Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag
Abstract
1. Introduction
2. Materials and Methods
2.1. FIV Strain and Nucleotide Designations
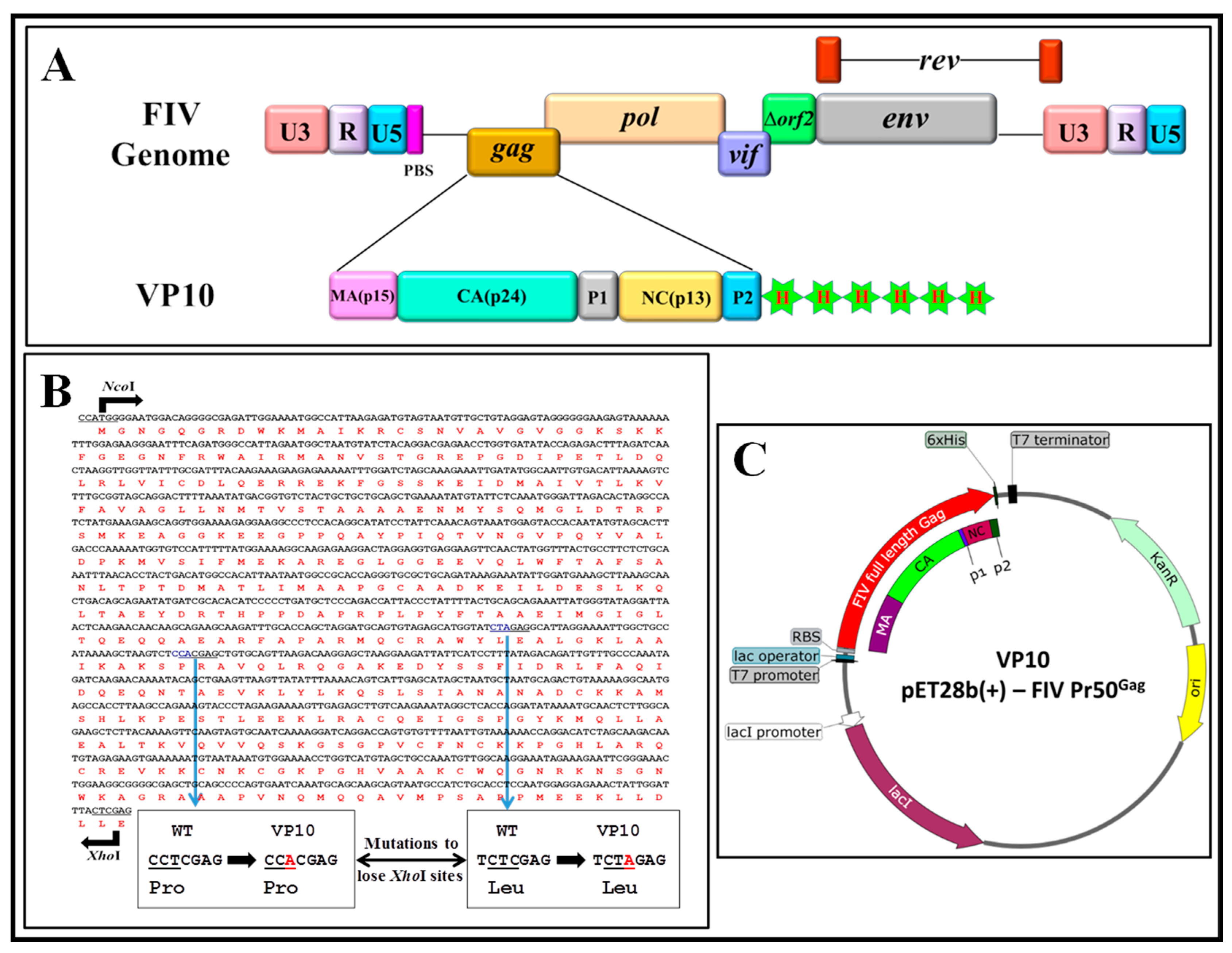
2.2. Prokaryotic FIV Gag Expression Plasmid Construction
2.3. Eukaryotic FIV Gag Expression Plasmid Construction
2.4. Bacterial Strains and Culture Media for Cloning and Expressing Full-Length Recombinant FIV Pr50Gag-His6-Tag Protein
2.5. Eukaryotic Expression of Recombinant Full-Length FIV Pr50Gag Protein
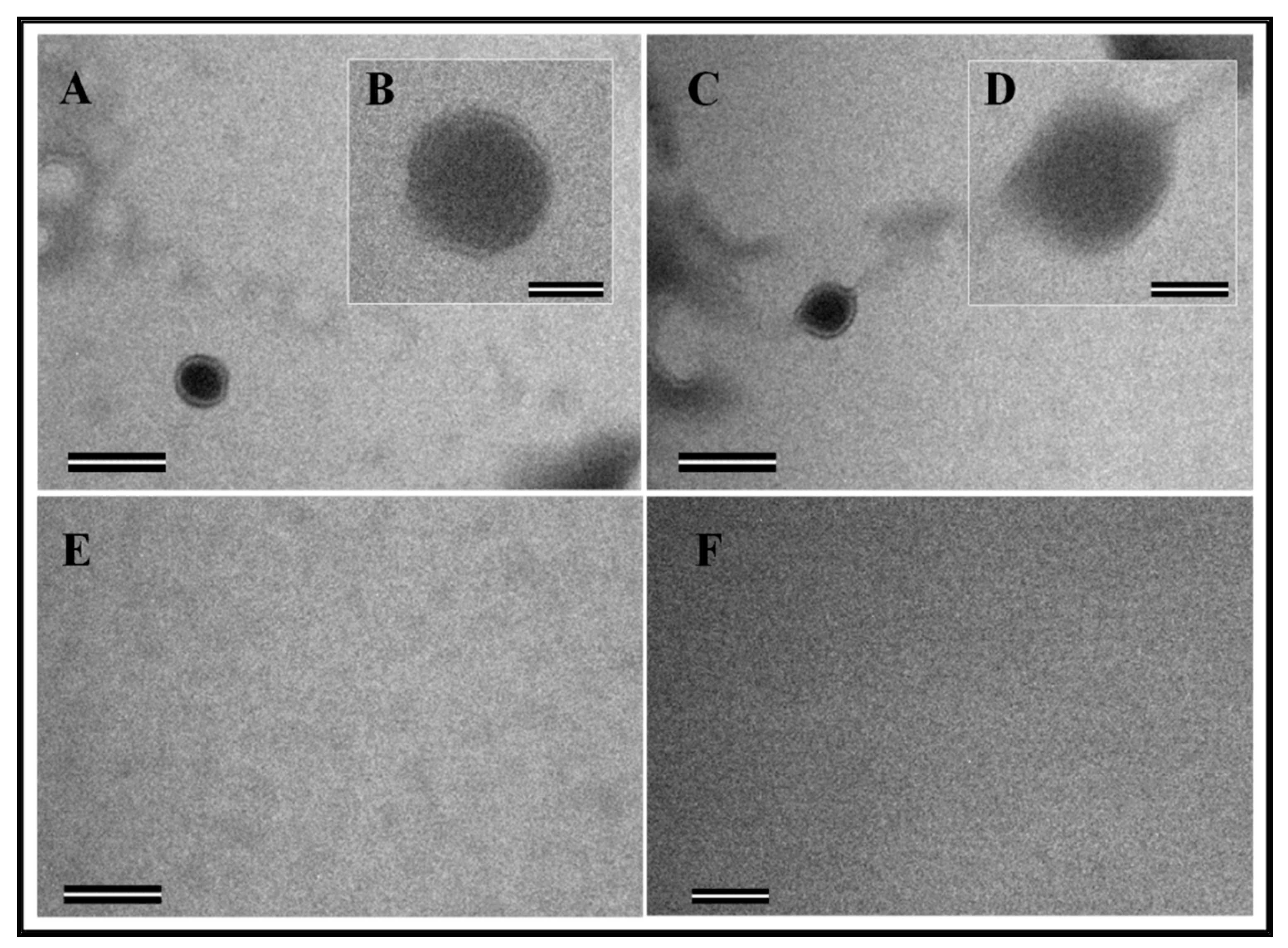
2.6. Transmission Electron Microscopy (TEM) of Eukaryotically-Expressed Virus-Like Particles (VLPs)
2.7. Evaluation of RNA Packaging by FIV Gag Virus-Like Particles (VLPs) Using Reverse Transcriptase PCR (RT-PCR) and qPCR Using SYBR Green
2.8. Expression of Recombinant FIV Pr50Gag-His6-tag Protein in E. coli
2.9. Affinity Purification and Size Exclusion Chromatography of Recombinant FIV Pr50Gag-His6-Tag Protein
2.10. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting
2.11. In Vitro Assembly to Form Virus-Like Particles (VLPs) by the Recombinant Full-Length Pr50Gag-His6-Tag Fusion Protein Expressed in E. coli
3. Results and Discussion
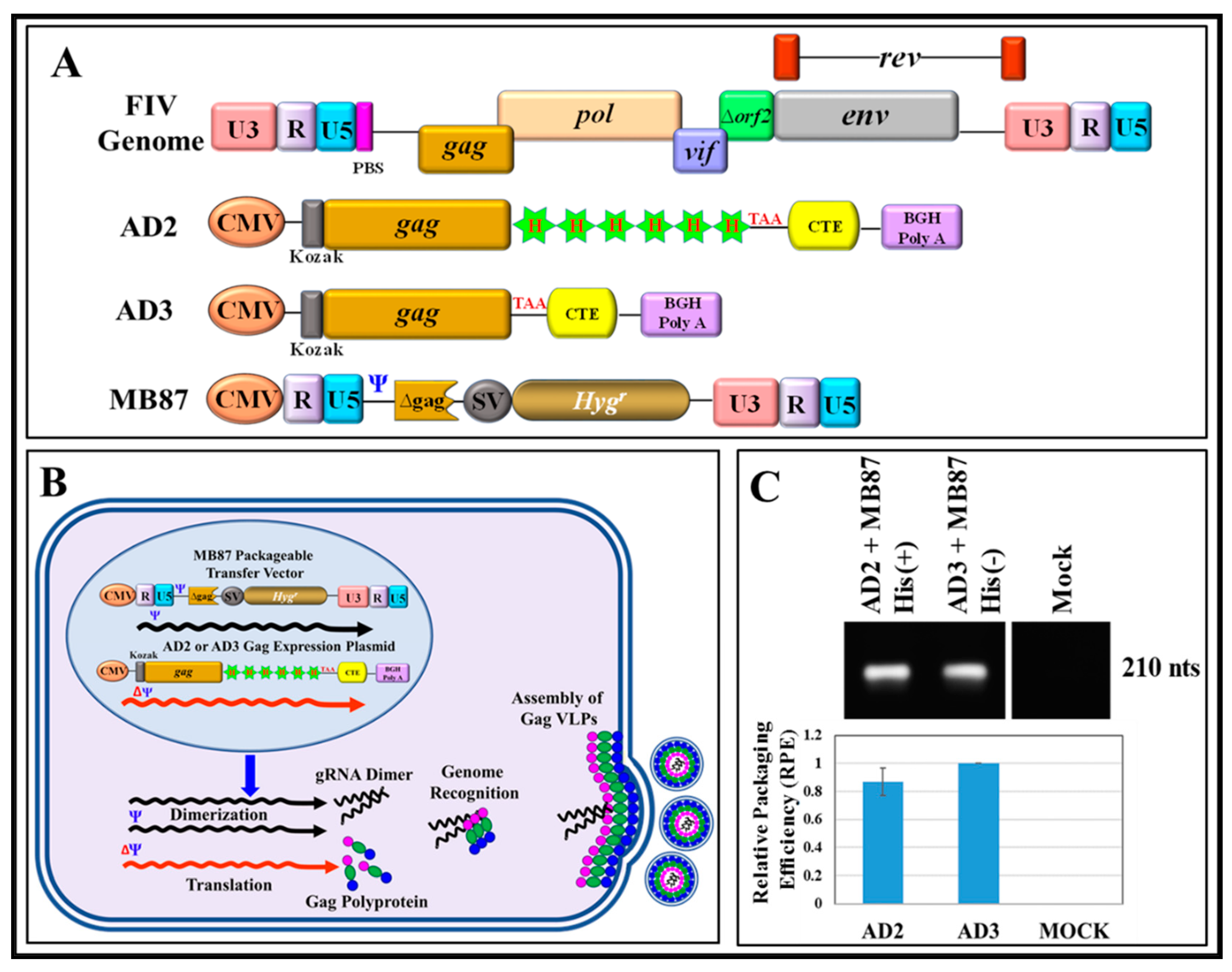
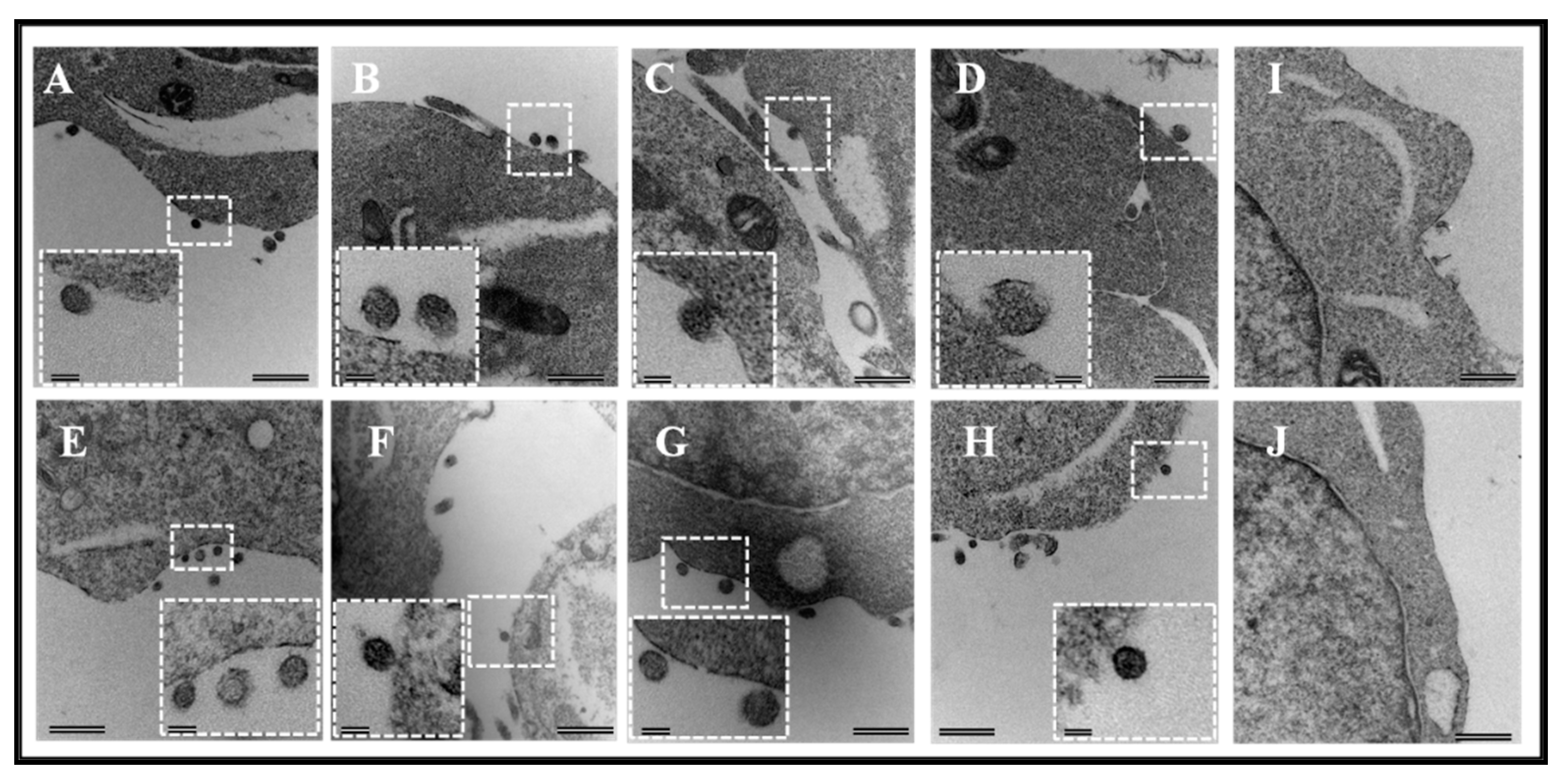
3.1. Full-Length FIV Pr50Gag Protein, Both with and without a His6-Tag Can Be Expressed in Eukaryotic Cells, Capable of Making Virus-Like Particles (VLPs) That Can Package FIV gRNA
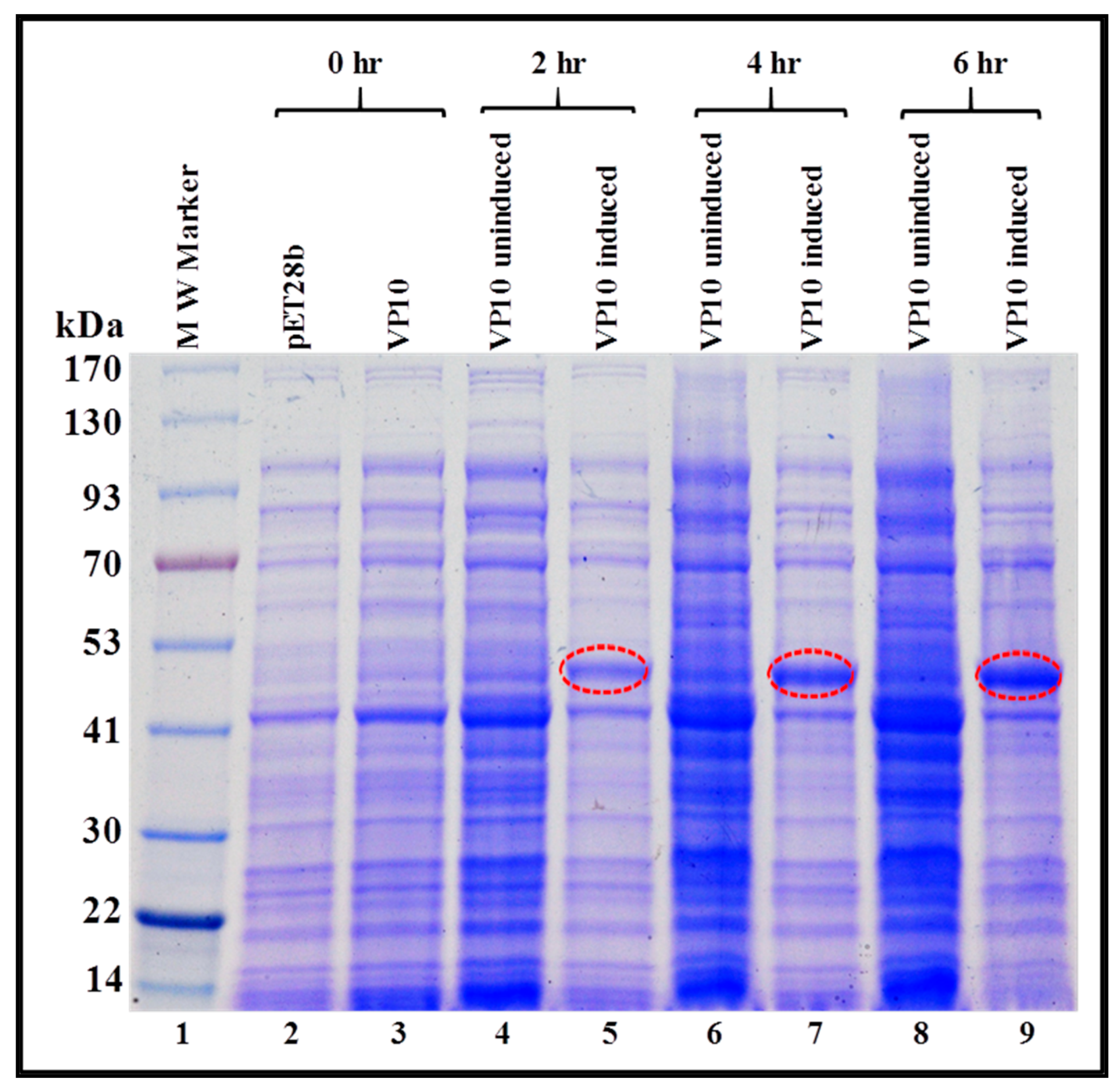
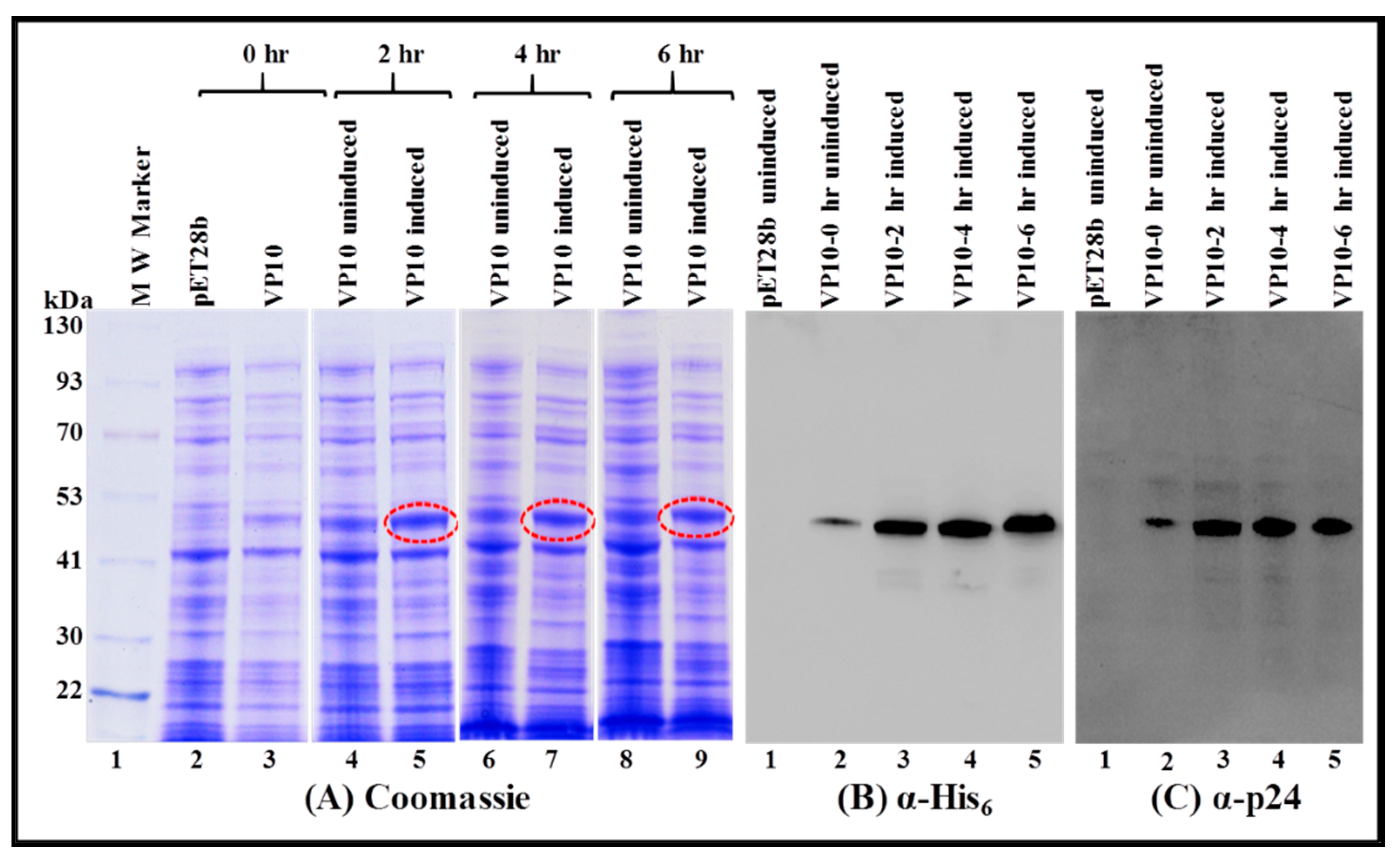
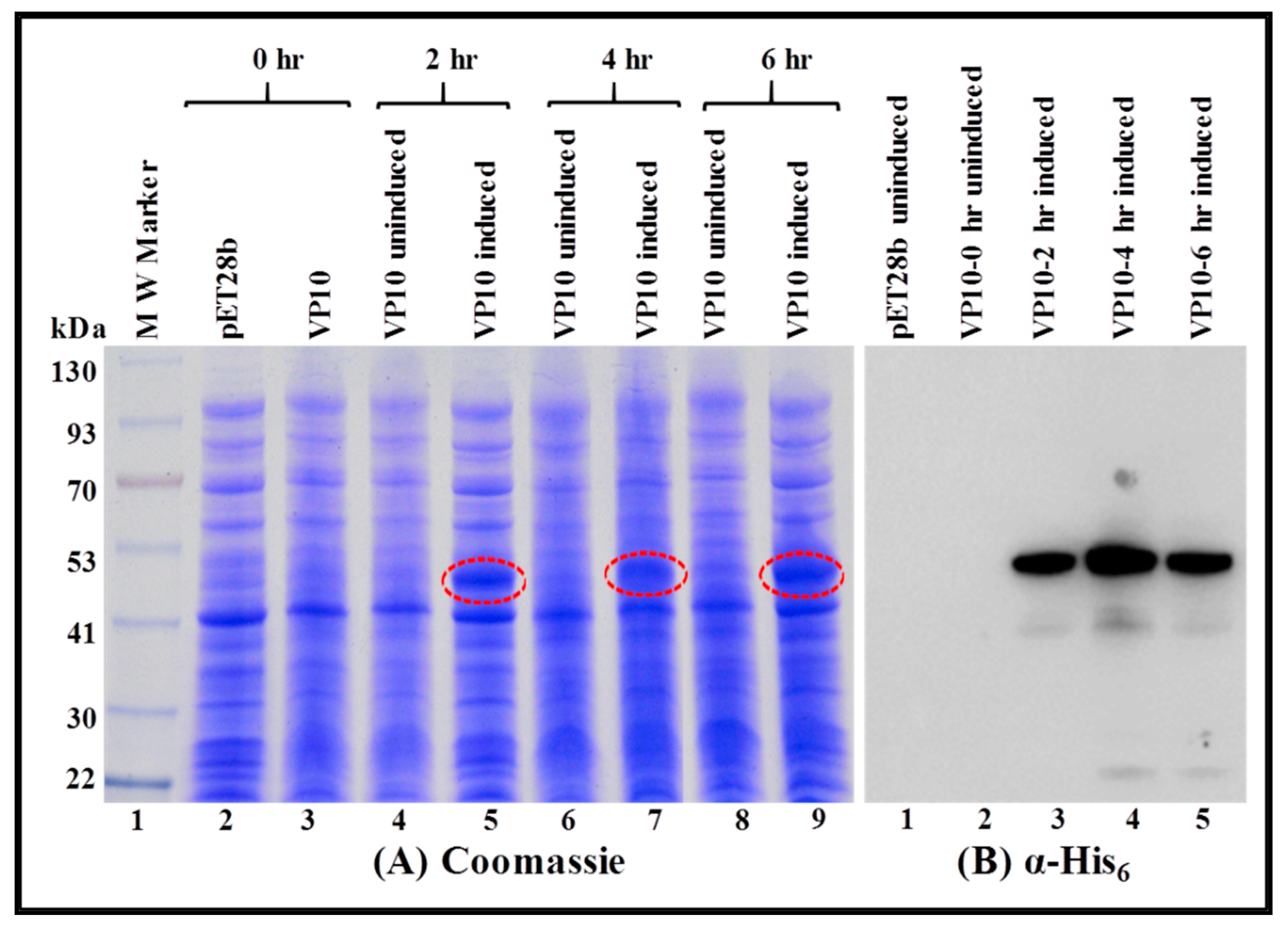
3.2. Expression of Full-Length Recombinant FIV Pr50Gag-His6-Tagged Fusion Protein in Prokaryotic Cells
3.3. FIV Pr50Gag-His6-Tagged Fusion Protein Is Expressed in the Soluble Fraction in Bacteria
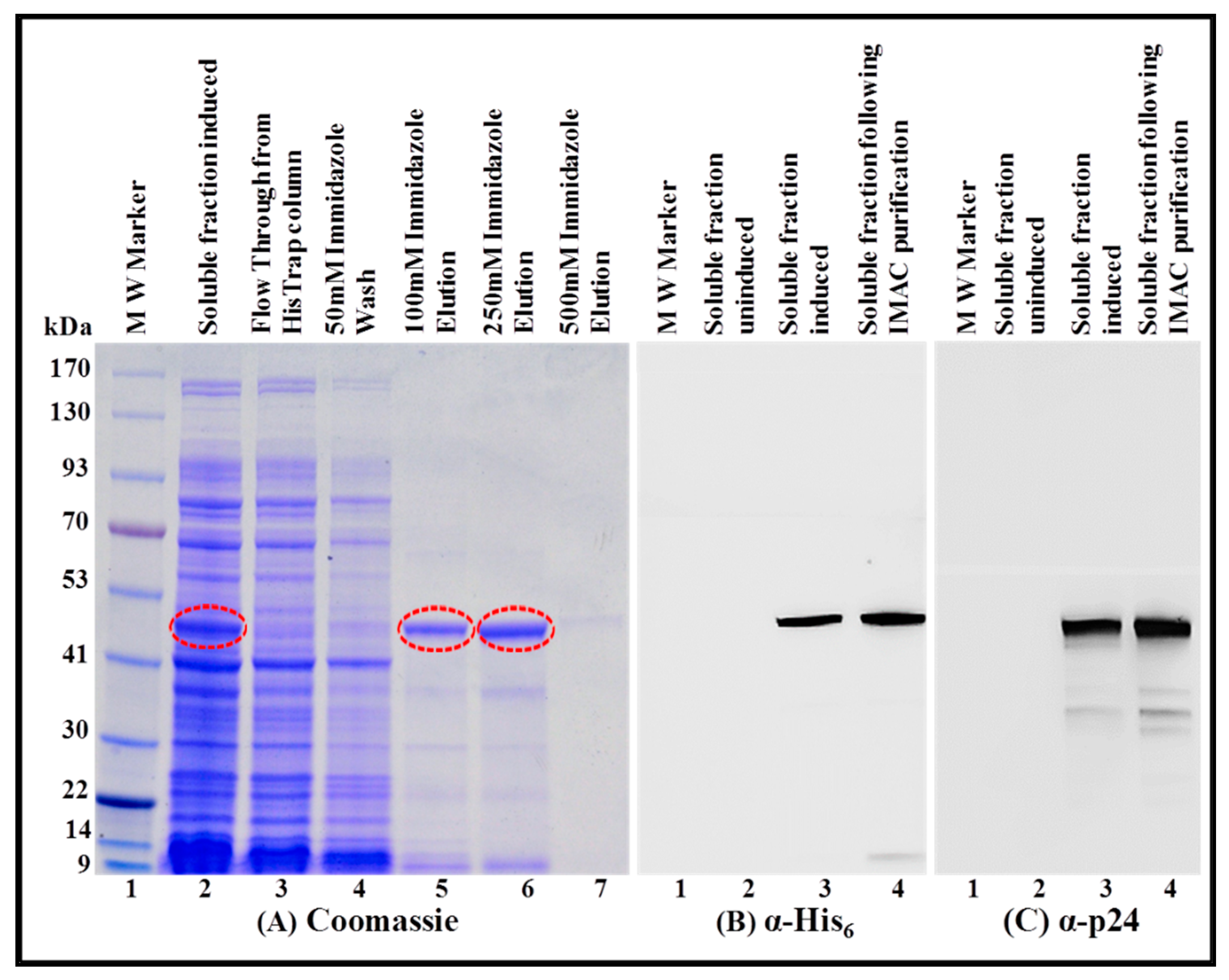
3.4. Purification of the Recombinant Full-Length Pr50Gag-His6-Tagged Fusion Protein from Soluble Fraction by Immobilized Metal Affinity Chromatography (IMAC)
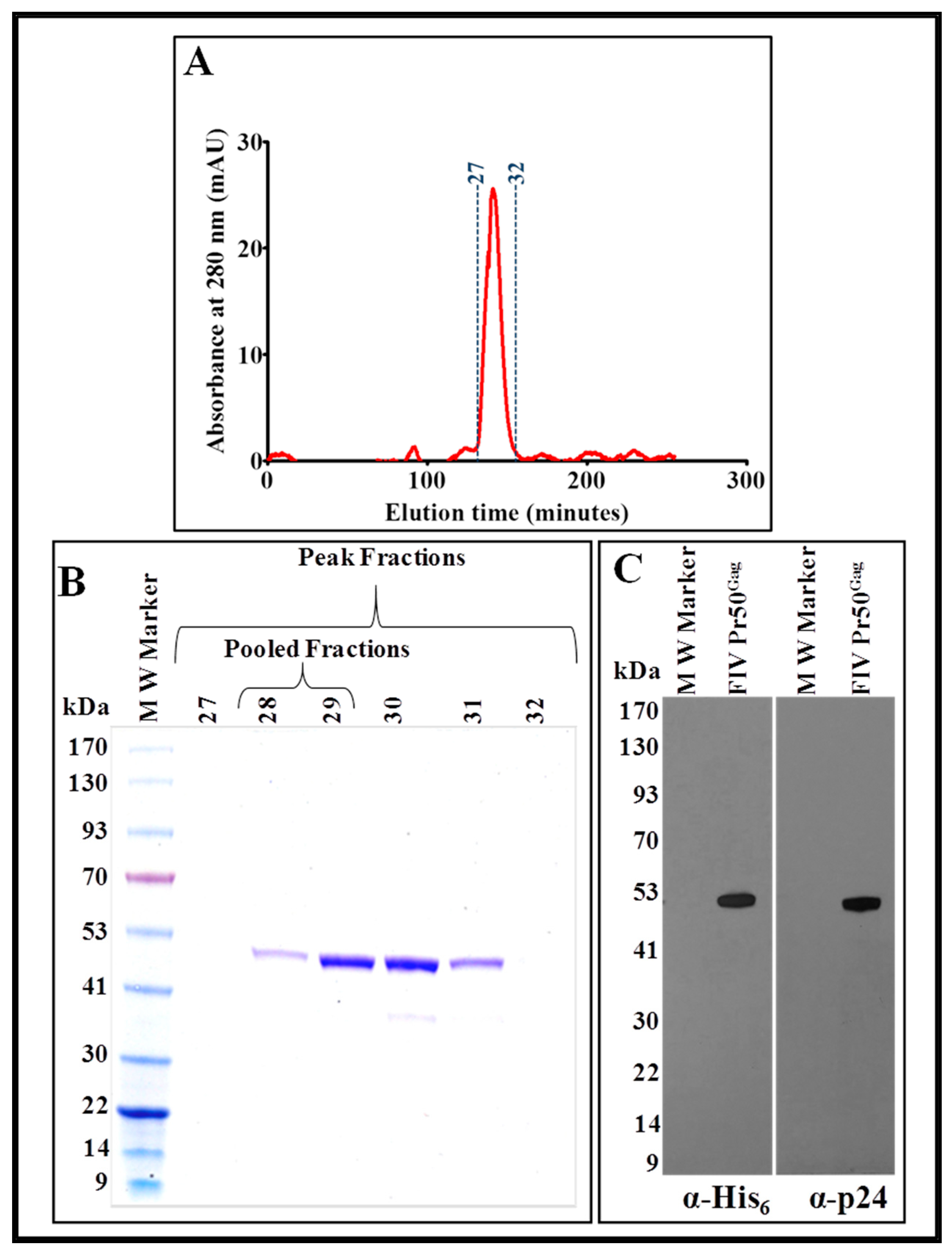
3.5. Further Purification of the IMAC-Purified Pr50Gag-His6-Tag Protein by Gel Filtration/Size Exclusion Chromatography
3.6. The Recombinant Full-Length Pr50Gag-His6-Tagged Fusion Protein Can Assemble In Vitro to Form Virus-Like Particles (VLPs)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- D’Souza, V.; Summers, M.F. How retroviruses select their genomes. Nat. Rev. Microbiol. 2005, 3, 643–655. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.M.L. HIV-1 RNA packaging. Adv. Pharmacol. San Diego Calif. 2007, 55, 1–32. [Google Scholar]
- Johnson, S.F.; Telesnitsky, A. Retroviral RNA Dimerization and Packaging: The What, How, When, Where, and Why. PLoS Pathog. 2010, 6, e1001007. [Google Scholar] [CrossRef] [PubMed]
- Ali, L.M.; Rizvi, T.A.; Mustafa, F. Cross- and Co-Packaging of Retroviral RNAs and Their Consequences. Viruses 2016, 8, 276. [Google Scholar] [CrossRef] [PubMed]
- Comas-Garcia, M.; Davis, S.R.; Rein, A. On the Selective Packaging of Genomic RNA by HIV-1. Viruses 2016, 8, 246. [Google Scholar] [CrossRef]
- Maldonado, R.J.K.; Parent, L.J.; Maldonado, R.K. Orchestrating the Selection and Packaging of Genomic RNA by Retroviruses: An Ensemble of Viral and Host Factors. Viruses 2016, 8, 257. [Google Scholar] [CrossRef] [PubMed]
- Dubois, N.; Marquet, R.; Paillart, J.-C.; Bernacchi, S. Retroviral RNA Dimerization: From Structure to Functions. Front. Microbiol. 2018, 9, 527. [Google Scholar] [CrossRef]
- Mailler, E.; Bernacchi, S.; Marquet, R.; Paillart, J.-C.; Vivet-Boudou, V.; Smyth, R.P. The Life-Cycle of the HIV-1 Gag–RNA Complex. Viruses 2016, 8, 248. [Google Scholar] [CrossRef]
- El-Wahab, E.W.A.; Smyth, R.P.; Mailler, E.; Bernacchi, S.; Vivet-Boudou, V.; Hijnen, M.; Jossinet, F.; Mak, J.; Paillart, J.-C.; Marquet, R. Specific recognition of the HIV-1 genomic RNA by the Gag precursor. Nat. Commun. 2014, 5, 4304. [Google Scholar] [CrossRef]
- Bernacchi, S.; Abd El-Wahab, E.W.; Dubois, N.; Hijnen, M.; Smyth, R.P.; Mak, J.; Marquet, R.; Paillart, J.-C. HIV-1 Pr55Gagbinds genomic and spliced RNAs with different affinity and stoichiometry. RNA Biol. 2017, 14, 90–103. [Google Scholar] [CrossRef]
- Smyth, R.P.; Despons, L.; Huili, G.; Bernacchi, S.; Hijnen, M.; Mak, J.; Jossinet, F.; Weixi, L.; Paillart, J.-C.; Von Kleist, M.; et al. Mutational interference mapping experiment (MIME) for studying RNA structure and function. Nat. Methods 2015, 12, 866–872. [Google Scholar] [CrossRef] [PubMed]
- Smyth, R.P.; Smith, M.R.; Jousset, A.-C.; Despons, L.; Laumond, G.; Decoville, T.; Cattenoz, P.; Moog, C.; Jossinet, F.; Mougel, M.; et al. In cell mutational interference mapping experiment (in cell MIME) identifies the 5′ polyadenylation signal as a dual regulator of HIV-1 genomic RNA production and packaging. Nucleic Acids Res. 2018, 46, e57. [Google Scholar] [CrossRef] [PubMed]
- McKinstry, W.J.; Hijnen, M.; Tanwar, H.S.; Sparrow, L.G.; Nagarajan, S.; Pham, S.T.; Mak, J. Expression and purification of soluble recombinant full length HIV-1 Pr55Gag protein in Escherichia coli. Protein Expr. Purif. 2014, 100, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Pitchai, F.N.N.; Ali, L.; Pillai, V.N.; Chameettachal, A.; Ashraf, S.S.; Mustafa, F.; Marquet, R.; Rizvi, T.A. Expression, purification, and characterization of biologically active full-length Mason-Pfizer monkey virus (MPMV) Pr78Gag. Sci. Rep. 2018, 8, 11793. [Google Scholar] [CrossRef] [PubMed]
- Chameettachal, A.; Pillai, V.N.; Ali, L.M.; Pitchai, F.N.N.; Ardah, M.T.; Mustafa, F.; Marquet, R.; Rizvi, T.A. Biochemical and Functional Characterization of Mouse Mammary Tumor Virus Full-Length Pr77Gag Expressed in Prokaryotic and Eukaryotic Cells. Viruses 2018, 10, 334. [Google Scholar] [CrossRef]
- Coffin, J.M.; Hughes, S.H.; Varmus, H.E. Purification, Composition, and Morphology of Virions; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 1997. [Google Scholar]
- De Guzman, R.N.; Wu, Z.R.; Stalling, C.C.; Pappalardo, L.; Borer, P.N.; Summers, M.F. Structure of the HIV-1 nucleocapsid protein bound to the SL3 psi-RNA recognition element. Science 1998, 279, 384–388. [Google Scholar] [CrossRef] [PubMed]
- Kuzembayeva, M.; Dilley, K.; Sardo, L.; Hu, W.-S. Life of Psi: How full-length HIV-1 RNAs become packaged genomes in the viral particles. Virology 2014, 454–455, 362–370. [Google Scholar] [CrossRef]
- Didierlaurent, L.; Racine, P.J.; Houzet, L.; Chamontin, C.; Berkhout, B.; Mougel, M. Role of HIV-1 RNA and protein determinants for the selective packaging of spliced and unspliced viral RNA and host U6 and 7SL RNA in virus particles. Nucleic Acids Res. 2011, 39, 8915–8927. [Google Scholar] [CrossRef]
- Dubois, N.; Khoo, K.K.; Ghossein, S.; Seissler, T.; Wolff, P.; McKinstry, W.J.; Mak, J.; Paillart, J.-C.; Marquet, R.; Bernacchi, S. The C-terminal p6 domain of the HIV-1 Pr55Gag precursor is required for specific binding to the genomic RNA. RNA Biol. 2018, 15, 923–936. [Google Scholar] [CrossRef]
- Kenyon, J.C.; Lever, A.M.L. The Molecular Biology of Feline Immunodeficiency Virus (FIV). Viruses 2011, 3, 2192–2213. [Google Scholar] [CrossRef]
- Luttge, B.G.; Freed, E.O. FIV Gag: Virus assembly and host-cell interactions. Vet. Immunol. Immunopathol. 2010, 134, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.; Ho, E.; Brown, M.; Yamamoto, J. Isolation of a T-lymphotropic virus from domestic cats with an immunodeficiency-like syndrome. Science 1987, 235, 790–793. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. The feline immunodeficiency virus. In The Retroviridae; Springer: Berlin/Heidelberg, Germany, 1993; pp. 181–228. [Google Scholar]
- Sparger, E.E.; Luciw, P.A.; Elder, J.H.; Yamamoto, J.K.; Lowenstine, L.J.; Pedersen, N.C. Feline immunodeficiency virus is a lentivirus associated with an AIDS-like disease in cats. AIDS 1989, 3, S43–S50. [Google Scholar] [CrossRef] [PubMed]
- Elder, J.H.; Phillips, T.R. Molecular properties of feline immunodeficiency virus (FIV). Infect. Agents Dis. 1993, 2, 361–374. [Google Scholar] [PubMed]
- Elder, J.H.; Sundström, M.; De Rozières, S.; De Parseval, A.; Grant, C.K.; Lin, Y.-C. Molecular Mechanisms of FIV Infection. Vet. Immunol. Immunopathol. 2008, 123, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.J.; Cairns, J.S.; Bridges, S.; Sarver, N. Human Immunodeficiency Virus and AIDS: Insights from Animal Lentiviruses. J. Virol. 2000, 74, 7187–7195. [Google Scholar] [CrossRef] [PubMed]
- Olmsted, R.A.; Barnes, A.K.; Yamamoto, J.K.; Hirsch, V.M.; Purcell, R.H.; Johnson, P.R. Molecular cloning of feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1989, 86, 2448–2452. [Google Scholar] [CrossRef] [PubMed]
- Bienzle, D. FIV in cats—A useful model of HIV in people? Vet. Immunol. Immunopathol. 2014, 159, 171–179. [Google Scholar] [CrossRef] [PubMed]
- Poeschla, E.M.; Wong-Staal, F.; Looney, D.J. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat. Med. 1998, 4, 354–357. [Google Scholar] [CrossRef]
- Poeschla, E.M. Primate and Feline Lentiviruses in Current Intrinsic Immunity Research: The Cat is Back. Vet. Immunol. Immunopathol. 2011, 143, 215–220. [Google Scholar] [CrossRef]
- Barraza, R.A.; Poeschla, E.M. Human Gene Therapy Vectors Derived from Feline Lentiviruses. Vet. Immunol. Immunopathol. 2008, 123, 23–31. [Google Scholar] [CrossRef]
- Saenz, D.T.; Poeschla, E.M. FIV: From lentivirus to lentivector. J. Gene Med. 2004, 6, S95–S104. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Slepushkin, V.; Zabner, J.; Keshavjee, S.; Johnston, J.C.; Sauter, S.L.; Jolly, D.J.; Dubensky, T.W.; Davidson, B.L.; McCray, P.B. Feline immunodeficiency virus vectors persistently transduce nondividing airway epithelia and correct the cystic fibrosis defect. J. Clin. Investig. 1999, 104, R55–R62. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.T.; Schmidt, R.D.; Lew, K.A.; Rizvi, T.A. Primate and Feline Lentivirus Vector RNA Packaging and Propagation by Heterologous Lentivirus Virions. J. Virol. 2001, 75, 5129–5140. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.T.; Schmidt, R.D.; Lew, K.A.; Mustafa, F.; Rizvi, T.A. Delineation of sequences important for efficient packaging of feline immunodeficiency virus RNA. J. Gen. Virol. 2003, 84, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Browning, M.T.; Mustafa, F.; Schmidt, R.D.; Lew, K.A.; Rizvi, T.A. Sequences within the gag gene of feline immunodeficiency virus (FIV) are important for efficient RNA encapsidation. Virus Res. 2003, 93, 199–209. [Google Scholar] [CrossRef]
- Ghazawi, A.; Mustafa, F.; Phillip, P.S.; Jayanth, P.; Ali, J.; Rizvi, T.A. Both the 5′ and 3′ LTRs of FIV contain minor RNA encapsidation determinants compared to the two core packaging determinants within the 5′ untranslated region and gag. Microbes Infect. 2006, 8, 767–778. [Google Scholar] [CrossRef]
- Kemler, I.; Barraza, R.; Poeschla, E.M. Mapping the Encapsidation Determinants of Feline Immunodeficiency Virus. J. Virol. 2002, 76, 11889–11903. [Google Scholar] [CrossRef]
- Kemler, I.; Azmi, I.; Poeschla, E.M. The critical role of proximal gag sequences in feline immunodeficiency virus genome encapsidation. Virology 2004, 327, 111–120. [Google Scholar] [CrossRef]
- Kenyon, J.C.; Tanner, S.J.; Legiewicz, M.; Phillip, P.S.; Rizvi, T.A.; Le Grice, S.F.J.; Lever, A.M.L. SHAPE analysis of the FIV Leader RNA reveals a structural switch potentially controlling viral packaging and genome dimerization. Nucleic Acids Res. 2011, 39, 6692–6704. [Google Scholar] [CrossRef]
- Mustafa, F.; Ghazawi, A.; Jayanth, P.; Phillip, P.S.; Ali, J.; Rizvi, T.A. Sequences Intervening between the Core Packaging Determinants Are Dispensable for Maintaining the Packaging Potential and Propagation of Feline Immunodeficiency Virus Transfer Vector RNAs. J. Virol. 2005, 79, 13817–13821. [Google Scholar] [CrossRef] [PubMed]
- Kenyon, J.C.; Ghazawi, A.; Cheung, W.K.; Phillip, P.S.; Rizvi, T.A.; Lever, A.M. The secondary structure of the 5′ end of the FIV genome reveals a long-range interaction between R/U5 and gag sequences, and a large, stable stem–loop. RNA 2008, 14, 2597–2608. [Google Scholar] [CrossRef] [PubMed]
- Rizvi, T.A.; Kenyon, J.C.; Ali, J.; Aktar, S.J.; Phillip, P.S.; Ghazawi, A.; Mustafa, F.; Lever, A.M. Optimal Packaging of FIV Genomic RNA Depends upon a Conserved Long-range Interaction and a Palindromic Sequence within gag. J. Mol. Biol. 2010, 403, 103–119. [Google Scholar] [CrossRef] [PubMed]
- Greatorex, J. The retroviral RNA dimer linkage: Different structures may reflect different roles. Retrovirology 2004, 1, 22. [Google Scholar] [CrossRef] [PubMed]
- Paillart, J.-C.; Shehu-Xhilaga, M.; Marquet, R.; Mak, J. Dimerization of retroviral RNA genomes: An inseparable pair. Nat. Rev. Microbiol. 2004, 2, 461–472. [Google Scholar] [CrossRef] [PubMed]
- Lever, A.M. RNA packaging in lentiviruses. Retrovirology 2009, 6, I13. [Google Scholar] [CrossRef][Green Version]
- Moore, M.D.; Hu, W.S. HIV-1 RNA Dimerization: It Takes Two to Tango. Aids Rev. 2009, 11, 91–102. [Google Scholar] [PubMed]
- Moore, M.D.; Nikolaitchik, O.A.; Chen, J.; Hammarskjold, M.-L.; Rekosh, D.; Hu, W.-S. Probing the HIV-1 Genomic RNA Trafficking Pathway and Dimerization by Genetic Recombination and Single Virion Analyses. PLoS Pathog. 2009, 5, e1000627. [Google Scholar] [CrossRef] [PubMed]
- Zeffman, A.; Hassard, S.; Varani, G.; Lever, A. The major HIV-1 packaging signal is an extended bulged stem loop whose structure is altered on interaction with the gag polyprotein. J. Mol. Biol. 2000, 297, 877–893. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Brik, A.; de Parseval, A.; Tam, K.; Torbett, B.E.; Wong, C.-H.; Elder, J.H. Altered Gag Polyprotein Cleavage Specificity of Feline Immunodeficiency Virus/Human Immunodeficiency Virus Mutant Proteases as Demonstrated in a Cell-Based Expression System. J. Virol. 2006, 80, 7832–7843. [Google Scholar] [CrossRef]
- Barajas, B.C.; Tanaka, M.; Robinson, B.A.; Phuong, D.J.; Chutiraka, K.; Reed, J.C.; Lingappa, J.R. Identifying the assembly intermediate in which Gag first associates with unspliced HIV-1 RNA suggests a novel model for HIV-1 RNA packaging. PLoS Pathog. 2018, 14, e1006977. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.T.; Sherer, N.M. Subcellular Localization of HIV-1 gag-pol mRNAs Regulates Sites of Virion Assembly. J. Virol. 2017, 91, e02315-16. [Google Scholar] [CrossRef] [PubMed]
- Behrens, R.T.; Aligeti, M.; Pocock, G.M.; Higgins, C.A.; Sherer, N.M. Nuclear Export Signal Masking Regulates HIV-1 Rev Trafficking and Viral RNA Nuclear Export. J. Virol. 2017, 91, e02107-16. [Google Scholar] [CrossRef] [PubMed]
- Brandt, S.; Blißenbach, M.; Grewe, B.; Konietzny, R.; Grunwald, T.; Überla, K. Rev Proteins of Human and Simian Immunodeficiency Virus Enhance RNA Encapsidation. PLoS Pathog. 2007, 3, e54. [Google Scholar] [CrossRef] [PubMed]
- Jouvenet, N.; Lainé, S.; Pessel-Vivares, L.; Mougel, M. Cell biology of retroviral RNA packaging. RNA Biol. 2011, 8, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, M.; Barajas, B.C.; Robinson, B.A.; Phuong, D.J.; Chutiraka, K.; Reed, J.C.; Lingappa, J.R. HIV-1 initiates genomic RNA packaging in a unique subset of host RNA granules. bioRxiv 2017. [Google Scholar] [CrossRef]
- Affranchino, J.L.; González, S.A. In vitro assembly of the feline immunodeficiency virus Gag polyprotein. Virus Res. 2010, 150, 153–157. [Google Scholar] [CrossRef]
- Tanwar, H.S.; Khoo, K.K.; Garvey, M.; Waddington, L.; Leis, A.; Hijnen, M.; Velkov, T.; Dumsday, G.J.; McKinstry, W.J.; Mak, J. The thermodynamics of Pr55Gag-RNA interaction regulate the assembly of HIV. PLoS Pathog. 2017, 13, e1006221. [Google Scholar] [CrossRef]
- Bewley, M.C.; Reinhart, L.; Stake, M.S.; Nadaraia-Hoke, S.; Parent, L.J.; Flanagan, J.M. A non-cleavable hexahistidine affinity tag at the carboxyl-terminus of the HIV-1 Pr55Gag polyprotein alters nucleic acid binding properties. Protein Expr. Purif. 2017, 130, 137–145. [Google Scholar] [CrossRef]
- Talbott, R.L.; Sparger, E.E.; Lovelace, K.M.; Fitch, W.M.; Pedersen, N.C.; Luciw, P.A.; Elder, J.H. Nucleotide sequence and genomic organization of feline immunodeficiency virus. Proc. Natl. Acad. Sci. USA 1989, 86, 5743–5747. [Google Scholar] [CrossRef]
- Bray, M.; Prasad, S.; Dubay, J.W.; Hunter, E.; Jeang, K.T.; Rekosh, D.; Hammarskjold, M.L. A small element from the Mason-Pfizer monkey virus genome makes human immunodeficiency virus type 1 expression and replication Rev-independent. Proc. Natl. Acad. Sci. USA 1994, 91, 1256–1260. [Google Scholar] [CrossRef] [PubMed]
- Tan, W.; Felber, B.K.; Zolotukhin, A.S.; Pavlakis, G.N.; Schwartz, S. Efficient expression of the human papillomavirus type 16 L1 protein in epithelial cells by using Rev and the Rev-responsive element of human immunodeficiency virus or the cis-acting transactivation element of simian retrovirus type 1. J. Virol. 1995, 69, 5607–5620. [Google Scholar] [PubMed]
- Luttge, B.G.; Shehu-Xhilaga, M.; Demirov, D.G.; Adamson, C.S.; Soheilian, F.; Nagashima, K.; Stephen, A.G.; Fisher, R.J.; Freed, E.O. Molecular characterization of feline immunodeficiency virus budding. J. Virol. 2008, 82, 2106–2119. [Google Scholar] [CrossRef] [PubMed]
- Manrique, M.L.; Celma, C.C.; González, S.A.; Affranchino, J.L. Mutational analysis of the feline immunodeficiency virus matrix protein. Virus Res. 2001, 76, 103–113. [Google Scholar] [CrossRef]
- Klikova, M.; Rhee, S.S.; Hunter, E.; Ruml, T. Efficient in vivo and in vitro assembly of retroviral capsids from Gag precursor proteins expressed in bacteria. J. Virol. 1995, 69, 1093–1098. [Google Scholar]
- Bell, P.A. E. coli Expression Systems. In Molecular Biology Problem Solver; Gerstein, A.S., Ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001; pp. 461–490. ISBN 978-0-471-22390-0. [Google Scholar]
- Campbell, S.; Rein, A. In Vitro Assembly Properties of Human Immunodeficiency Virus Type 1 Gag Protein Lacking the p6 Domain. J. Virol. 1999, 73, 2270–2279. [Google Scholar] [PubMed]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krishnan, A.; Pillai, V.N.; Chameettachal, A.; Mohamed Ali, L.; Nuzra Nagoor Pitchai, F.; Tariq, S.; Mustafa, F.; Marquet, R.; A. Rizvi, T. Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag. Viruses 2019, 11, 689. https://doi.org/10.3390/v11080689
Krishnan A, Pillai VN, Chameettachal A, Mohamed Ali L, Nuzra Nagoor Pitchai F, Tariq S, Mustafa F, Marquet R, A. Rizvi T. Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag. Viruses. 2019; 11(8):689. https://doi.org/10.3390/v11080689
Chicago/Turabian StyleKrishnan, Anjana, Vineeta N. Pillai, Akhil Chameettachal, Lizna Mohamed Ali, Fathima Nuzra Nagoor Pitchai, Saeed Tariq, Farah Mustafa, Roland Marquet, and Tahir A. Rizvi. 2019. "Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag" Viruses 11, no. 8: 689. https://doi.org/10.3390/v11080689
APA StyleKrishnan, A., Pillai, V. N., Chameettachal, A., Mohamed Ali, L., Nuzra Nagoor Pitchai, F., Tariq, S., Mustafa, F., Marquet, R., & A. Rizvi, T. (2019). Purification and Functional Characterization of a Biologically Active Full-Length Feline Immunodeficiency Virus (FIV) Pr50Gag. Viruses, 11(8), 689. https://doi.org/10.3390/v11080689





