ZIKV Envelope Domain-Specific Antibodies: Production, Purification and Characterization
Abstract
1. Introduction
2. Materials and Methods
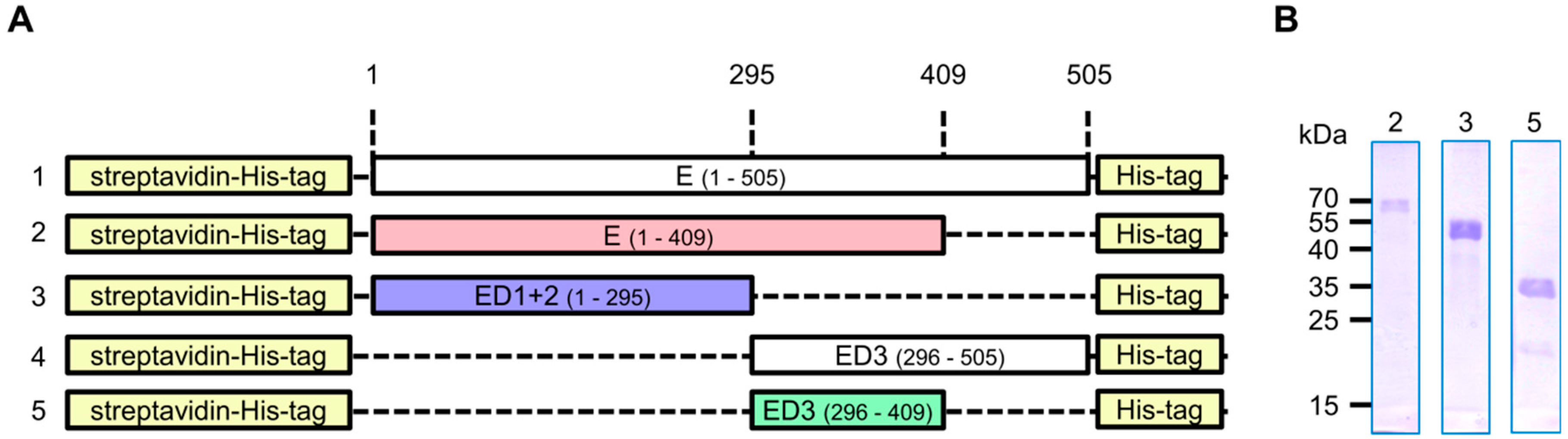
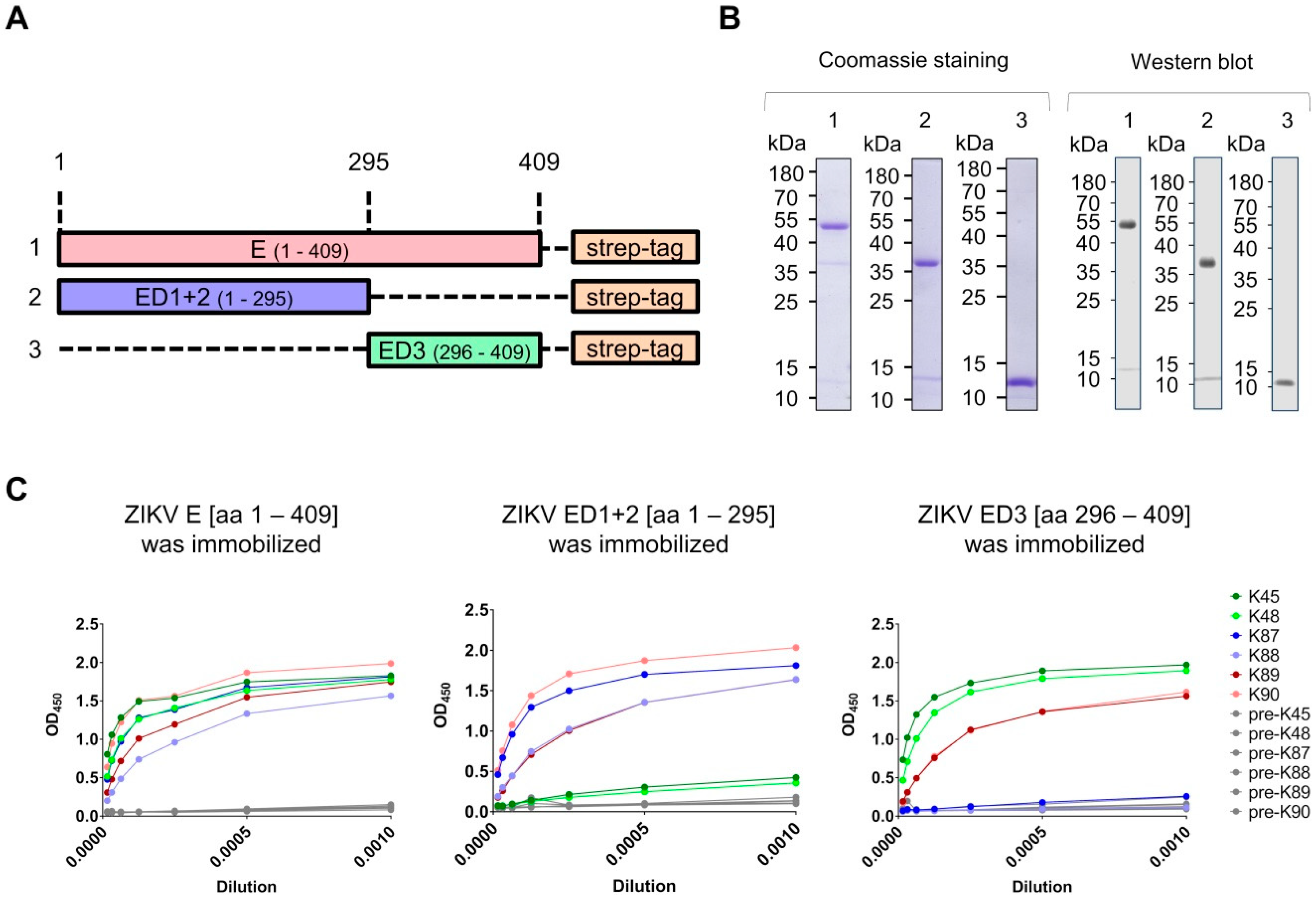
2.1. Construct Design
2.2. Protein Production and Rabbit Immunization
2.3. Antibody Purification
2.3.1. Preparation of the Affinity Beads
2.3.2. Antibody Purification
2.4. SDS-PAGE and Western Blot Analysis
2.5. Indirect Immunofluorescence Microscopy Analysis
2.6. Peptide Arrays for Determination of the Antibody Binding Sites
2.6.1. Multipeptide Microarray Preparation
2.6.2. Binding Sites Determination
2.7. Viruses
2.8. ZIKV Purification
2.9. ELISA
2.9.1. Coating with Purified ZIKV E Proteins
2.9.2. Coating with Purified Virus Particles
2.10. Plaque Reduction Neutralization Test (PRNT)
2.11. Surface Plasmon Resonance (SPR)
2.12. Software and Statistical Analyses
3. Results
3.1. Production of Recombinant Proteins and Immunization of Rabbits
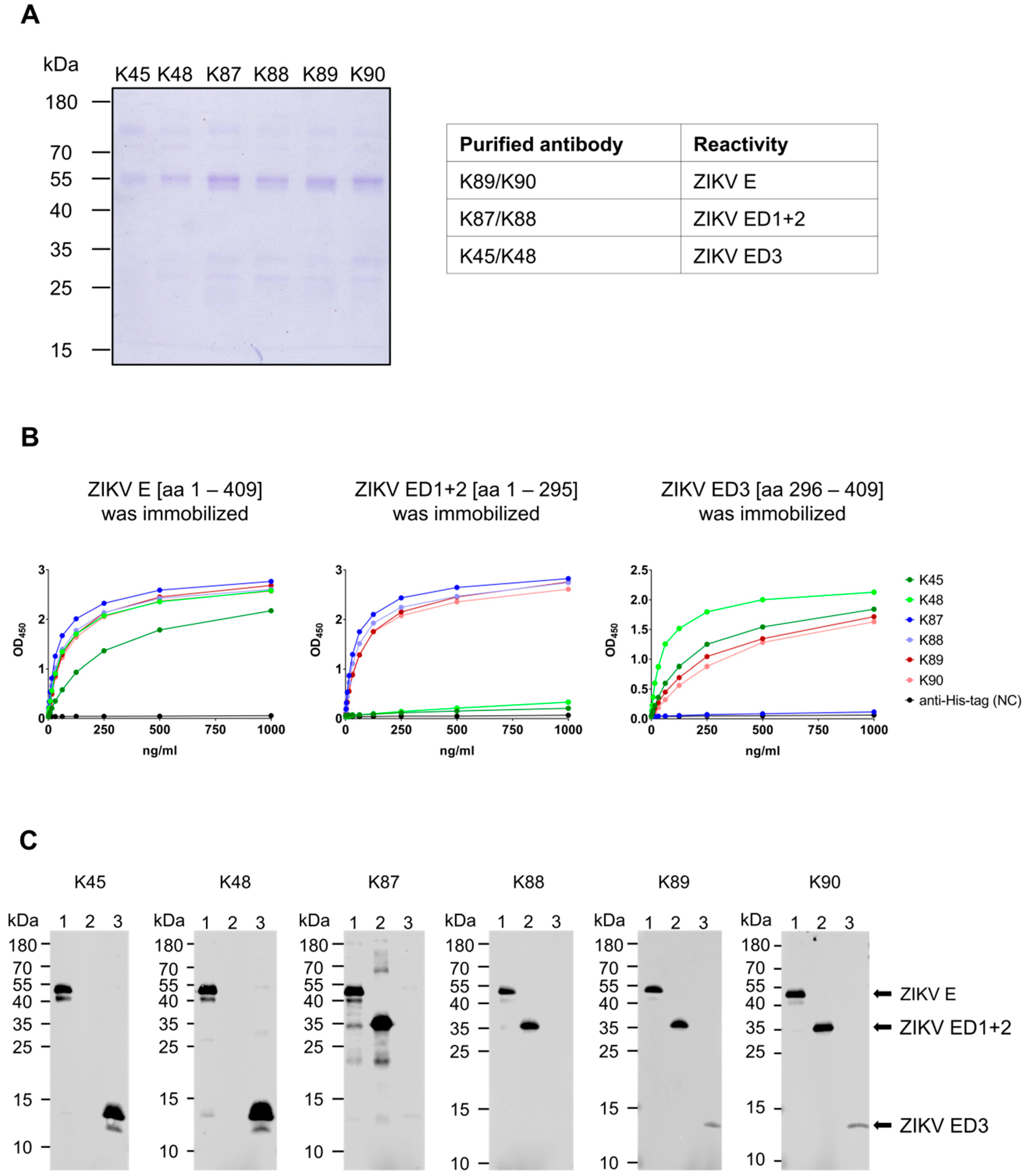
3.2. Generation and Purification of ZIKV E-Specific Antisera
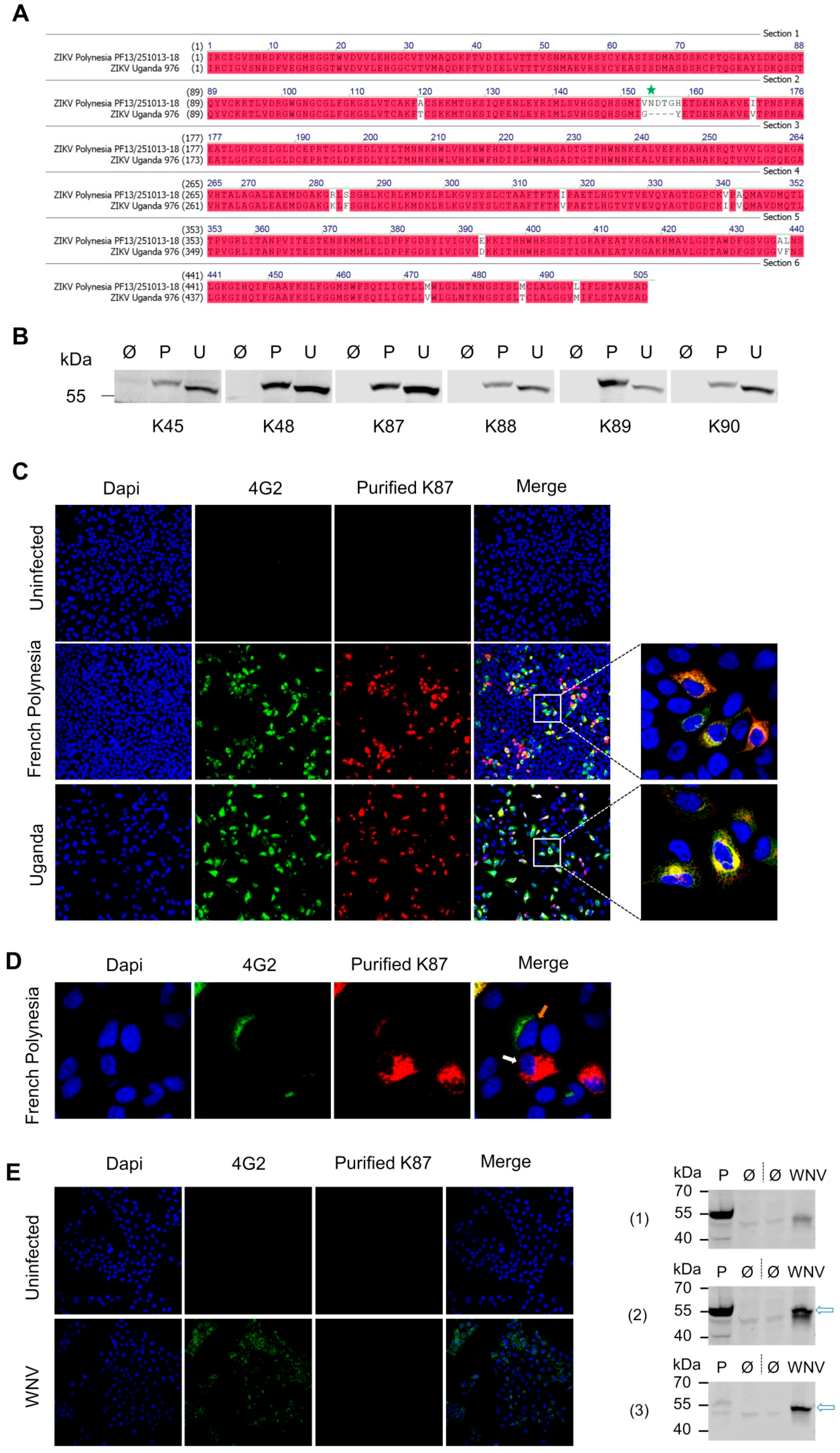
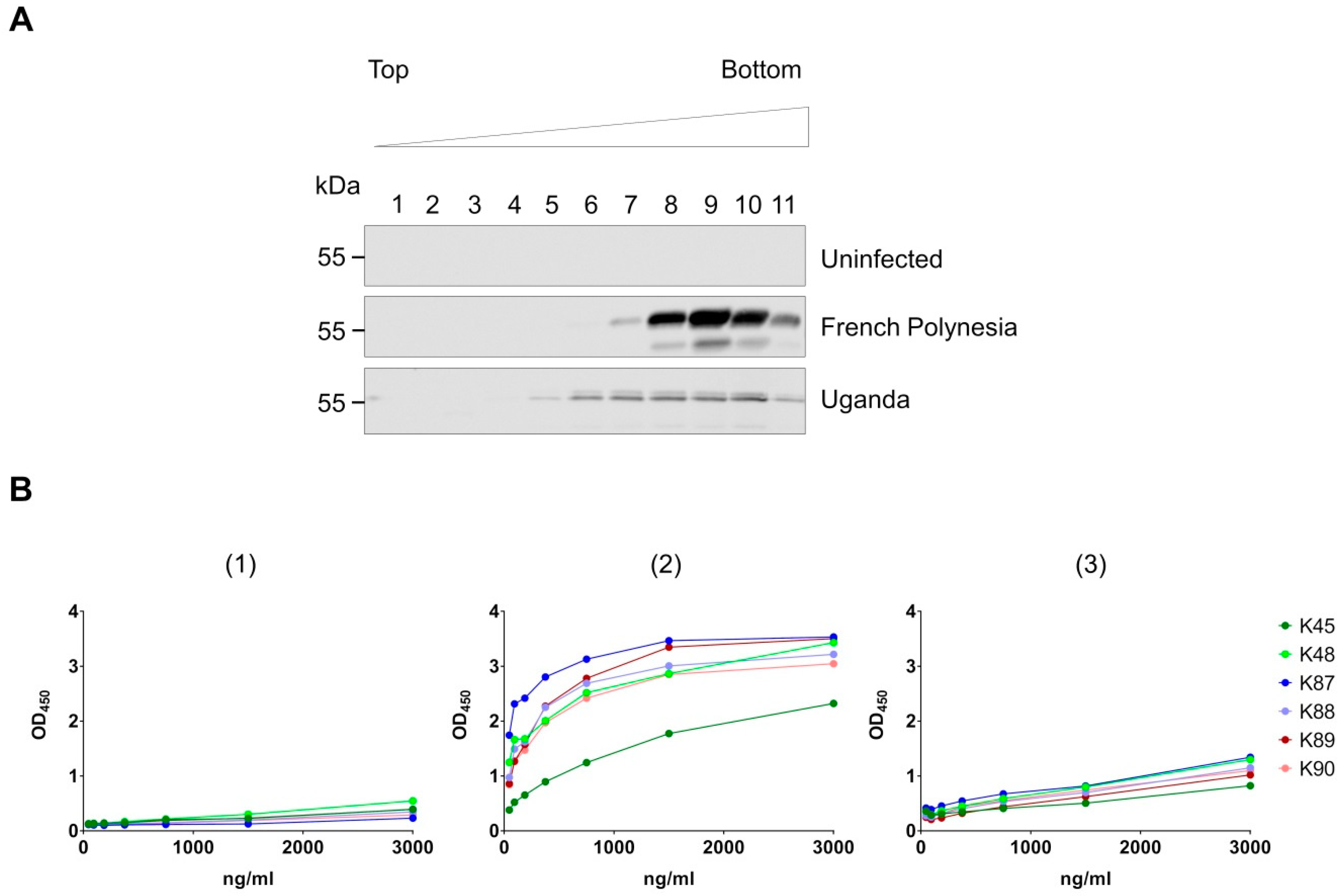
3.3. Recognition of Denatured ZIKV E Protein from the Asian and the African Virus Strains by the Purified Antibodies
3.4. Binding of the Purified Antibodies to the Surface of ZIKV Particles
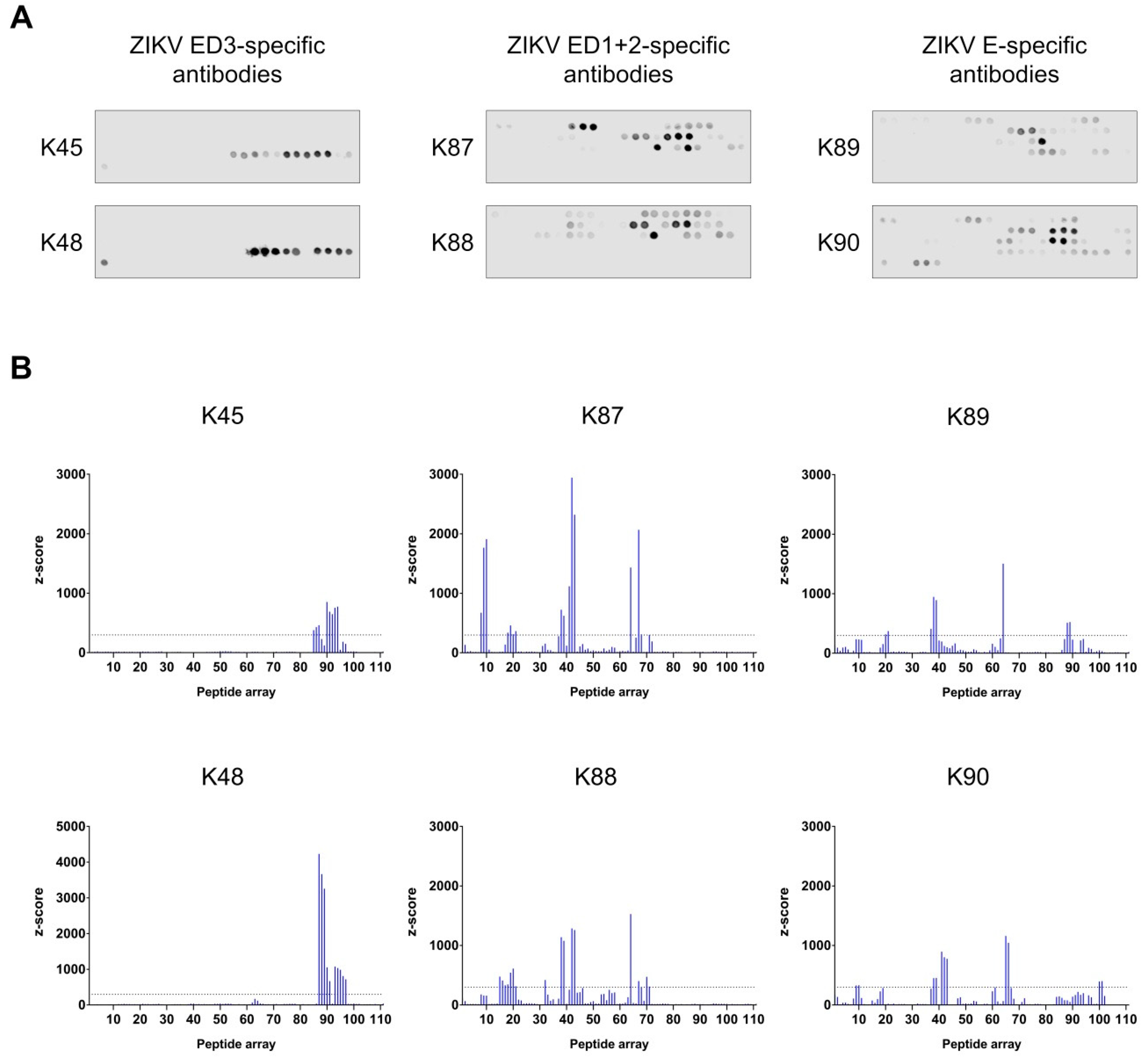
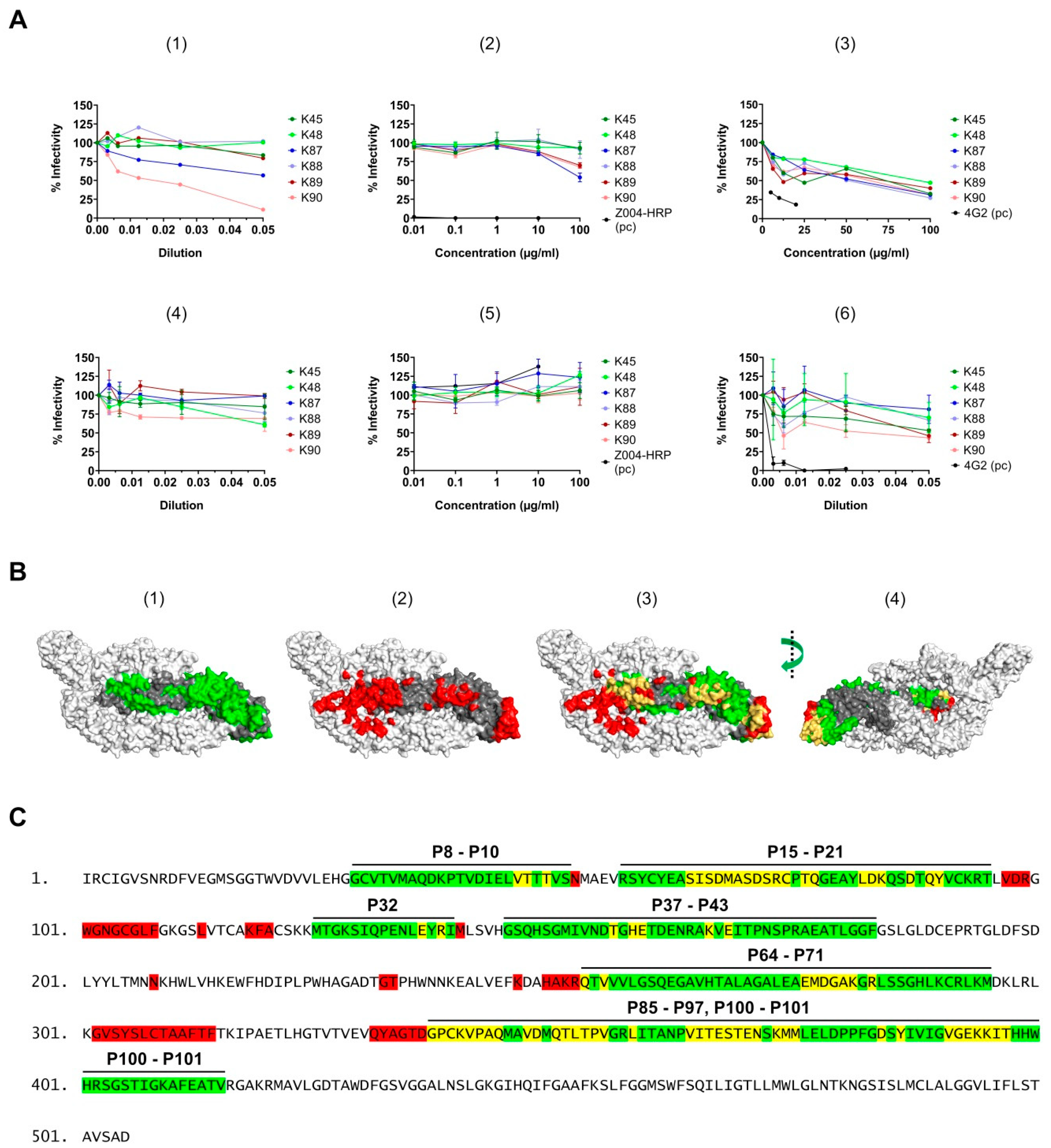
3.5. Binding Sites Determination of the Purified Antibodies
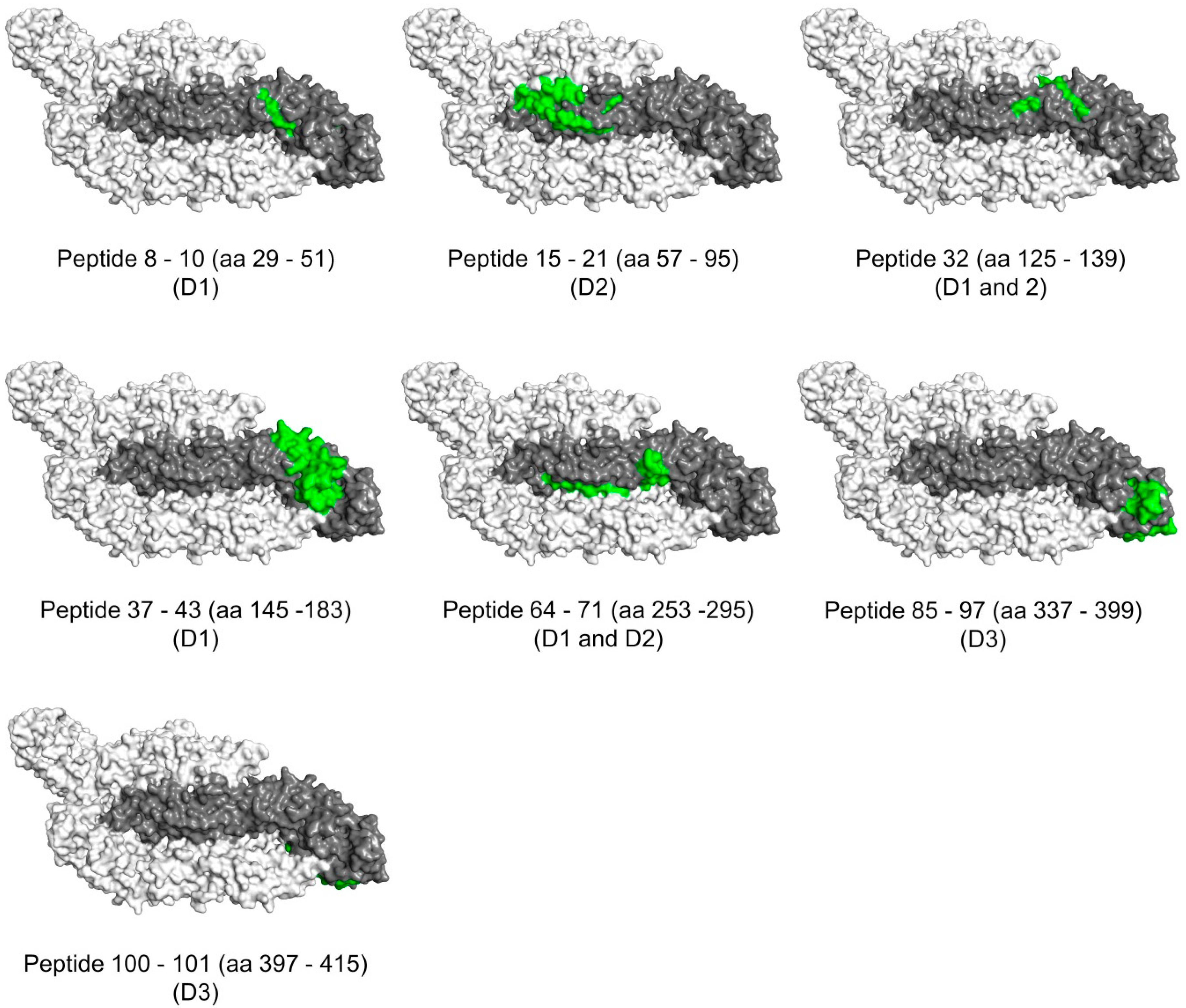
3.6. Surface Exposure of the Binding Sites
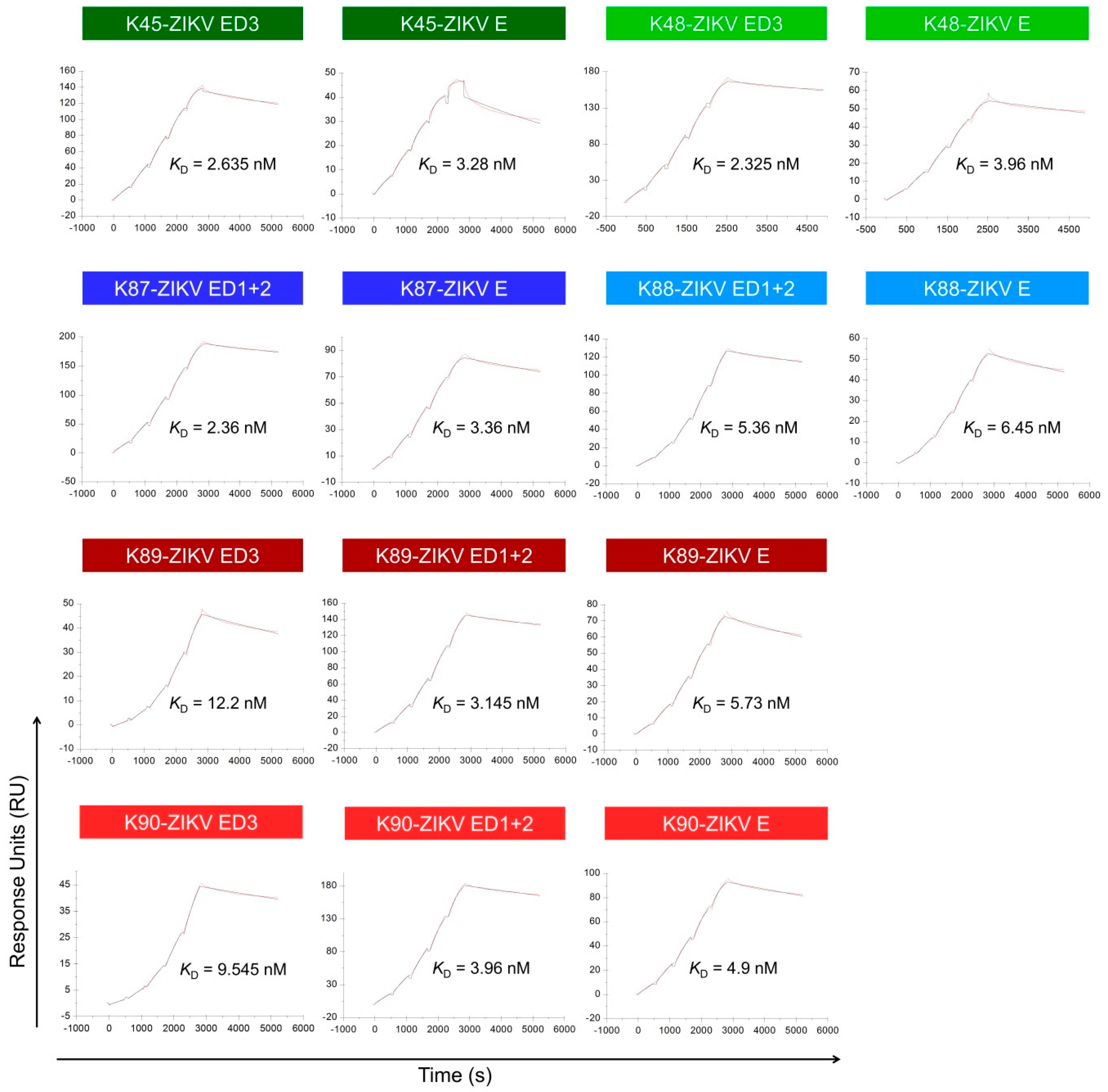
3.7. Binding Affinity to the ZIKV E Domains
3.8. Neutralizing Activity of the Purified Antibodies
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Dick, G.W.A. Zika Virus (I). Isolations and serological specificity. Trans. R. Soc. Trop. Med. Hyg. 1952, 46, 509–520. [Google Scholar] [CrossRef]
- MacNamara, F.N. Zika virus: A report on three cases of human infection during an epidemic of jaundice in Nigeria. Trans. R. Soc. Trop. Med. Hyg. 1954, 48, 139–145. [Google Scholar] [CrossRef]
- Duffy, M.R.; Chen, T.-H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika Virus Outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Cao-Lormeau, V.-M.; Musso, D. Emerging arboviruses in the Pacific. Lancet 2014, 384, 1571–1572. [Google Scholar] [CrossRef]
- Oehler, E.; Watrin, L.; Larre, P.; Leparc-Goffart, I.; Lastere, S.; Valour, F.; Baudouin, L.; Mallet, H.P.; Musso, D.; Ghawche, F. Zika virus infection complicated by Guillain-Barre syndrome—Case report, French Polynesia, December 2013. Eurosurveillance 2014, 19, 20720. [Google Scholar] [CrossRef]
- Mlakar, J.; Korva, M.; Tul, N.; Popović, M.; Poljšak-Prijatelj, M.; Mraz, J.; Kolenc, M.; Resman Rus, K.; Vesnaver Vipotnik, T.; Fabjan Vodušek, V.; et al. Zika Virus Associated with Microcephaly. N. Engl. J. Med. 2016, 374, 951–958. [Google Scholar] [CrossRef]
- Calvet, G.; Aguiar, R.S.; Melo, A.S.O.; Sampaio, S.A.; de Filippis, I.; Fabri, A.; Araujo, E.S.M.; de Sequeira, P.C.; de Mendonça, M.C.L.; de Oliveira, L.; et al. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: A case study. Lancet Infect. Dis. 2016, 16, 653–660. [Google Scholar] [CrossRef]
- Schuler-Faccini, L.; Ribeiro, E.M.; Feitosa, I.M.L.; Horovitz, D.D.G.; Cavalcanti, D.P.; Pessoa, A.; Doriqui, M.J.R.; Neri, J.I.; de Pina Neto, J.M.; Wanderley, H.Y.C.; et al. Possible Association Between Zika Virus Infection and Microcephaly—Brazil, 2015. Morb. Mortal. Wkly. Rep. 2016, 65, 59–62. [Google Scholar] [CrossRef]
- Kuno, G.; Chang, G.J.J. Full-length sequencing and genomic characterization of Bagaza, Kedougou, and Zika viruses. Arch. Virol. 2007, 152, 687–696. [Google Scholar] [CrossRef]
- Dai, L.; Song, J.; Lu, X.; Deng, Y.Q.; Musyoki, A.M.; Cheng, H.; Zhang, Y.; Yuan, Y.; Song, H.; Haywood, J.; et al. Structures of the Zika Virus Envelope Protein and Its Complex with a Flavivirus Broadly Protective Antibody. Cell Host Microbe 2016, 19, 696–704. [Google Scholar] [CrossRef]
- Barba-Spaeth, G.; Dejnirattisai, W.; Rouvinski, A.; Vaney, M.C.; Medits, I.; Sharma, A.; Simon-Lorière, E.; Sakuntabhai, A.; Cao-Lormeau, V.M.; Haouz, A.; et al. Structural basis of potent Zika-dengue virus antibody cross-neutralization. Nature 2016, 536, 48–53. [Google Scholar] [CrossRef]
- Wang, L.; Wang, R.; Wang, L.; Ben, H.; Yu, L.; Gao, F.; Shi, X.; Yin, C.; Zhang, F.; Xiang, Y.; et al. Structural Basis for Neutralization and Protection by a Zika Virus-Specific Human Antibody. Cell Rep. 2019, 26, 3360–3368.e5. [Google Scholar] [CrossRef]
- Zhao, H.; Fernandez, E.; Dowd, K.A.; Speer, S.D.; Platt, D.J.; Gorman, M.J.; Govero, J.; Nelson, C.A.; Pierson, T.C.; Diamond, M.S.; et al. Structural Basis of Zika Virus-Specific Antibody Protection. Cell 2016, 166, 1016–1027. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Bardelli, M.; Espinosa, D.A.; Pedotti, M.; Ng, T.S.; Bianchi, S.; Simonelli, L.; Lim, E.X.Y.; Foglierini, M.; Zatta, F.; et al. A Human Bi-specific Antibody against Zika Virus with High Therapeutic Potential. Cell 2017, 171, 229–241.e15. [Google Scholar] [CrossRef]
- Wang, Q.; Yang, H.; Liu, X.; Dai, L.; Ma, T.; Qi, J.; Wong, G.; Peng, R.; Liu, S.; Li, J.; et al. Molecular determinants of human neutralizing antibodies isolated from a patient infected with Zika virus. Sci. Transl. Med. 2016, 8, 369ra179. [Google Scholar] [CrossRef]
- Robbiani, D.F.; Bozzacco, L.; Keeffe, J.R.; Khouri, R.; Olsen, P.C.; Gazumyan, A.; Schaefer-Babajew, D.; Avila-Rios, S.; Nogueira, L.; Patel, R.; et al. Recurrent Potent Human Neutralizing Antibodies to Zika Virus in Brazil and Mexico. Cell 2017, 169, 597–609.e11. [Google Scholar] [CrossRef] [PubMed]
- Keeffe, J.R.; Van Rompay, K.K.A.; Olsen, P.C.; Wang, Q.; Gazumyan, A.; Azzopardi, S.A.; Schaefer-Babajew, D.; Lee, Y.E.; Stuart, J.B.; Singapuri, A.; et al. A Combination of Two Human Monoclonal Antibodies Prevents Zika Virus Escape Mutations in Non-human Primates. Cell Rep. 2018, 25, 1385–1394.e7. [Google Scholar] [CrossRef]
- Long, F.; Doyle, M.; Fernandez, E.; Miller, A.S.; Klose, T.; Sevvana, M.; Bryan, A.; Davidson, E.; Doranz, B.J.; Kuhn, R.J.; et al. Structural basis of a potent human monoclonal antibody against Zika virus targeting a quaternary epitope. Proc. Natl. Acad. Sci. USA 2019, 116, 1591–1596. [Google Scholar] [CrossRef]
- Hasan, S.S.; Miller, A.; Sapparapu, G.; Fernandez, E.; Klose, T.; Long, F.; Fokine, A.; Porta, J.C.; Jiang, W.; Diamond, M.S.; et al. A human antibody against Zika virus crosslinks the E protein to prevent infection. Nat. Commun. 2017, 8, 14722. [Google Scholar] [CrossRef]
- Zhang, S.; Kostyuchenko, V.A.; Ng, T.-S.; Lim, X.-N.; Ooi, J.S.G.; Lambert, S.; Tan, T.Y.; Widman, D.G.; Shi, J.; Baric, R.S.; et al. Neutralization mechanism of a highly potent antibody against Zika virus. Nat. Commun. 2016, 7, 13679. [Google Scholar] [CrossRef]
- Campos, G.S.; Bandeira, A.C.; Sardi, S.I. Zika Virus Outbreak, Bahia, Brazil. Emerg. Infect. Dis. 2015, 21, 1885–1886. [Google Scholar] [CrossRef]
- Howarth, M.; Chinnapen, D.J.F.; Gerrow, K.; Dorrestein, P.C.; Grandy, M.R.; Kelleher, N.L.; El-Husseini, A.; Ting, A.Y. A monovalent streptavidin with a single femtomolar biotin binding site. Nat. Methods 2006, 3, 267–273. [Google Scholar] [CrossRef]
- Kühne, Y.; Reese, G.; Ballmer-Weber, B.K.; Niggemann, B.; Hanschmann, K.M.; Vieths, S.; Holzhauser, T. A novel multipeptide microarray for the specific and sensitive mapping of linear IgE-binding epitopes of food allergens. Int. Arch. Allergy Immunol. 2015, 166, 213–224. [Google Scholar] [CrossRef]
- Yang, M.; Dent, M.; Lai, H.; Sun, H.; Chen, Q. Immunization of Zika virus envelope protein domain III induces specific and neutralizing immune responses against Zika virus. Vaccine 2017, 35, 4287–4294. [Google Scholar] [CrossRef]
- Premkumar, L.; Collins, M.; Graham, S.; Liou, G.J.A.; Lopez, C.A.; Jadi, R.; Balmaseda, A.; Brackbill, J.A.; Dietze, R.; Camacho, E.; et al. Development of envelope protein antigens to serologically differentiate zika virus infection from dengue virus infection. J. Clin. Microbiol. 2018, 56, e01504-e17. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Heinz, F.X.; Stiasny, K. The Antigenic Structure of Zika Virus and Its Relation to Other Flaviviruses: Implications for Infection and Immunoprophylaxis. Microbiol. Mol. Biol. Rev. 2017, 81, e00055-16. [Google Scholar] [CrossRef]
| ZIKV E Protein Domain (S) | Serum |
|---|---|
| ZIKV E protein (aa 1–409) | K89 and K90 |
| ZIKV ED1+2 protein (aa 1–295) | K87 and K88 |
| ZIKV ED3 protein (aa 296–409) | K45 and K48 |
| 10% Sucrose | 20% Sucrose | 30% Sucrose | 40% Sucrose | 50% Sucrose | 60% Sucrose |
|---|---|---|---|---|---|
| 2 mL | 1.5 mL | 1.5 mL | 1.5 mL | 1.5 mL | 1.5 mL |
| Protein Used for Immunization | Serum |
|---|---|
| Streptavidin-ZIKV E (aa 1–409) | K89/K90 |
| Streptavidin-ZIKV E domains 1+2 (aa 1–295) | K87/K88 |
| Streptavidin-ZIKV E Domain 3 (aa 296–409) | K45/K48 |
| Peptide | Amino Acid | Corresponding Sequence | Domain | Recognition | Surface Exposure |
|---|---|---|---|---|---|
| P8–P10 | 29–51 | GCVTVMAQDKPTVDIELVTTTVS | 1 | K87, K90 | partially |
| P15–P21 | 57–95 | RSYCYEASISDMASDSRCPTQGEAYLDKQSDTQYVCKRT | 2 | K87, K88, K89 | mostly |
| P32 | 125–139 | MTGKSIQPENLEYRI | 1 and 2 | K88 | partially |
| P37–P43 | 145–183 | GSQHSGMIVNDTGHETDENRAKVEITPNSPRAEATLGGF | 1 | K87, K88, K89, K90 | mostly |
| P64–P71 | 253–295 | QTVVVLGSQEGAVHTALAGALEAEMDGAKGRLSSGHLKCRLKM | 1 and 2 | K87, K88, K89, K90 | partially |
| P85–P97 | 337–399 | GPCKVPAQMAVDMQTLTPVGRLITANPVITESTENSKMMLELDPPFGDSYIVIGVGEKKITHH | 3 | K45, K48, K89 | partially |
| P100–P101 | 397–415 | THHWHRSGSTIGKAFEATV | 3 | K90 | no |
| Binding to ZIKV ED3 | Binding to ZIKV ED1+2 | Binding to ZIKV E | ||||
|---|---|---|---|---|---|---|
| Antibody | KD (nM) | Mean Value of KD (nM) | KD (nM) | Mean Value of KD (nM) | KD (nM) | Mean Value of KD (nM) |
| K45 | 2.93 | 2.635 | - | - | 4.04 | 3.28 |
| 2.34 | - | 2.52 | ||||
| K48 | 1.79 | 2.325 | - | - | 2.96 | 3.96 |
| 2.86 | - | 4.96 | ||||
| K87 | - | - | 2.43 | 2.36 | 3.25 | 3.36 |
| - | 2.29 | 3.47 | ||||
| K88 | - | - | 4.85 | 5.36 | 6.06 | 6.45 |
| - | 5.87 | 6.84 | ||||
| K89 | 11.3 | 12.2 | 3.2 | 3.145 | 5.8 | 5.73 |
| 13.1 | 3.09 | 5.66 | ||||
| K90 | 9.42 | 9.545 | 3.13 | 3.96 | 3.85 | 4.9 |
| 9.67 | 4.79 | 5.95 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akhras, S.; Herrlein, M.-L.; Elgner, F.; Holzhauser, T.; Hildt, E. ZIKV Envelope Domain-Specific Antibodies: Production, Purification and Characterization. Viruses 2019, 11, 748. https://doi.org/10.3390/v11080748
Akhras S, Herrlein M-L, Elgner F, Holzhauser T, Hildt E. ZIKV Envelope Domain-Specific Antibodies: Production, Purification and Characterization. Viruses. 2019; 11(8):748. https://doi.org/10.3390/v11080748
Chicago/Turabian StyleAkhras, Sami, Marie-Luise Herrlein, Fabian Elgner, Thomas Holzhauser, and Eberhard Hildt. 2019. "ZIKV Envelope Domain-Specific Antibodies: Production, Purification and Characterization" Viruses 11, no. 8: 748. https://doi.org/10.3390/v11080748
APA StyleAkhras, S., Herrlein, M.-L., Elgner, F., Holzhauser, T., & Hildt, E. (2019). ZIKV Envelope Domain-Specific Antibodies: Production, Purification and Characterization. Viruses, 11(8), 748. https://doi.org/10.3390/v11080748






