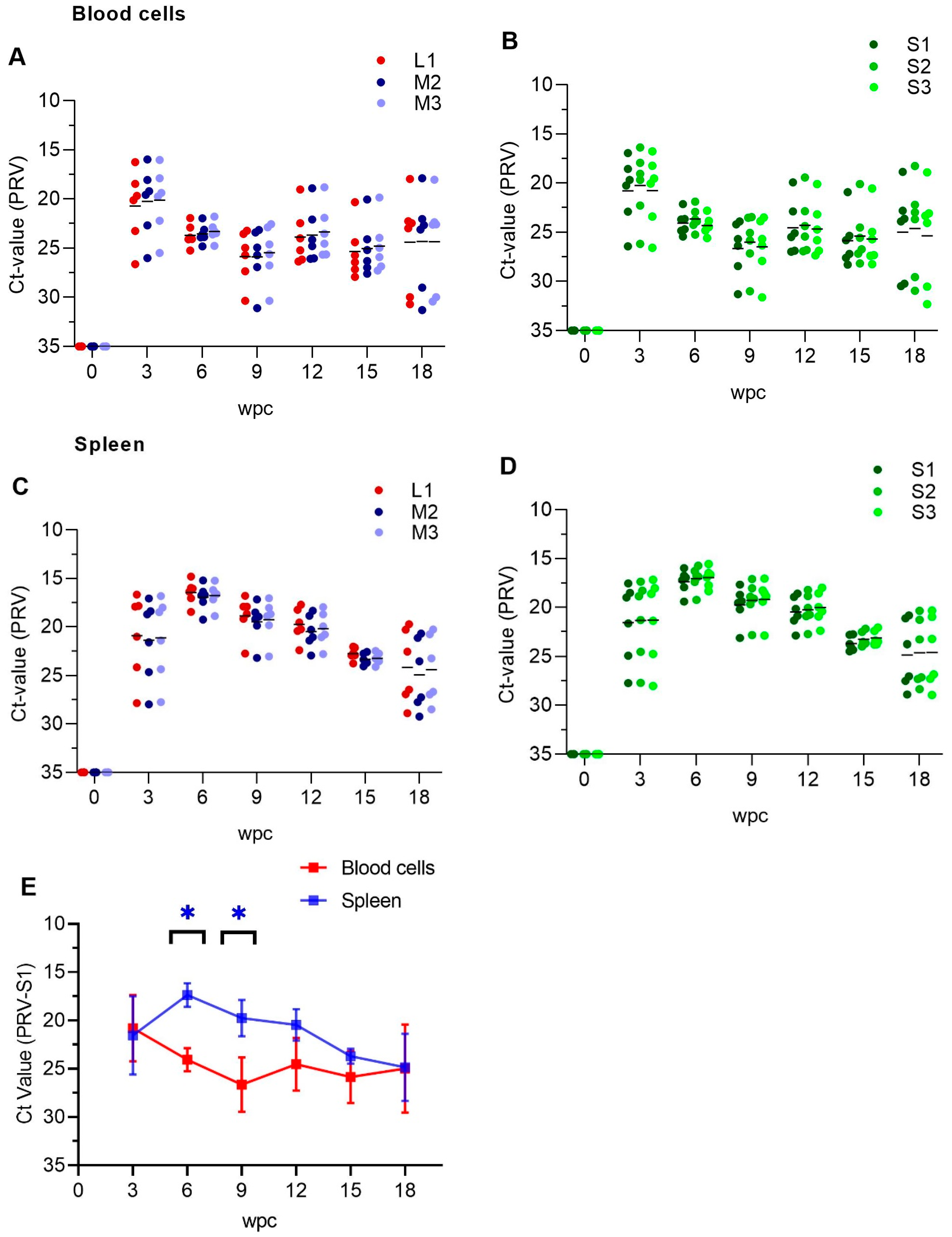
3.1. PRV-1 Segments Have Similar Expression Pattern
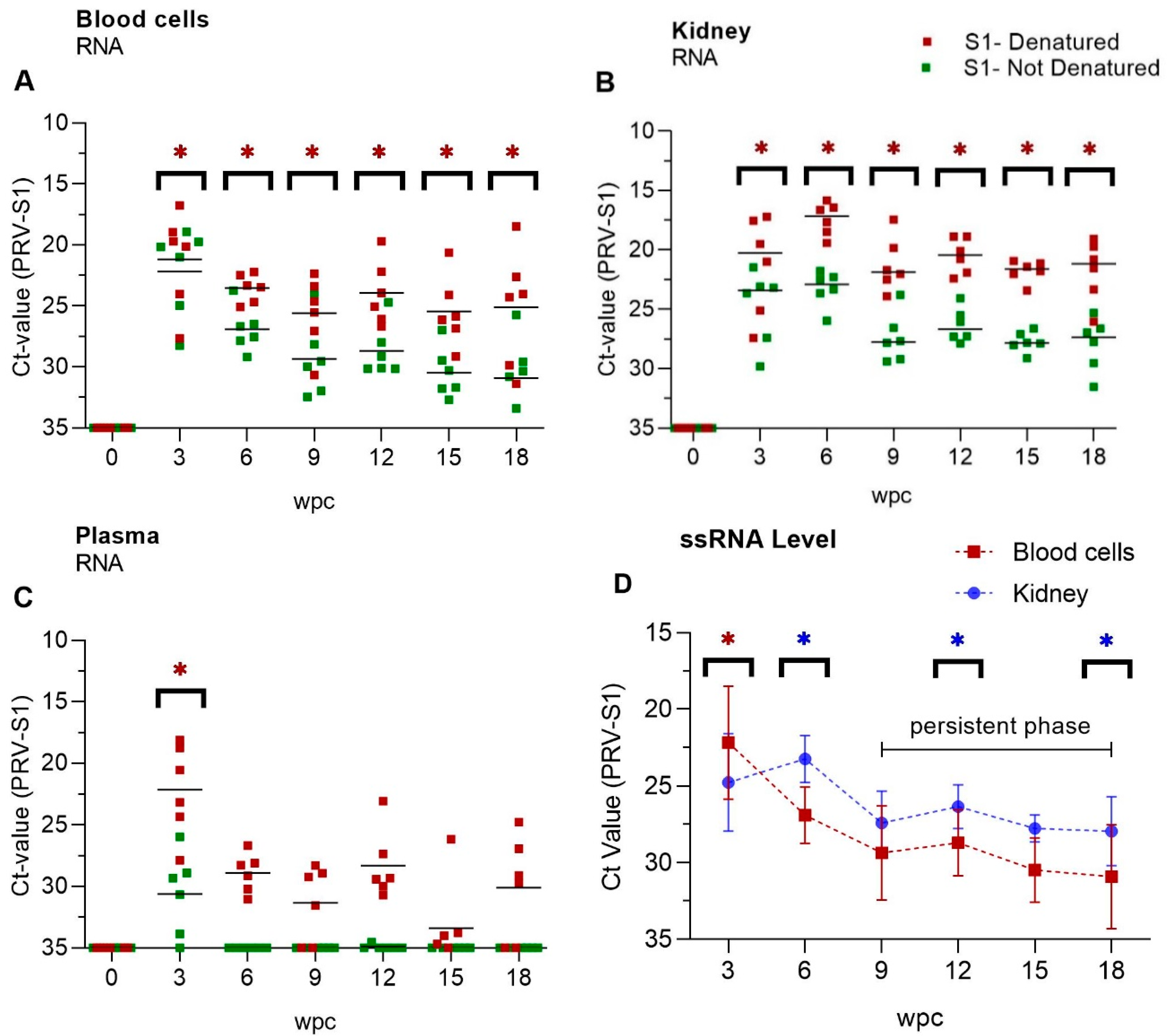
3.2. Viral Genomic RNA Versus Viral Transcripts
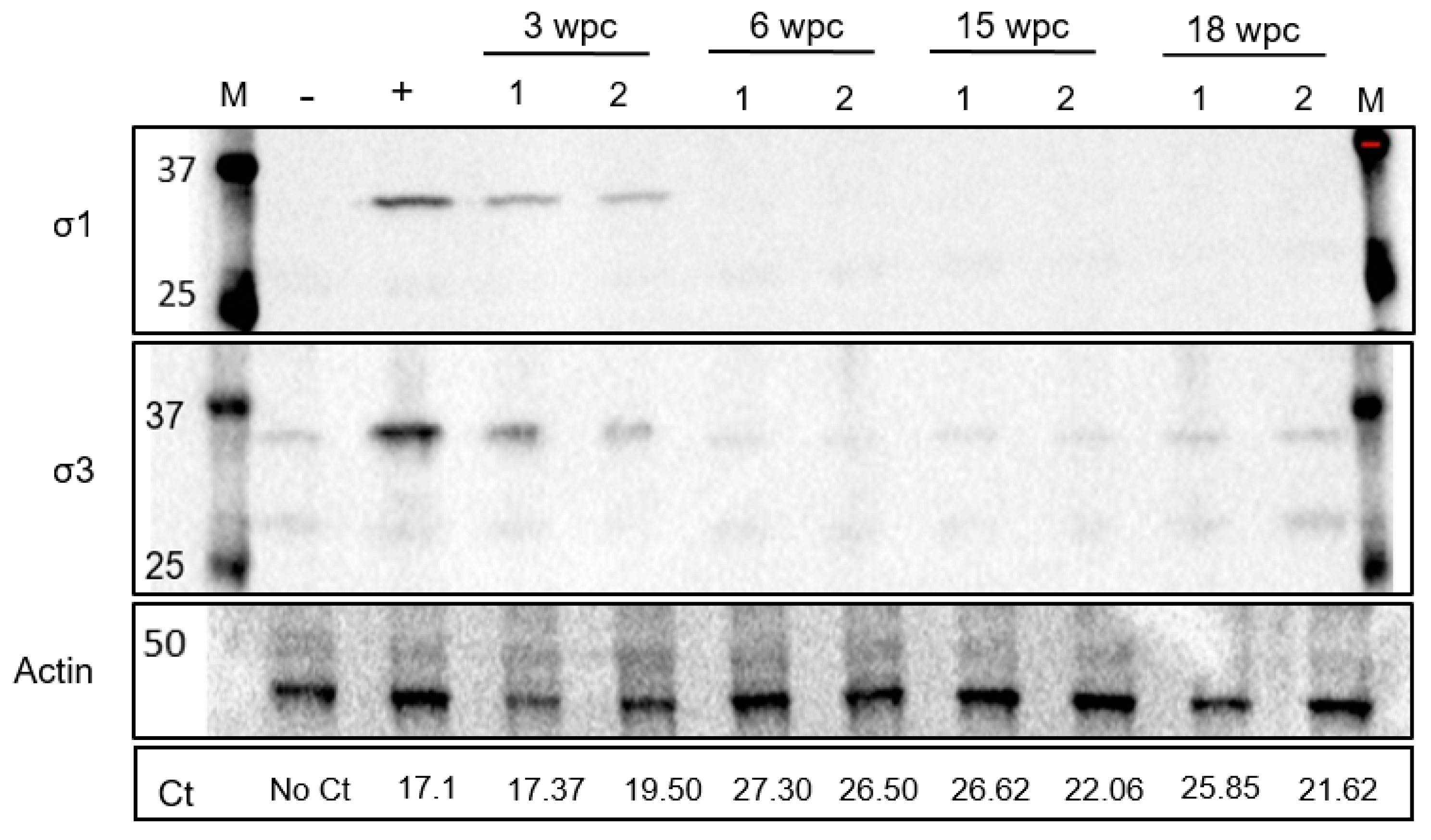
3.3. Low Level of PRV-1 Protein Synthesis in the Persistent Phase
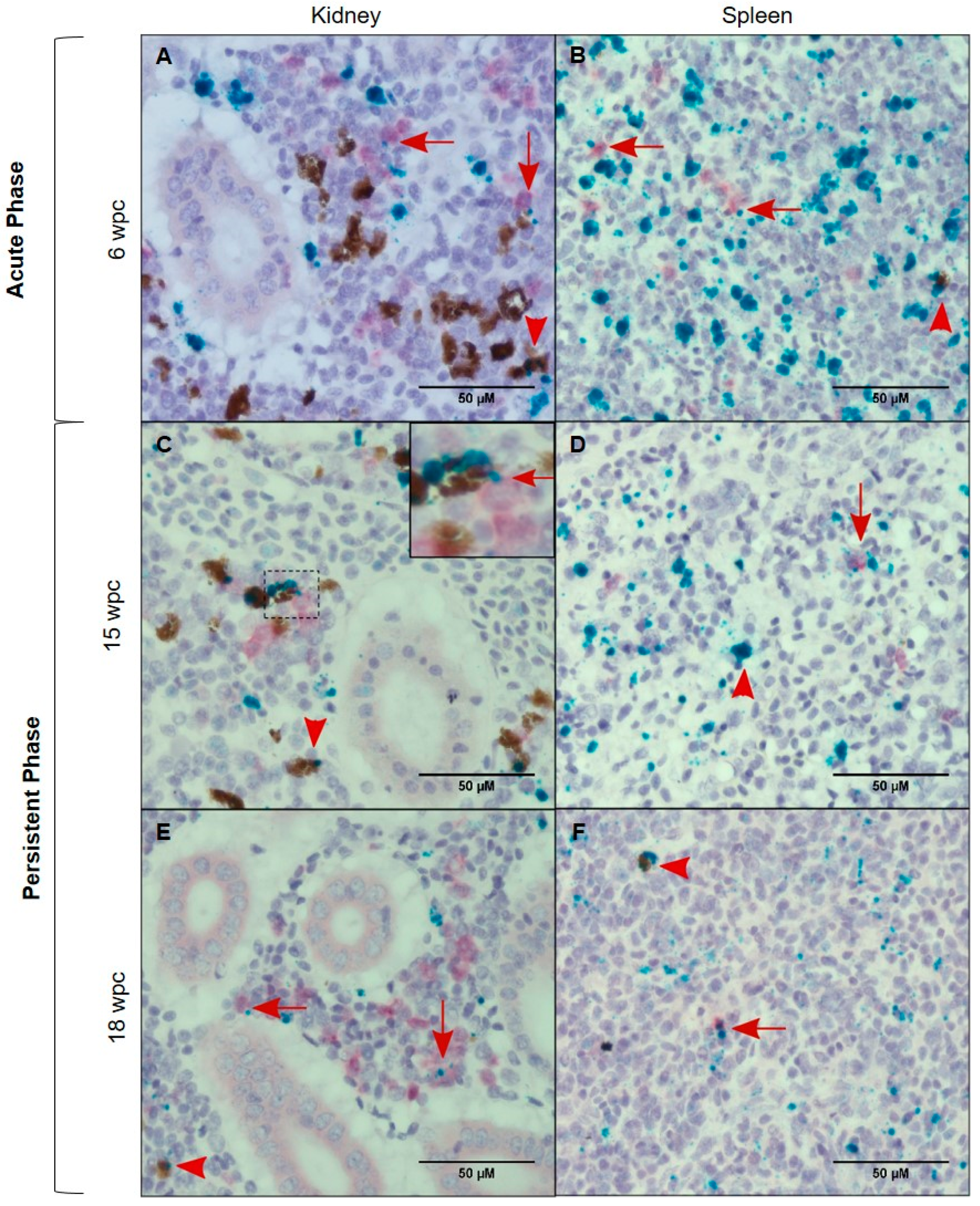
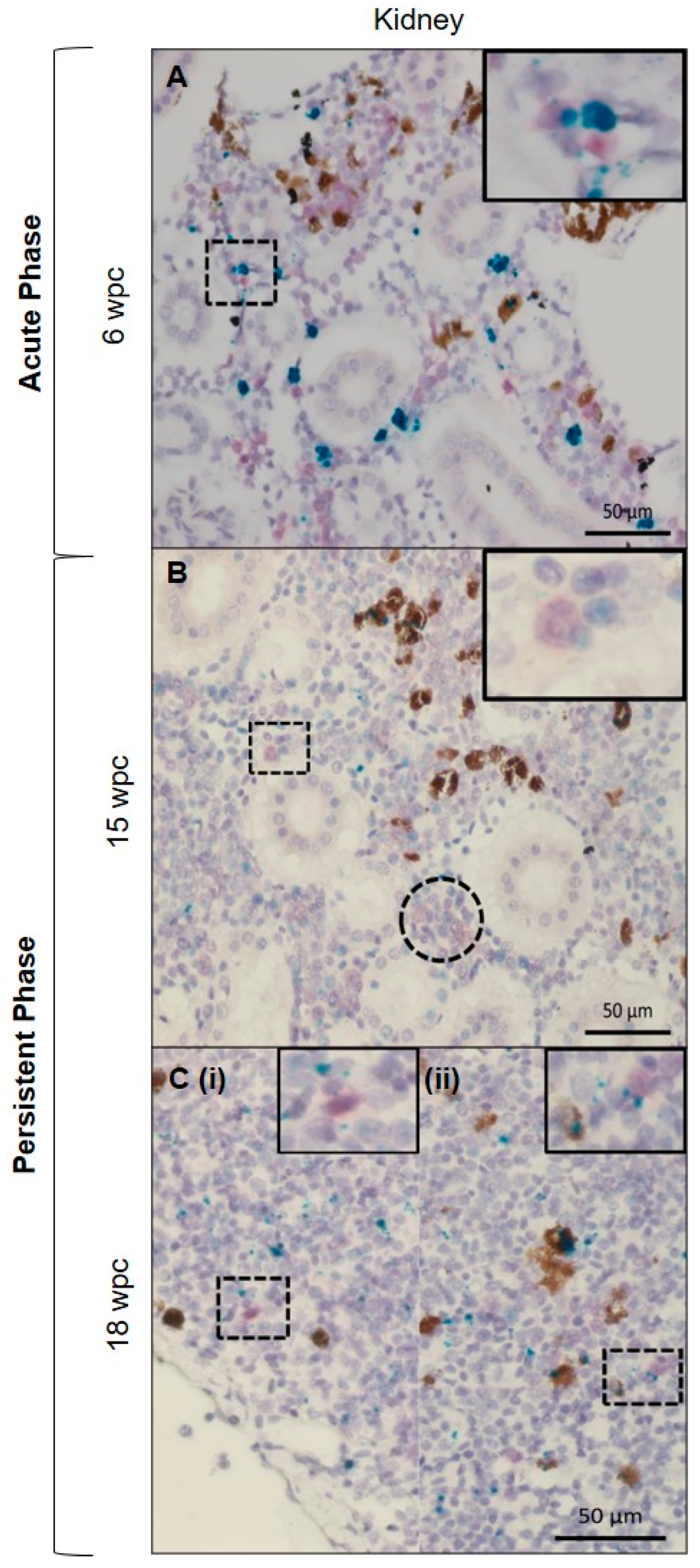
3.4. Tissue Localization of PRV-1 in the Acute Phase
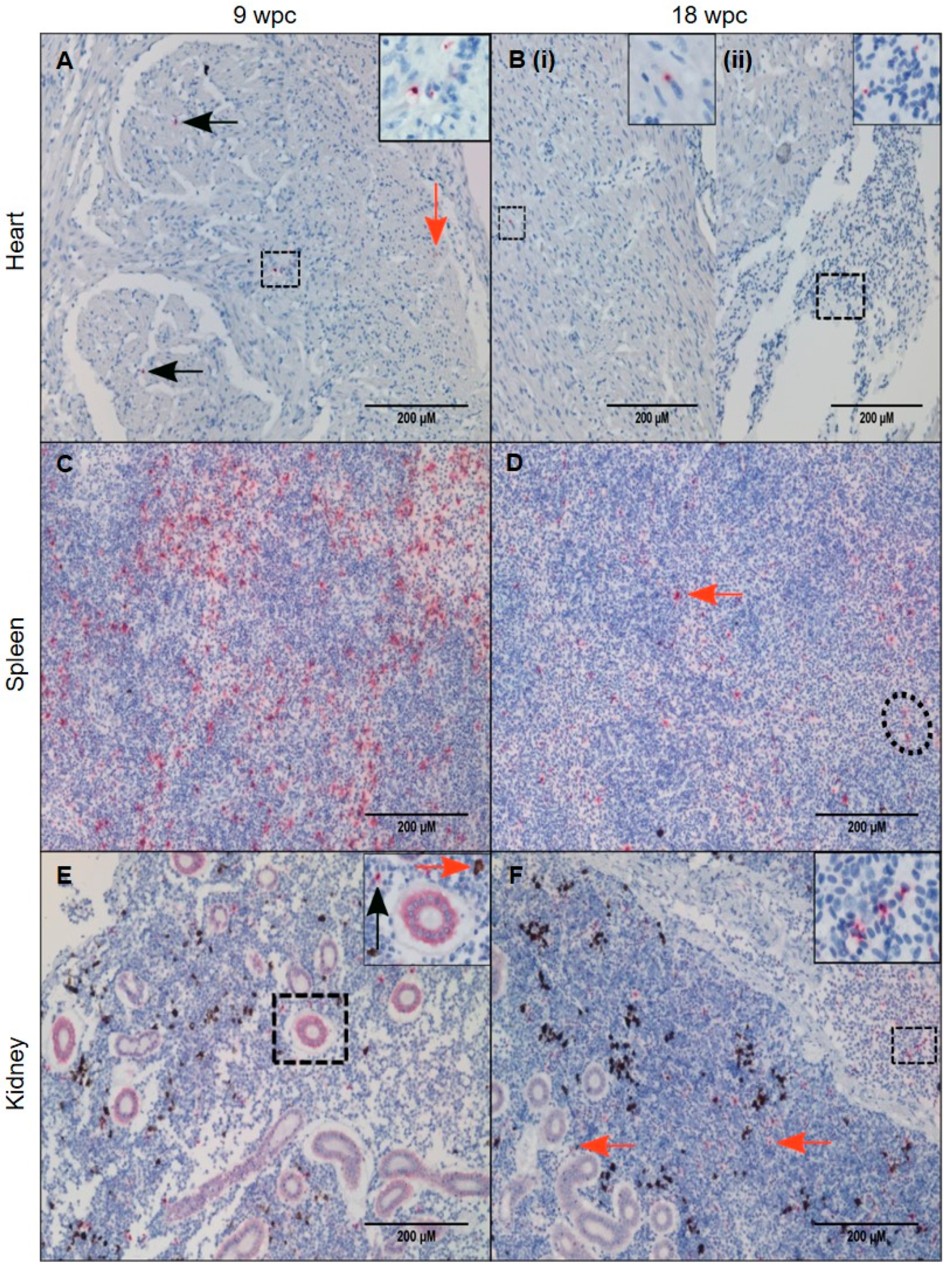
3.5. Tissue Localization of PRV-1 in the Persistent Phase
3.6. Characterization of the PRV-1 Infected Cell Populations in Kidney and Spleen
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Taranger, G.L.; Karlsen, O.; Bannister, R.J.; Glover, K.A.; Husa, V.; Karlsbakk, E.; Kvamme, B.O.; Boxaspen, K.K.; Bjorn, P.A.; Finstad, B.; et al. Risk assessment of the environmental impact of norwegian atlantic salmon farming. ICES J. Mar. Sci. 2015, 72, 997–1021. [Google Scholar] [CrossRef]
- Lovoll, M.; Wiik-Nielsen, J.; Grove, S.; Wiik-Nielsen, C.R.; Kristoffersen, A.B.; Faller, R.; Poppe, T.; Jung, J.; Pedamallu, C.S.; Nederbragt, A.J.; et al. A novel totivirus and piscine reovirus (prv) in atlantic salmon (salmo salar) with cardiomyopathy syndrome (cms). Virol. J. 2010, 7, 309. [Google Scholar] [CrossRef]
- Palacios, G.; Lovoll, M.; Tengs, T.; Hornig, M.; Hutchison, S.; Hui, J.; Kongtorp, R.T.; Savji, N.; Bussetti, A.V.; Solovyov, A.; et al. Heart and skeletal muscle inflammation of farmed salmon is associated with infection with a novel reovirus. PLoS ONE 2010, 5, e11487. [Google Scholar] [CrossRef]
- Wessel, O.; Braaen, S.; Alarcon, M.; Haatveit, H.; Roos, N.; Markussen, T.; Tengs, T.; Dahle, M.K.; Rimstad, E. Infection with purified piscine orthoreovirus demonstrates a causal relationship with heart and skeletal muscle inflammation in atlantic salmon. PLoS ONE 2017, 12, e0183781. [Google Scholar] [CrossRef]
- Kongtorp, R.T.; Taksdal, T.; Lyngoy, A. Pathology of heart and skeletal muscle inflammation (hsmi) in farmed atlantic salmon salmo salar. Dis. Aquat. Org. 2004, 59, 217–224. [Google Scholar] [CrossRef]
- Dhamotharan, K.; Tengs, T.; Wessel, O.; Braaen, S.; Nyman, I.B.; Hansen, E.F.; Christiansen, D.H.; Dahle, M.K.; Rimstad, E.; Markussen, T. Evolution of the piscine orthoreovirus genome linked to emergence of heart and skeletal muscle inflammation in farmed atlantic salmon (salmo salar). Viruses 2019, 11, 465. [Google Scholar] [CrossRef]
- Lund, M.; Krudtaa Dahle, M.; Timmerhaus, G.; Alarcon, M.; Powell, M.; Aspehaug, V.; Rimstad, E.; Jorgensen, S.M. Hypoxia tolerance and responses to hypoxic stress during heart and skeletal muscle inflammation in atlantic salmon (salmo salar). PLoS ONE 2017, 12, e0181109. [Google Scholar] [CrossRef]
- Polinski, M.P.; Marty, G.D.; Snyman, H.N.; Garver, K.A. Piscine orthoreovirus demonstrates high infectivity but low virulence in atlantic salmon of pacific canada. Sci. Rep. 2019, 9, 3297. [Google Scholar] [CrossRef]
- Coombs, K.M. Reovirus structure and morphogenesis. In Reoviruses: Entry, Assembly and Morphogenesis; Roy, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; pp. 117–167. [Google Scholar]
- Takano, T.; Nawata, A.; Sakai, T.; Matsuyama, T.; Ito, T.; Kurita, J.; Terashima, S.; Yasuike, M.; Nakamura, Y.; Fujiwara, A.; et al. Full-genome sequencing and confirmation of the causative agent of erythrocytic inclusion body syndrome in coho salmon identifies a new type of piscine orthoreovirus. PLoS ONE 2016, 11, e0165424. [Google Scholar] [CrossRef]
- Hauge, H.; Vendramin, N.; Taksdal, T.; Olsen, A.B.; Wessel, O.; Mikkelsen, S.S.; Alencar, A.L.F.; Olesen, N.J.; Dahle, M.K. Infection experiments with novel piscine orthoreovirus from rainbow trout (oncorhynchus mykiss) in salmonids. PLoS ONE 2017, 12, e0180293. [Google Scholar] [CrossRef]
- Finstad, O.W.; Dahle, M.K.; Lindholm, T.H.; Nyman, I.B.; Lovoll, M.; Wallace, C.; Olsen, C.M.; Storset, A.K.; Rimstad, E. Piscine orthoreovirus (prv) infects atlantic salmon erythrocytes. Vet. Res. 2014, 45, 35. [Google Scholar] [CrossRef]
- Gotting, M.; Nikinmaa, M.J. Transcriptomic analysis of young and old erythrocytes of fish. Front. Physiol. 2017, 8, 1046. [Google Scholar] [CrossRef]
- Takahashi, K.; Okamoto, N.; Maita, M.; Rohovec, J.S.; Ikeda, Y. Progression of erythrocytic inclusion body syndrome in artificially infected coho salmon. Fish Pathol. 1992, 27, 89–95. [Google Scholar] [CrossRef]
- Haatveit, H.M.; Wessel, O.; Markussen, T.; Lund, M.; Thiede, B.; Nyman, I.B.; Braaen, S.; Dahle, M.K.; Rimstad, E. Viral protein kinetics of piscine orthoreovirus infection in atlantic salmon blood cells. Viruses 2017, 9, 49. [Google Scholar] [CrossRef]
- Garver, K.A.; Johnson, S.C.; Polinski, M.P.; Bradshaw, J.C.; Marty, G.D.; Snyman, H.N.; Morrison, D.B.; Richard, J. Piscine orthoreovirus from western north america is transmissible to atlantic salmon and sockeye salmon but fails to cause heart and skeletal muscle inflammation. PLoS ONE 2016, 11, e0146229. [Google Scholar] [CrossRef]
- Dahle, M.K.; Wessel, O.; Timmerhaus, G.; Nyman, I.B.; Jorgensen, S.M.; Rimstad, E.; Krasnov, A. Transcriptome analyses of atlantic salmon (salmo salar l.) erythrocytes infected with piscine orthoreovirus (prv). Fish Shellfish Immunol. 2015, 45, 780–790. [Google Scholar] [CrossRef]
- Di Cicco, E.; Ferguson, H.W.; Schulze, A.D.; Kaukinen, K.H.; Li, S.; Vanderstichel, R.; Wessel, O.; Rimstad, E.; Gardner, I.A.; Hammell, K.L.; et al. Heart and skeletal muscle inflammation (hsmi) disease diagnosed on a british columbia salmon farm through a longitudinal farm study. PLoS ONE 2017, 12, e0171471. [Google Scholar] [CrossRef]
- Zuniga, E.I.; Macal, M.; Lewis, G.M.; Harker, J.A. Innate and adaptive immune regulation during chronic viral infections. Annu. Rev. Virol. 2015, 2, 573–597. [Google Scholar] [CrossRef]
- Lemay, G. Synthesis and translation of viral mrna in reovirus-infected cells: Progress and remaining questions. Viruses 2018, 10, 671. [Google Scholar] [CrossRef]
- Patton, J.T.; Vasquez-Del Carpio, R.; Tortorici, M.A.; Taraporewala, Z.F. Coupling of rotavirus genome replication and capsid assembly. In Advances in Virus Research; Academic Press: Cambridge, MA, USA, 2006; Volume 69, pp. 167–201. [Google Scholar]
- Urquhart, K.; Murray, A.G.; Gregory, A.; O’Dea, M.; Munro, L.A.; Smail, D.A.; Shanks, A.M.; Raynard, R.S. Estimation of infectious dose and viral shedding rates for infectious pancreatic necrosis virus in atlantic salmon, salmo salar l., post-smolts. J. Fish Dis. 2008, 31, 879–887. [Google Scholar] [CrossRef]
- Madhun, A.S.; Isachsen, C.H.; Omdal, L.M.; Einen, A.C.B.; Maehle, S.; Wennevik, V.; Niemela, E.; Svasand, T.; Karlsbakk, E. Prevalence of piscine orthoreovirus and salmonid alphavirus in sea-caught returning adult atlantic salmon (salmo salar l.) in northern norway. J. Fish Dis. 2018, 41, 797–803. [Google Scholar] [CrossRef]
- Lovoll, M.; Austbo, L.; Jorgensen, J.B.; Rimstad, E.; Frost, P. Transcription of reference genes used for quantitative rt-pcr in atlantic salmon is affected by viral infection. Vet. Res. 2011, 42, 8. [Google Scholar] [CrossRef]
- Di Cicco, E.; Ferguson, H.W.; Kaukinen, K.H.; Schulze, A.D.; Li, S.; Tabata, A.; Guenther, O.P.; Mordecai, G.; Suttle, C.A.; Miller, K.M. The same strain of piscine orthoreovirus (prv-1) is involved in the development of different, but related, diseases in atlantic and pacific salmon in british columbia. Facets 2018, 3, 599–641. [Google Scholar] [CrossRef]
- Finstad, O.W.; Falk, K.; Lovoll, M.; Evensen, O.; Rimstad, E. Immunohistochemical detection of piscine reovirus (prv) in hearts of atlantic salmon coincide with the course of heart and skeletal muscle inflammation (hsmi). Vet. Res. 2012, 43, 27. [Google Scholar] [CrossRef]
- Smith, J.A.; Schmechel, S.C.; Williams, B.R.; Silverman, R.H.; Schiff, L.A. Involvement of the interferon-regulated antiviral proteins pkr and rnase l in reovirus-induced shutoff of cellular translation. J. Virol. 2005, 79, 2240–2250. [Google Scholar] [CrossRef]
- Chang, Y.H.; Lau, K.S.; Kuo, R.L.; Horng, J.T. Dsrna binding domain of pkr is proteolytically released by enterovirus a71 to facilitate viral replication. Front. Cell. Infect. Microbiol. 2017, 7, 284. [Google Scholar] [CrossRef]
- Samuel, C.E.; Duncan, R.; Knutson, G.S.; Hershey, J.W. Mechanism of interferon action. Increased phosphorylation of protein synthesis initiation factor eif-2 alpha in interferon-treated, reovirus-infected mouse l929 fibroblasts in vitro and in vivo. J. Biol. Chem. 1984, 259, 13451–13457. [Google Scholar]
- Li, M.M.; MacDonald, M.R.; Rice, C.M. To translate, or not to translate: Viral and host mrna regulation by interferon-stimulated genes. Trends Cell Biol. 2015, 25, 320–329. [Google Scholar] [CrossRef]
- Lund, M.; Rosaeg, M.V.; Krasnov, A.; Timmerhaus, G.; Nyman, I.B.; Aspehaug, V.; Rimstad, E.; Dahle, M.K. Experimental piscine orthoreovirus infection mediates protection against pancreas disease in atlantic salmon (salmo salar). Vet. Res. 2016, 47, 107. [Google Scholar] [CrossRef]
- Wessel, O.; Haugland, O.; Rode, M.; Fredriksen, B.N.; Dahle, M.K.; Rimstad, E. Inactivated piscine orthoreovirus vaccine protects against heart and skeletal muscle inflammation in atlantic salmon. J. Fish Dis. 2018, 41, 1411–1419. [Google Scholar] [CrossRef]
- Diaz-Rosales, P.; Munoz-Atienza, E.; Tafalla, C. Role of teleost b cells in viral immunity. Fish Shellfish Immunol. 2019, 86, 135–142. [Google Scholar] [CrossRef]
- Teige, L.H.; Lund, M.; Haatveit, H.M.; Rosaeg, M.V.; Wessel, O.; Dahle, M.K.; Storset, A.K. A bead based multiplex immunoassay detects piscine orthoreovirus specific antibodies in atlantic salmon (salmo salar). Fish Shellfish Immunol. 2017, 63, 491–499. [Google Scholar] [CrossRef]
- Reyes-Cerpa, S.; Reyes-Lopez, F.; Toro-Ascuy, D.; Montero, R.; Maisey, K.; Acuna-Castillo, C.; Sunyer, J.O.; Parra, D.; Sandino, A.M.; Imarai, M. Induction of anti-inflammatory cytokine expression by ipnv in persistent infection. Fish Shellfish Immunol. 2014, 41, 172–182. [Google Scholar] [CrossRef]
- Press, C.M.; Evensen, O. The morphology of the immune system in teleost fishes. Fish Shellfish Immunol. 1999, 9, 309–318. [Google Scholar] [CrossRef]
- Bautista, D.; Rodriguez, L.S.; Franco, M.A.; Angel, J.; Barreto, A. Caco-2 cells infected with rotavirus release extracellular vesicles that express markers of apoptotic bodies and exosomes. Cell Stress Chaperones 2015, 20, 697–708. [Google Scholar] [CrossRef]
- Santiana, M.; Ghosh, S.; Ho, B.A.; Rajasekaran, V.; Du, W.L.; Mutsafi, Y.; De Jesus-Diaz, D.A.; Sosnovtsev, S.V.; Levenson, E.A.; Parra, G.I.; et al. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 2018, 24, 208–220.e8. [Google Scholar] [CrossRef]
- Hauge, H.; Dahle, M.; Moldal, T.; Thoen, E.; Gjevre, A.G.; Weli, S.; Alarcon, M.; Grove, S. Piscine orthoreovirus can infect and shed through the intestine in experimentally challenged atlantic salmon (salmo salar l.). Vet. Res. 2016, 47, 57. [Google Scholar] [CrossRef]
- Tyler, K.L.; Virgin, H.W.; Fields, B.N.J.N. Sites of action of immune-mediated protection against neurotropic reoviruses. Neurology 1989, 39, 268. [Google Scholar]
- Miller, K.D.; Schnell, M.J.; Rall, G.F. Keeping it in check: Chronic viral infection and antiviral immunity in the brain. Nat. Rev. Neurosci. 2016, 17, 766. [Google Scholar] [CrossRef]
- Patton, J.; Silvestri, L.; Tortorici, M.; Vasquez-Del Carpio, R.; Taraporewala, Z. Rotavirus genome replication and morphogenesis: Role of the viroplasm. In Reoviruses: Entry, Assembly and Morphogenesis; Springer: Berlin/Heidelberg, Germany, 2006; pp. 169–187. [Google Scholar]
- Ellis, A. Antigen-trapping in the spleen and kidney of the plaice pleuronectes platessa L. J. Fish Dis. 1980, 3, 413–426. [Google Scholar] [CrossRef]
- Bjorgen, H.; Wessel, O.; Fjelldal, P.G.; Hansen, T.; Sveier, H.; Saebo, H.R.; Enger, K.B.; Monsen, E.; Kvellestad, A.; Rimstad, E.; et al. Piscine orthoreovirus (prv) in red and melanised foci in white muscle of atlantic salmon (salmo salar). Vet. Res. 2015, 46, 89. [Google Scholar] [CrossRef]
- Hodgkinson, J.W.; Grayfer, L.; Belosevic, M. Biology of bony fish macrophages. Biology 2015, 4, 881–906. [Google Scholar] [CrossRef]
- Wang, T.H.; Hanington, P.C.; Belosevic, M.; Secombes, C.J. Two macrophage colony-stimulating factor genes exist in fish that differ in gene organization and are differentially expressed. J. Immunol. 2008, 181, 3310–3322. [Google Scholar] [CrossRef]
- Mulero, I.; Sepulcre, M.P.; Roca, F.J.; Meseguer, J.; Garcia-Ayala, A.; Mulero, V. Characterization of macrophages from the bony fish gilthead seabream using an antibody against the macrophage colony-stimulating factor receptor. Dev. Comp. Immunol. 2008, 32, 1151–1159. [Google Scholar] [CrossRef]
- Haugland, G.T.; Jordal, A.E.O.; Wergeland, H.I. Characterization of small, mononuclear blood cells from salmon having high phagocytic capacity and ability to differentiate into dendritic like cells. PLoS ONE 2012, 7, e49260. [Google Scholar] [CrossRef]
- Grayfer, L.; Hanington, P.C.; Belosevic, M. Macrophage colony-stimulating factor (csf-1) induces pro-inflammatory gene expression and enhances antimicrobial responses of goldfish (Carassius auratus L.) macrophages. Fish Shellfish Immunol. 2009, 26, 406–413. [Google Scholar] [CrossRef]
- Hume, D.A.; MacDonald, K.P.A. Therapeutic applications of macrophage colony-stimulating factor-1 (csf-1) and antagonists of csf-1 receptor (csf-1r) signaling. Blood 2012, 119, 1810–1820. [Google Scholar] [CrossRef]
- Svensson, J.; Jenmalm, M.C.; Matussek, A.; Geffers, R.; Berg, G.; Ernerudh, J. Macrophages at the fetal-maternal interface express markers of alternative activation and are induced by m-csf and il-10. J. Immunol. 2011, 187, 3671–3682. [Google Scholar] [CrossRef]
- Haatveit, H.M.; Hodneland, K.; Braaen, S.; Hansen, E.F.; Nyman, I.B.; Dahle, M.K.; Frost, P.; Rimstad, E. DNA vaccine expressing the non-structural proteins of piscine orthoreovirus delay the kinetics of prv infection and induces moderate protection against heart -and skeletal muscle inflammation in atlantic salmon (salmo salar). Vaccine 2018, 36, 7599–7608. [Google Scholar] [CrossRef]
- Bjorgen, H.; Haldorsen, R.; Oaland, O.; Kvellestad, A.; Kannimuthu, D.; Rimstad, E.; Koppang, E.O. Melanized focal changes in skeletal muscle in farmed atlantic salmon after natural infection with piscine orthoreovirus (prv). J. Fish Dis. 2019, 42, 935–945. [Google Scholar] [CrossRef]
- O’Hara, D.; Patrick, M.; Cepica, D.; Coombs, K.M.; Duncan, R. Avian reovirus major μ-class outer capsid protein influences efficiency of productive macrophage infection in a virus strain-specific manner. J. Virol. 2001, 75, 5027–5035. [Google Scholar] [CrossRef]
- Paffett-Lugassy, N.; Hsia, N.; Fraenkel, P.G.; Paw, B.; Leshinsky, I.; Barut, B.; Bahary, N.; Caro, J.; Handin, R.; Zon, L.I. Functional conservation of erythropoietin signaling in zebrafish. Blood 2007, 110, 2718–2726. [Google Scholar] [CrossRef]
- Lai, J.C.C.; Kakuta, I.; Mok, H.O.L.; Rummer, J.L.; Randall, D. Effects of moderate and substantial hypoxia on erythropoietin levels in rainbow trout kidney and spleen. J. Exp. Biol. 2006, 209, 2734–2738. [Google Scholar] [CrossRef]
- Morceau, F.; Dicato, M.; Diederich, M. Pro-inflammatory cytokine-mediated anemia: Regarding molecular mechanisms of erythropoiesis. Mediat. Inflamm. 2009, 2009, 405016. [Google Scholar] [CrossRef]
- Kulkeaw, K.; Sugiyama, D. Zebrafish erythropoiesis and the utility of fish as models of anemia. Stem Cell Res. Ther. 2012, 3, 55. [Google Scholar] [CrossRef]
- Davidson, A.J.; Zon, L.I. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 2004, 23, 7233–7246. [Google Scholar] [CrossRef]
- Catton, W.T. Blood cell formation in certain teleost fishes. Blood 1951, 6, 39–60. [Google Scholar]
- Krasnov, A.; Timmerhaus, G.; Afanasyev, S.; Takle, H.; Jørgensen, S.M.J.G.; Endocrinology, C. Induced erythropoiesis during acute anemia in atlantic salmon: A transcriptomic survey. Gen. Comp. Endocrinol. 2013, 192, 181–190. [Google Scholar] [CrossRef]

| Target (PRV) | Primer/Probe | Concentration | Sequence (5′-3′) |
|---|---|---|---|
| L1 | Fwd | 400 nM | CGCACTCCCACAGATACAGTTC |
| Rev | CGCGAGGTGTTACGTATTGTGA | ||
| M2 | Fwd | 400 nM | AGACTGGGAAGATCGTTGCTTT |
| Rev | ATGCGTCTTGTTGAGTGTAGGT | ||
| M3 | Fwd | 400 nM | GGCCTGCATTGTGTCAACGT |
| Rev | TGCGTTCAAGGTCGTCGTCA | ||
| S1 [12] | Fwd | 400 nM | TGCGTCCTGCGTATGGCACC |
| Rev | GGCTGGCATGCCCGAATAGCA | ||
| Probe (FAM) | 300 nM | ATCACAACGCCTACCT | |
| S2 | Fwd | 400 nM | ATCAATGGCTTCGCTCTTCCTCTCTT |
| Rev | TCTATATCCATTGCCGCATTTCCAGC | ||
| S3 | Fwd | 300 nM | AGCATCCTCACCATTTCCAAGCACTT |
| Rev | AGAGGCACGATACACTAGAGCTTGA | ||
| EF1 α [24] | Fwd | 300 nM | TGCCCCTCCAGGATGTCTAC |
| Rev | CACGGCCCACAGGTACTG |
| Probe | Channel ** | Accession no. | Target Region (bp) | |
|---|---|---|---|---|
| Target | PRV-L3 | C1 | KY429945 | 415–1379 |
| MCSFR | C2 | NM_001140235 | 434–1425 | |
| EPOR | C2 | NM_001140235 | 764–1754 | |
| Control | PPIB * | C1 | NM_001140870 | 20–934 |
| DapB * | C1 | EF191515 | 414–862 | |
| DapB * | C2 | EF191515 | 414–862 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malik, M.S.; Bjørgen, H.; Dhamotharan, K.; Wessel, Ø.; Koppang, E.O.; Di Cicco, E.; Hansen, E.F.; Dahle, M.K.; Rimstad, E. Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV). Viruses 2019, 11, 824. https://doi.org/10.3390/v11090824
Malik MS, Bjørgen H, Dhamotharan K, Wessel Ø, Koppang EO, Di Cicco E, Hansen EF, Dahle MK, Rimstad E. Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV). Viruses. 2019; 11(9):824. https://doi.org/10.3390/v11090824
Chicago/Turabian StyleMalik, Muhammad Salman, Håvard Bjørgen, Kannimuthu Dhamotharan, Øystein Wessel, Erling Olaf Koppang, Emiliano Di Cicco, Elisabeth F. Hansen, Maria K. Dahle, and Espen Rimstad. 2019. "Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV)" Viruses 11, no. 9: 824. https://doi.org/10.3390/v11090824
APA StyleMalik, M. S., Bjørgen, H., Dhamotharan, K., Wessel, Ø., Koppang, E. O., Di Cicco, E., Hansen, E. F., Dahle, M. K., & Rimstad, E. (2019). Erythroid Progenitor Cells in Atlantic Salmon (Salmo salar) May Be Persistently and Productively Infected with Piscine Orthoreovirus (PRV). Viruses, 11(9), 824. https://doi.org/10.3390/v11090824







