Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems
Abstract
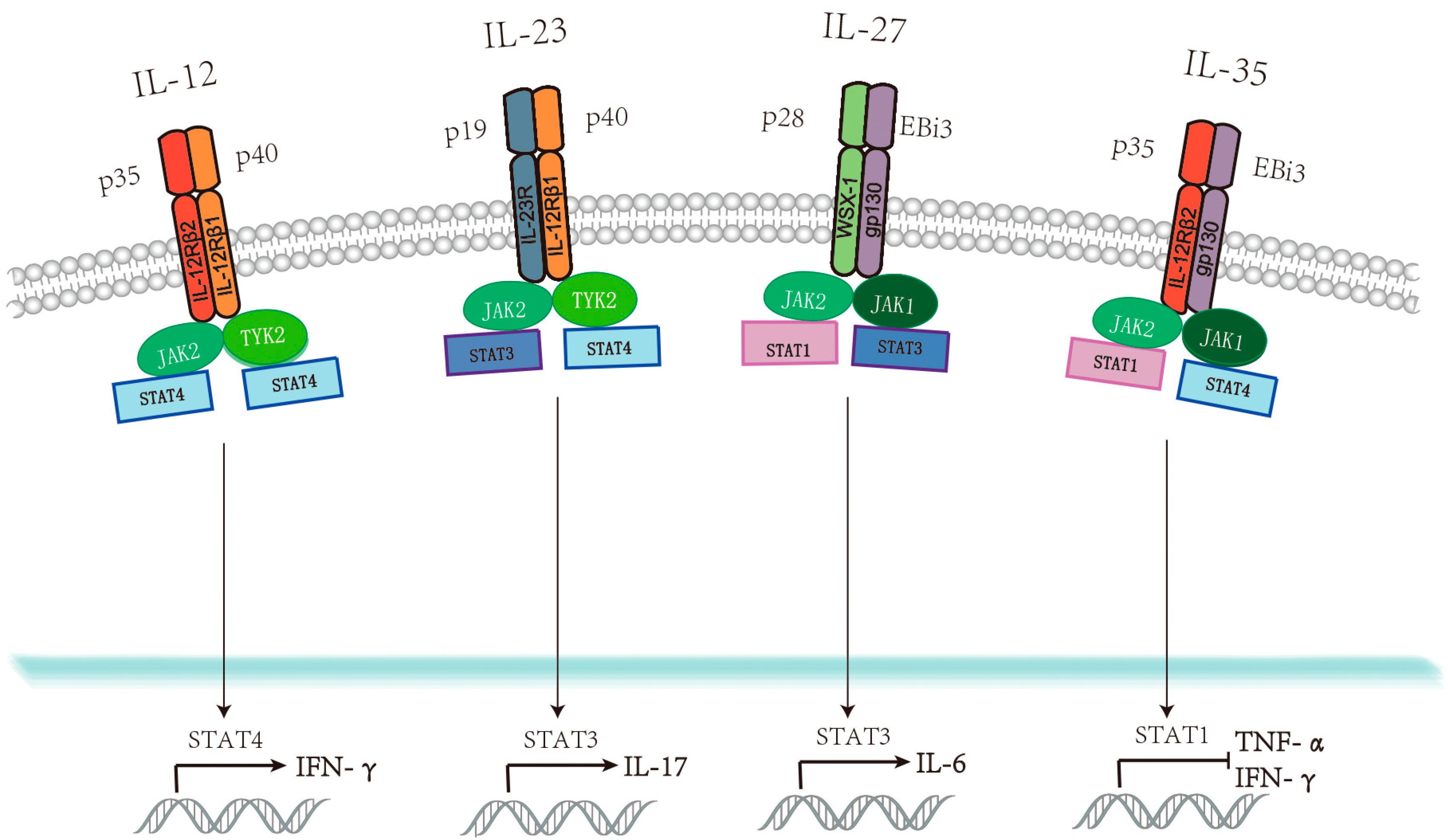
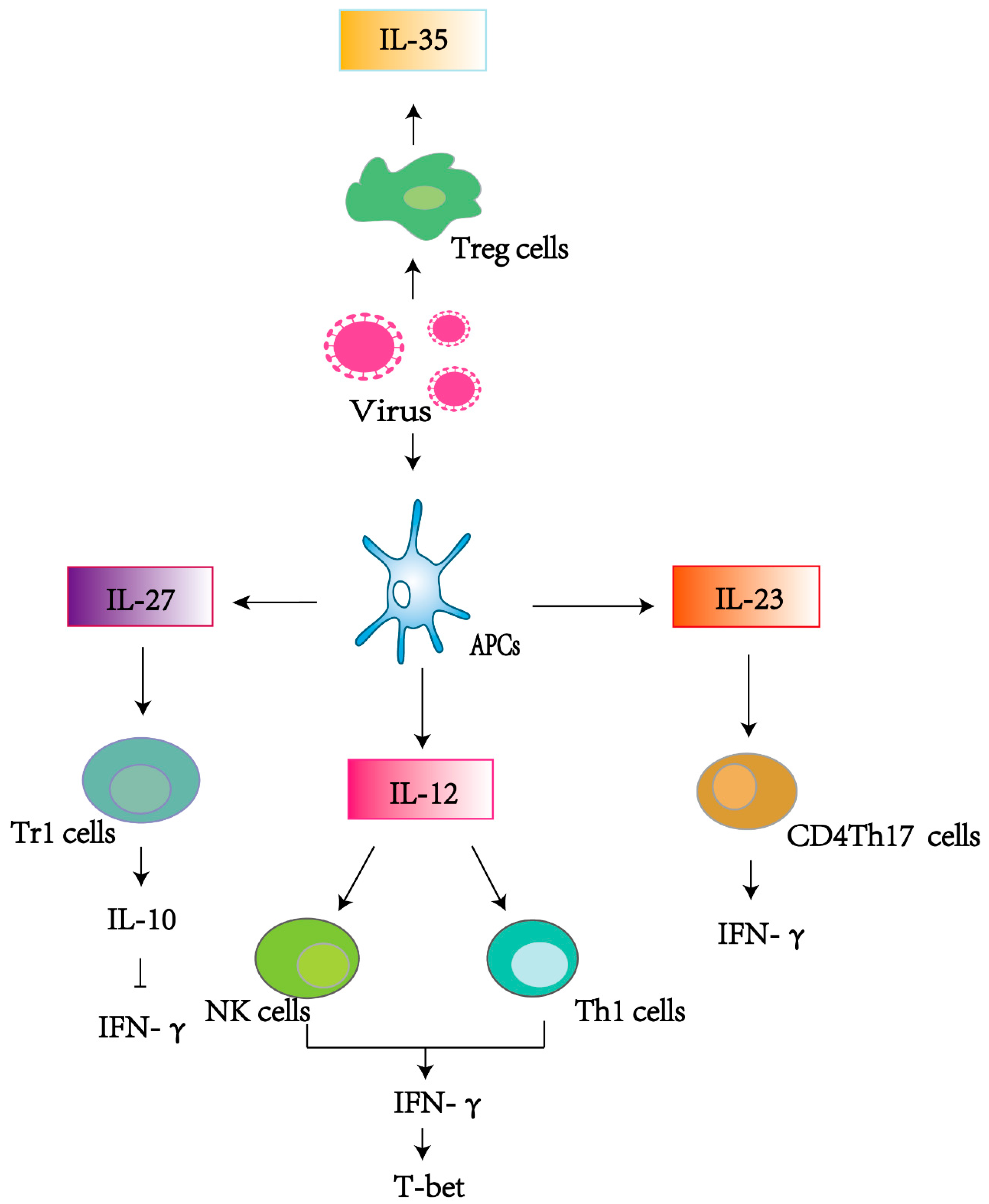
1. Introduction
2. Anti-Viral Functions
2.1. IL-12
2.2. IL-23
2.3. IL-27
2.4. IL-35
3. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Kobayashi, M.; Fitz, L.; Ryan, M.; Hewick, R.M.; Clark, S.C.; Chan, S.; Loudon, R.; Sherman, F.; Perussia, B.; Trinchieri, G. Identification and purification of natural killer cell stimulatory factor (NKSF), a cytokine with multiple biologic effects on human lymphocytes. J. Exp. Med. 1989, 170, 827–845. [Google Scholar] [CrossRef] [PubMed]
- Bastian, D.; Wu, Y.; Betts, B.C.; Yu, X.-Z. The IL-12 Cytokine and Receptor Family in Graft-vs.-Host Disease. Front. Immunol. 2019, 10, 988. [Google Scholar] [CrossRef] [PubMed]
- Behzadi, P.; Behzadi, E.; Ranjbar, R.; Information, R. IL-12 Family Cytokines: General Characteristics, Pathogenic Microorganisms, Receptors, and Signalling Pathways. Acta Microbiol. Immunol. Hung. 2016, 63, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Floss, D.M.; Schönberg, M.; Franke, M.; Horstmeier, F.C.; Engelowski, E.; Schneider, A.; Rosenfeldt, E.M.; Scheller, J. IL-6/IL-12 Cytokine Receptor Shuffling of Extra- and Intracellular Domains Reveals Canonical STAT Activation via Synthetic IL-35 and IL-39 Signaling. Sci. Rep. 2017, 7, 15172. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wei, Y.; Xiao, H.; Liu, X.; Zhang, Y.; Han, G.; Chen, G.; Hou, C.; Ma, N.; Shen, B.; et al. A novel IL-23p19/Ebi3 (IL-39) cytokine mediates inflammation in Lupus-like mice. Eur. J. Immunol. 2016, 46, 1343–1350. [Google Scholar] [CrossRef] [PubMed]
- Bridgewood, C.; Alase, A.; Watad, A.; Wittmann, M.; Cuthbert, R.; McGonagle, D. The IL-23p19/EBI3 heterodimeric cytokine termed IL-39 remains a theoretical cytokine in man. Inflamm. Res. 2019, 68, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Trinchieri, G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat. Rev. Immunol. 2003, 3, 133–146. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Mizoguchi, I.; Morishima, N.; Chiba, Y.; Mizuguchi, J.; Yoshimoto, T. Regulation of Antitumor Immune Responses by the IL-12 Family Cytokines, IL-12, IL-23, and IL-27. Clin. Dev. Immunol. 2010, 2010, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Aparicio-Siegmund, S.; Garbers, C. The biology of interleukin-27 reveals unique pro- and anti-inflammatory functions in immunity. Cytokine Growth Factor Rev. 2015, 26, 579–586. [Google Scholar] [CrossRef] [PubMed]
- Konjević, G.M.; Vuletić, A.M.; Martinović, K.M.M.; Larsen, A.K.; Jurišić, V.B. The role of cytokines in the regulation of NK cells in the tumor environment. Cytokine 2019, 117, 30–40. [Google Scholar] [CrossRef]
- Hamza, T.; Barnett, J.B.; Li, B. Interleukin 12 a Key Immunoregulatory Cytokine in Infection Applications. Int. J. Mol. Sci. 2010, 11, 789–806. [Google Scholar] [CrossRef] [PubMed]
- De Groen, R.A.; Boltjes, A.; Hou, J.; Liu, B.S.; McPhee, F.; Friborg, J.; Janssen, H.L.; Boonstra, A. IFN-lambda-mediated IL-12 production in macrophages induces IFN-gamma production in human NK cells. Eur. J. Immunol. 2015, 45, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Hu, Y.; Song, Z.; Liu, Y.; Guo, X.; Jie, Z. Interleukin-23 facilitates Th1 and Th2 cell differentiation in vitro following respiratory syncytial virus infection. J. Med. Virol. 2015, 87, 708–715. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, K.; Zhou, Z.; Wakamatsu, N.; Guerrero-Plata, A. Interleukin-12p40 Modulates Human Metapneumovirus-Induced Pulmonary Disease in an Acute Mouse Model of Infection. PLoS ONE 2012, 7, 37173. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Zandian, M.; Kuo, J.; Mott, K.R.; Chen, S.; Arditi, M.; Ghiasi, H. Suppression of IL-12p70 formation by IL-2 or following macrophage depletion causes T-cell autoreactivity leading to CNS demyelination in HSV-1-infected mice. PLoS Pathog. 2017, 13, e1006401. [Google Scholar] [CrossRef] [PubMed]
- Ugele, I.; Cárdenas-Conejo, Z.E.; Hammon, K.; Wehrstein, M.; Bruss, C.; Peter, K.; Singer, K.; Gottfried, E.; Boesch, J.; Oefner, P.; et al. D-2-Hydroxyglutarate and L-2-Hydroxyglutarate Inhibit IL-12 Secretion by Human Monocyte-Derived Dendritic Cells. Int. J. Mol. Sci. 2019, 20, 742. [Google Scholar] [CrossRef] [PubMed]
- Yu, Q.; Gu, J.X.; Kovacs, C.; Freedman, J.; Thomas, E.K.; Ostrowski, M.A. Cooperation of TNF family members CD40 ligand, receptor activator of NF-kappa B ligand, and TNF-alpha in the activation of dendritic cells and the expansion of viral specific CD8+ T cell memory responses in HIV-1-infected and HIV-1-uninfected individuals. J. Immunol. 2003, 170, 1797–1805. [Google Scholar] [CrossRef]
- Adalid-Peralta, L.; Godot, V.; Colin, C.; Krzysiek, R.; Tran, T.; Poignard, P.; Venet, A.; Hosmalin, A.; Lebon, P.; Rouzioux, C.; et al. Stimulation of the primary anti-HIV antibody response by IFN-{alpha} in patients with acute HIV-1 infection. J. Leukoc. Biol. 2008, 83, 1060–1067. [Google Scholar] [CrossRef]
- Garcia-Bates, T.M.; Palma, M.L.; Shen, C.; Gambotto, A.; Macatangay, B.J.C.; Ferris, R.L.; Rinaldo, C.R.; Mailliard, R.B. Contrasting Roles of the PD-1 Signaling Pathway in Dendritic Cell-Mediated Induction and Regulation of HIV-1-Specific Effector T Cell Functions. J. Virol. 2019, 93, 5. [Google Scholar] [CrossRef]
- Perdomo-Celis, F.; Feria, M.G.; Taborda, N.A.; Rugeles, M.T. Induction of Follicular-Like CXCR5(+) CD8(+) T Cells by TGF-beta1/IL-23 Is Limited During HIV Infection. Viral. Immunol. 2019. [Google Scholar] [CrossRef]
- Chuai, X.; Xie, B.; Deng, Y.; Zhao, Y.; Wang, W.; Gao, Z.; Wang, W.; Qiu, X.; Tan, W. HBV antigen and DNA loss from mouse serum is associated with novel vaccine-induced HBV surface antigen-specific cell-mediated immunity and cytokine production. Antivir. Res. 2019, 161, 20–27. [Google Scholar] [CrossRef] [PubMed]
- Schurich, A.; Pallett, L.J.; Lubowiecki, M.; Singh, H.D.; Gill, U.S.; Kennedy, P.T.; Nastouli, E.; Tanwar, S.; Rosenberg, W.; Maini, M.K. The third signal cytokine IL-12 rescues the anti-viral function of exhausted HBV-specific CD8 T cells. PLoS Pathog. 2013, 9, e1003208. [Google Scholar] [CrossRef] [PubMed]
- Bloch, Y.; Bouchareychas, L.; Merceron, R.; Skladanowska, K.; Van den Bossche, L.; Detry, S.; Govindarajan, S.; Elewaut, D.; Haerynck, F.; Dullaers, M.; et al. Structural Activation of Pro-inflammatory Human Cytokine IL-23 by Cognate IL-23 Receptor Enables Recruitment of the Shared Receptor IL-12Rbeta1. Immunity 2018, 48, 45–58. [Google Scholar] [CrossRef] [PubMed]
- Wojno, E.D.T.; Hunter, C.A.; Stumhofer, J.S. The Immunobiology of the Interleukin-12 Family: Room for Discovery. Immunity 2019, 50, 851–870. [Google Scholar] [CrossRef] [PubMed]
- Yannam, G.R.; Gutti, T.; Poluektova, L.Y. IL-23 in infections, inflammation, autoimmunity and cancer: Possible role in HIV-1 and AIDS. J. Neuroimmune Pharm. 2012, 7, 95–112. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ziblat, A.; Nunez, S.Y.; Raffo Iraolagoitia, X.L.; Spallanzani, R.G.; Torres, N.I.; Sierra, J.M.; Secchiari, F.; Domaica, C.I.; Fuertes, M.B.; Zwirner, N.W. Interleukin (IL)-23 Stimulates IFN-gamma Secretion by CD56(bright) Natural Killer Cells and Enhances IL-18-Driven Dendritic Cells Activation. Front. Immunol. 2017, 8, 1959. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, M.; Lahdou, I.; Opelz, G.; Mehrabi, A.; Zeier, M.; Schnitzler, P.; Daniel, V. IL-23 plasma level is strongly associated with CMV status and reactivation of CMV in renal transplant recipients. BMC Immunol. 2016, 17, 35. [Google Scholar] [CrossRef]
- Garg, A.; Rawat, P.; Spector, S.A. Interleukin 23 produced by myeloid dendritic cells contributes to T-cell dysfunction in HIV type 1 infection by inducing SOCS1 expression. J. Infect. Dis. 2015, 211, 755–768. [Google Scholar] [CrossRef]
- Barjaktarevic, I.Z.; Crystal, R.G.; Kaner, R.J. The Role of Interleukin-23 in the Early Development of Emphysema in HIV1(+) Smokers. J. Immunol. Res. 2016, 2016, 3463104. [Google Scholar] [CrossRef]
- Meng, P.; Zhao, S.; Niu, X.; Fu, N.; Su, S.; Wang, R.; Zhang, Y.; Nan, Y.; Qiao, L. Involvement of the Interleukin-23/Interleukin-17 Axis in Chronic Hepatitis C Virus Infection and Its Treatment Responses. Int. J. Mol. Sci. 2016, 17, 1070. [Google Scholar] [CrossRef]
- Zhu, H.; Lou, F.; Yin, Q.; Gao, Y.; Sun, Y.; Bai, J.; Xu, Z.; Liu, Z.; Cai, W.; Ke, F.; et al. RIG-I antiviral signaling drives interleukin-23 production and psoriasis-like skin disease. EMBO Mol. Med. 2017, 9, 589–604. [Google Scholar] [CrossRef]
- Odigie, M.; Osinusi, A.; Barrett, L.; Townsend, K.; Wang, H.; Suffredini, A.F.; Masur, H.; Polis, M.A.; Kottilil, S. Inteleukin-23 promotes interferon-alpha responsiveness in hepatitis C virus/HIV-coinfected patients. AIDS Res. Hum. Retrovir. 2014, 30, 775–782. [Google Scholar] [CrossRef]
- Meng, Z.; Zhang, Y.; Wei, Z.; Liu, P.; Kang, J.; Zhang, Y.; Ma, D.; Ke, C.; Chen, Y.; Luo, J.; et al. High serum resistin associates with intrahepatic inflammation and necrosis: An index of disease severity for patients with chronic HBV infection. BMC Gastroenterol. 2017, 17, 2053. [Google Scholar] [CrossRef]
- Meng, Z.; Wang, J.; Yuan, Y.; Cao, G.; Fan, S.; Gao, C.; Wang, L.; Li, Z.; Wu, X.; Wu, Z.; et al. γδ T cells are indispensable for interleukin-23-mediated protection against Concanavalin A-induced hepatitis in hepatitis B virus transgenic mice. Immunology 2017, 151, 43–55. [Google Scholar] [CrossRef]
- Shen, H.; Gu, J.; Xiao, H.; Liang, S.; Yang, E.; Yang, R.; Huang, D.; Chen, C.; Wang, F.; Shen, L.; et al. Selective Destruction of Interleukin 23-Induced Expansion of a Major Antigen-Specific gammadelta T-Cell Subset in Patients with Tuberculosis. J. Infect. Dis. 2017, 215, 420–430. [Google Scholar]
- Bao, S.; Zhao, Q.; Zheng, J.-M.; Li, N.; Huang, C.; Chen, M.; Cheng, Q.; Zhu, M.; Yu, K.; Liu, C.; et al. Interleukin-23 mediates the pathogenesis of LPS/GalN-induced liver injury in mice. Int. Immunopharmacol. 2017, 46, 97–104. [Google Scholar] [CrossRef]
- Tamassia, N.; Arruda-Silva, F.; Wright, H.L.; Moots, R.J.; Gardiman, E.; Bianchetto-Aguilera, F.; Gasperini, S.; Capone, M.; Maggi, L.; Annunziato, F.; et al. Human neutrophils activated via TLR8 promote Th17 polarization through IL-23. J. Leukoc. Biol. 2019. [Google Scholar] [CrossRef]
- Deng, J.; Yu, X.-Q.; Wang, P.-H. Inflammasome activation and Th17 responses. Mol. Immunol. 2019, 107, 142–164. [Google Scholar] [CrossRef]
- Jiang, Q.; Sun, Y.; Guo, Z.; Chen, R.; Ma, S.; Fu, M.; Zhu, H.; Ning, Q.; Lei, P.; Shen, G. IL-23 enhances the malignant properties of hepatoma cells by attenuation of HNF4alpha. Oncotarget 2018, 9, 28309–28321. [Google Scholar] [CrossRef]
- Miralles, M.; Eixarch, H.; Tejero, M.; Costa, C.; Hirota, K.; Castaño, A.R.; Puig, M.; Stockinger, G.; Montalban, X.; Bosch, A.; et al. Clinical and Histopathological Amelioration of Experimental Autoimmune Encephalomyelitis by AAV Vectors Expressing a Soluble Interleukin-23 Receptor. Neurotherapeutics 2017, 14, 1095–1106. [Google Scholar] [CrossRef]
- Gee, K.; Guzzo, C.; Mat, N.C.; Ma, W.; Kumar, A. The IL-12 Family of Cytokines in Infection, Inflammation and Autoimmune Disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef]
- Méndez-Samperio, P. Role of interleukin-12 family cytokines in the cellular response to mycobacterial disease. Int. J. Infect. Dis. 2010, 14, e366–e371. [Google Scholar] [CrossRef]
- Trinchieri, G.; Pflanz, S.; Kastelein, R.A. The IL-12 family of heterodimeric cytokines: New players in the regulation of T cell responses. Immunity 2003, 19, 641–644. [Google Scholar] [CrossRef]
- Nelson, D.A.; Tolbert, M.D.; Clemens, M.G.; Bost, K.L. Interleukin-27 expression following infection with the murine gammaherpesvirus 68. Cytokine 2010, 51, 184–194. [Google Scholar] [CrossRef]
- Gajanayaka, N.; O’Hara, S.; Konarski, Y.; Fernandes, J.; Muthumani, K.; Kozlowski, M.; Angel, J.B.; Kumar, A. HIV and HIV-Tat inhibit LPS-induced IL-27 production in human macrophages by distinct intracellular signaling pathways. J. Leukoc. Biol. 2017, 102, 925–939. [Google Scholar] [CrossRef]
- Chen, Q.; Swaminathan, S.; Yang, D.; Dai, L.; Sui, H.; Yang, J.; Hornung, R.L.; Wang, Y.; Huang da, W.; Hu, X.; et al. Interleukin-27 is a potent inhibitor of cis HIV-1 replication in monocyte-derived dendritic cells via a type I interferon-independent pathway. PLoS ONE 2013, 8, e59194. [Google Scholar] [CrossRef]
- Orii, N.; Mizoguchi, I.; Chiba, Y.; Hasegawa, H.; Ohashi, M.; Xu, M.; Nagai, T.; Ochiai, N.; Mochizuki, Y.; Owaki, T.; et al. Protective effects against tumors and infection by interleukin-27 through promotion of expansion and differentiation of hematopoietic stem cells into myeloid progenitors. OncoImmunology 2018, 7, e1421892. [Google Scholar] [CrossRef]
- Zheng, Y.H.; Xiao, S.L.; He, B.; He, Y.; Zhou, H.Y.; Chen, Z.; Zheng, L.W.; He, M.; Wang, H.Y.; Lin, Y.H.; et al. The Role of IL-27 and its Receptor in the Pathogenesis of HIV/AIDS and Anti-viral Immune Response. Curr. HIV Res. 2017, 15, 279–284. [Google Scholar] [CrossRef]
- Frank, A.C.; Zhang, X.; Katsounas, A.; Bharucha, J.P.; Kottilil, S.; Imamichi, T. Interleukin-27, an Anti-HIV-1 Cytokine, Inhibits Replication of Hepatitis C Virus. J. Interferon Cytokine Res. 2010, 30, 427–431. [Google Scholar] [CrossRef]
- Ashrafi Hafez, A.; Ahmadi Vasmehjani, A.; Baharlou, R.; Mousavi Nasab, S.D.; Davami, M.H.; Najafi, A.; Joharinia, N.; Rezanezhad, H.; Ahmadi, N.A.; Imanzad, M. Analytical assessment of interleukin—23 and—27 cytokines in healthy people and patients with hepatitis C virus infection (genotypes 1 and 3a). Hepat. Mon. 2014, 14, e21000. [Google Scholar] [CrossRef]
- Liu, L.; Cao, Z.; Chen, J.; Li, R.; Cao, Y.; Zhu, C.; Wu, K.; Wu, J.; Liu, F.; Zhu, Y. Influenza A Virus Induces Interleukin-27 through Cyclooxygenase-2 and Protein Kinase A Signaling. J. Biol. Chem. 2012, 287, 11899–11910. [Google Scholar] [CrossRef]
- Cao, Y.; Zhang, R.; Zhang, W.; Zhu, C.; Yu, Y.; Song, Y.; Wang, Q.; Bai, L.; Liu, Y.; Wu, K.; et al. IL-27, a cytokine, and IFN-lambda1, a type III IFN, are coordinated to regulate virus replication through type I IFN. J. Immunol. 2014, 192, 691–703. [Google Scholar] [CrossRef]
- Takeda, A.; Hamano, S.; Shiraishi, H.; Yoshimura, T.; Ogata, H.; Ishii, K.; Ishibashi, T.; Yoshimura, A.; Yoshida, H. WSX-1 over-expression in CD4(+) T cells leads to hyperproliferation and cytokine hyperproduction in response to TCR stimulation. Int. Immunol. 2005, 17, 889–897. [Google Scholar] [CrossRef]
- Yang, X.; Hao, H.; Xia, Z.; Xu, G.; Cao, Z.; Chen, X.; Liu, S.; Zhu, Y. Soluble IL-6 Receptor and IL-27 Subunit p28 Protein Complex Mediate the Antiviral Response through the Type III IFN Pathway. J. Immunol. 2016, 197, 2369–2381. [Google Scholar] [CrossRef]
- Zwirner, N.W.; Ziblat, A. Regulation of NK Cell Activation and Effector Functions by the IL-12 Family of Cytokines: The Case of IL-27. Front. Immunol. 2017, 8, 279. [Google Scholar] [CrossRef]
- Zare, A.; Karimi, M.H.; Rashki, A.; Geramizadeh, B.; Afshari, A.; Miri, H.R.; Yaghobi, R. Association of the Interleukin-27 Gene Expression and Hepatitis B Virus Infection in Liver Transplanted Patients. Exp. Clin. Transplant. 2017, 15, 554–560. [Google Scholar]
- De Aquino, M.T.P.; Kapil, P.; Hinton, D.R.; Phares, T.W.; Puntambekar, S.S.; Savarin, C.; Bergmann, C.C.; Stohlman, S.A. IL-27 Limits Central Nervous System Viral Clearance by Promoting IL-10 and Enhances Demyelination. J. Immunol. 2014, 193, 285–294. [Google Scholar] [CrossRef]
- Ramamurthy, N.; Boninsegna, S.; Adams, R.; Sahgal, N.; Lockstone, H.; Baban, D.; Marchi, E.; Klenerman, P. Impact of IL-27 on hepatocyte antiviral gene expression and function. Wellcome Open Res. 2016, 1, 17. [Google Scholar] [CrossRef]
- Zhang, G.L.; Xie, D.Y.; Ye, Y.N.; Lin, C.S.; Zhang, X.H.; Zheng, Y.B.; Huang, Z.L.; Peng, L.; Gao, Z.L. High level of IL-27 positively correlated with Th17 cells may indicate liver injury in patients infected with HBV. Liver Int. 2014, 34, 266–273. [Google Scholar] [CrossRef]
- Gehlert, T.; Devergne, O.; Niedobitek, G. Epstein-Barr Virus (EBV) infection and expression of the interleukin-12 family member EBV-induced gene 3 (EBI3) in chronic inflammatory bowel disease. J. Med. Virol. 2004, 73, 432–438. [Google Scholar] [CrossRef]
- Yang, L.; Shao, X.; Jia, S.; Zhang, Q.; Jin, Z. Interleukin-35 Dampens CD8(+) T Cells Activity in Patients With Non-viral Hepatitis-Related Hepatocellular Carcinoma. Front. Immunol. 2019, 10, 1032. [Google Scholar] [CrossRef]
- Xiang, X.G.; Xie, Q. IL-35: A potential therapeutic target for controlling hepatitis B virus infection. J. Dig. Dis. 2015, 16, 1–6. [Google Scholar] [CrossRef]
- Choi, J.; Leung, P.S.C.; Bowlus, C.; Gershwin, M.E. IL-35 and Autoimmunity: A Comprehensive Perspective. Clin. Rev. Allergy Immunol. 2015, 49, 327–332. [Google Scholar] [CrossRef]
- Liu, M.X.; Liu, Q.Y.; Liu, Y.; Cheng, Z.M.; Liu, L.; Zhang, L.; Sun, D.H. Interleukin-35 suppresses antitumor activity of circulating CD8(+) T cells in osteosarcoma patients. Connect. Tissue Res. 2019, 60, 367–375. [Google Scholar] [CrossRef]
- Catalan-Dibene, J.; McIntyre, L.L.; Zlotnik, A. Interleukin 30 to Interleukin 40. J. Interferon Cytokine Res. 2018, 38, 423–439. [Google Scholar] [CrossRef]
- Chen, X.; Hao, S.; Zhao, Z.; Liu, J.; Shao, Q.; Wang, F.; Sun, D.; He, Y.; Gao, W.; Mao, H. Interleukin 35: Inhibitory regulator in monocyte-derived dendritic cell maturation and activation. Cytokine 2018, 108, 43–52. [Google Scholar] [CrossRef]
- Teymouri, M.; Pirro, M.; Fallarino, F.; Gargaro, M.; Sahebkar, A. IL-35, a hallmark of immune-regulation in cancer progression, chronic infections and inflammatory diseases. Int. J. Cancer 2018, 143, 2105–2115. [Google Scholar] [CrossRef]
- Tao, N.N.; Gong, R.; Chen, X.; He, L.; Ren, F.; Yu, H.B.; Chen, J.; Ren, J.H. Interleukin-35 stimulates hepatitis B virus transcription and replication by targeting transcription factor HNF4alpha. J. Gen. Virol. 2018, 99, 645–654. [Google Scholar] [CrossRef]
- Wu, S.; Li, Y.; Yao, L.; Li, Y.; Jiang, S.; Gu, W.; Shen, H.; Xia, L.; Lu, J. Interleukin-35 inhibits angiogenesis through STAT1 signalling in rheumatoid synoviocytes. Clin. Exp. Rheumatol. 2018, 36, 223–227. [Google Scholar]
- Shao, X.; Ma, J.; Jia, S.; Yang, L.; Wang, W.; Jin, Z. Interleukin-35 Suppresses Antiviral Immune Response in Chronic Hepatitis B Virus Infection. Front. Microbiol. 2017, 7, 472. [Google Scholar] [CrossRef]
- Wang, L.; Zhu, S.; Xu, G.; Feng, J.; Han, T.; Zhao, F.; She, Y.-L.; Liu, S.; Ye, L.; Zhu, Y. Gene Expression and Antiviral Activity of Interleukin-35 in Response to Influenza A Virus Infection. J. Biol. Chem. 2016, 291, 16863–16876. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Zhang, Q.; Shao, X.; Wang, W.; Zhang, C.; Jin, Z. An immunosuppressive function of interleukin-35 in chronic hepatitis C virus infection. Int. Immunopharmacol. 2017, 50, 87–94. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pan, X.; Peng, X.; Li, S.; Zhou, Y.; Zheng, X.; Li, M. Adenovirus-mediated interleukin-35 gene transfer suppresses allergic airway inflammation in a murine model of asthma. Inflamm. Res. 2015, 64, 767–774. [Google Scholar] [CrossRef] [PubMed]
| HIV | HBV | |
|---|---|---|
| IL-12 | CD40L+TNF-α  IL-12 IL-12 Th1  IFN-γ IFN-γ  T-bet T-bet  IL-12R IL-12R IFN-α2b, RANKL suppress HIV | IL-12  CD4/8 T cells CD4/8 T cells  IFN-γ IFN-γ TNF-α, T-bet suppress HIV |
| IL-23 | Th17  IL-17/22 IL-17/22  IL-23 IL-23 NK  IL-23R IL-23R IFN-α, SOCS1 suppress HIV | IL-23  IL-17 IL-17  γδT cells γδT cells IL-23  IFN-γ, TNF-α, SOCS1 IFN-γ, TNF-α, SOCS1 Th17, PD-1 suppress HIV |
| IL-27 | MDMs + Tat  p38 MAPKs p38 MAPKs IFN-λ1 suppresses HIV | IL-27  Tr1-like CD4 T cells Tr1-like CD4 T cells IL-27  GATA3, T-bet GATA3, T-bet IFN-λ1, NF-κb suppress HIV |
| IL-35 | Unknown | IL-35  Th1/17 Th1/17 IL-35  IFN-γ, TNF-α IFN-γ, TNF-α Promoting HBV |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, Y.; Cao, W.; Zhu, Y. Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems. Viruses 2019, 11, 772. https://doi.org/10.3390/v11090772
Guo Y, Cao W, Zhu Y. Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems. Viruses. 2019; 11(9):772. https://doi.org/10.3390/v11090772
Chicago/Turabian StyleGuo, Yifei, Wei Cao, and Ying Zhu. 2019. "Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems" Viruses 11, no. 9: 772. https://doi.org/10.3390/v11090772
APA StyleGuo, Y., Cao, W., & Zhu, Y. (2019). Immunoregulatory Functions of the IL-12 Family of Cytokines in Antiviral Systems. Viruses, 11(9), 772. https://doi.org/10.3390/v11090772




