Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation
Abstract
:1. Introduction
2. Impact of AM Symbiosis on Viral Development
2.1. AM Fungi
2.2. Viruses
2.3. Plants
2.4. Environment Conditions
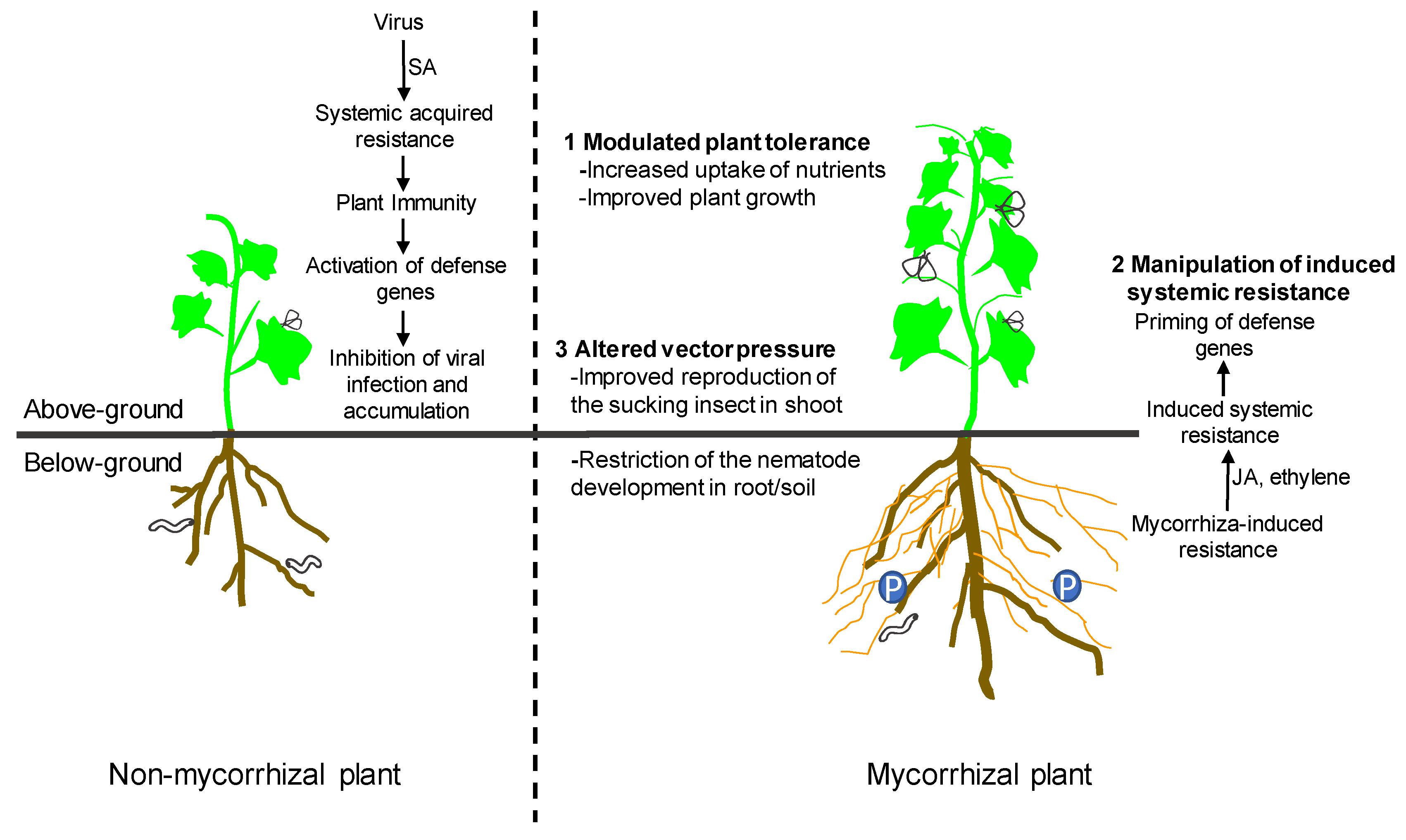
3. Mechanisms of Interactions between AM Fungi and Plant Viruses
3.1. Modulated Plant Tolerance
3.2. Manipulation of Induced Systemic Resistance
3.3. Altered Vector Pressure
4. Conclusions and Future Directions
Author Contributions
Funding
Conflicts of Interest
References
- Parniske, M. Arbuscular mycorrhiza: The mother of plant root endosymbioses. Nat. Rev. Microbiol. 2008, 6, 763–775. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.E.; Facelli, E.; Pope, S.; Smith, F.A. Plant performance in stressful environments: Interpreting new and established knowledge of the roles of arbuscular mycorrhizas. Plant Soil 2010, 326, 3–20. [Google Scholar] [CrossRef]
- Berruti, A.; Lumini, E.; Balestrini, R.; Bianciotto, V. Arbuscular mycorrhizal fungi as natural biofertilizers: let’s benefit from past successes. Front. Microbiol. 2016, 6, 1559. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, W.; Xie, Q.; Liu, N.; Liu, L.; Wang, D.; Zhang, X.; Yang, C.; Chen, X.; Tang, D.; et al. Plants transfer lipids to sustain colonization by mutualistic mycorrhizal and parasitic fungi. Science 2017, 356, 1172–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, S.E.; Smith, F.A. Roles of arbuscular mycorrhizas in plant nutrition and growth: New paradigms from cellular to ecosystem scales. Annu. Rev. Plant Biol. 2011, 62, 227–250. [Google Scholar] [CrossRef]
- Li, T.; Hu, Y.J.; Hao, Z.P.; Li, H.; Wang, Y.S.; Chen, B.D. First cloning and characterization of two functional aquaporin genes from an arbuscular mycorrhizal fungus Glomus intraradices. New Phytol. 2013, 197, 617–630. [Google Scholar] [CrossRef] [PubMed]
- Pozo, M.J.; Azcon-Aguilar, C. Unraveling mycorrhiza-induced resistance. Curr. Opin. Plant Biol. 2007, 10, 393–398. [Google Scholar] [CrossRef]
- Chandrasekaran, M.; Chanratana, M.; Kim, K.; Seshadri, S.; Sa, T. Impact of arbuscular mycorrhizal fungi on photosynthesis, water status, and gas exchange of plants under salt stress-A meta-analysis. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef]
- French, K.E. Engineering Mycorrhizal symbioses to alter plant metabolism and improve crop health. Front. Microbiol. 2017, 8, 1403. [Google Scholar] [CrossRef]
- Santander, C.; Aroca, R.; Manuel Ruiz-Lozano, J.; Olave, J.; Cartes, P.; Borie, F.; Cornejo, P. Arbuscular mycorrhiza effects on plant performance under osmotic stress. Mycorrhiza 2017, 27, 639–657. [Google Scholar] [CrossRef]
- Hao, Z.P.; Christie, P.; Qin, L.; Wang, C.X.; Li, X.L. Control of fusarium wilt of cucumber seedlings by inoculation with an arbuscular mycorrhizal fungus. J. Plant Nutr. 2005, 28, 1961–1974. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-induced resistance and priming of plant defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef] [PubMed]
- Whipps, J.M. Prospects and limitations for mycorrhizas in biocontrol of root pathogens. Can. J. Bot. 2004, 82, 1198–1227. [Google Scholar] [CrossRef]
- Schouteden, N.; de Waele, D.; Panis, B.; Vos, C.M. Arbuscular mycorrhizal fungi for the biocontrol of plant-parasitic nematodes: A review of the mechanisms involved. Front. Microbiol. 2015, 6, 1280. [Google Scholar] [CrossRef] [PubMed]
- Comby, M.; Mustafa, G.; Magnin-Robert, M.; Randoux, B.; Fontaine, J.; Reignault, P.; Lounès-Hadj Sahraoui, A. Arbuscular mycorrhizal fungi as potential bioprotectants against aerial phytopathogens and pests. In Arbuscular Mycorrhizas and Stress Tolerance of Plants; Wu, Q.S., Ed.; Springer Singapore: Singapore, 2017; pp. 195–223. [Google Scholar]
- Singh, I.; Giri, B. Arbuscular Mycorrhiza Mediated Control of Plant Pathogens. In Mycorrhiza - Nutrient Uptake, Biocontrol, Ecorestoration; Varma, A., Prasad, R., Tuteja, N., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 131–160. [Google Scholar]
- Kreuze, J.F.; Valkonen, J.P.T. Utilization of engineered resistance to viruses in crops of the developing world, with emphasis on sub-Saharan Africa. Curr. Opin. Virol. 2017, 26, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Faoro, F.; Gozzo, F. Is modulating virus virulence by induced systemic resistance realistic? Plant Sci. 2015, 234, 1–13. [Google Scholar] [CrossRef]
- Palukaitis, P.; Yoon, J.Y.; Choi, S.K.; Carr, J.P. Manipulation of induced resistance to viruses. Curr. Opin. Virol. 2017, 26, 141–148. [Google Scholar] [CrossRef] [Green Version]
- Shaul, O.; Galili, S.; Volpin, H.; Ginzberg, I.; Elad, Y.; Chet, I.; Kapulnik, Y. Mycorrhiza-induced changes in disease severity and PR protein expression in tobacco leaves. Mol. Plant Microbe In. 1999, 12, 1000–1007. [Google Scholar] [CrossRef]
- Nemec, S.; Myhre, D. Virus-Glomus etunicatum interactions in Citrus rootstocks. Plant Dis. 1984, 68, 311–314. [Google Scholar] [CrossRef]
- Daft, M.J.; Okusanya, B.O. Effect of endogone-mycorrhiza on plant-growth.5. influence of infection on multiplication of viruses in tomato, petunia and strawberry. New Phytol. 1973, 72, 975–983. [Google Scholar] [CrossRef]
- Sipahioglu, M.H.; Demir, S.; Usta, M.; Akkopru, A. Biological relationship of potato virus Y and arbuscular mycorrhizal fungus Glomus intraradices in potato. Pest Tec. 2009, 3, 63–66. [Google Scholar]
- Miozzi, L.; Catoni, M.; Fiorilli, V.; Mullineaux, P.M.; Accotto, G.P.; Lanfranco, L. Arbuscular mycorrhizal symbiosis limits foliar transcriptional responses to viral infection and favors long-term virus accumulation. Mol. Plant Microbe In. 2011, 24, 1562–1572. [Google Scholar] [CrossRef] [PubMed]
- Maffei, G.; Miozzi, L.; Fiorilli, V.; Novero, M.; Lanfranco, L.; Accotto, G.P. The arbuscular mycorrhizal symbiosis attenuates symptom severity and reduces virus concentration in tomato infected by Tomato yellow leaf curl Sardinia virus (TYLCSV). Mycorrhiza 2014, 24, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; van Tuinen, D.; Fayolle, L.; Chatagnier, O.; Li, X.; Chen, B.; Gianinazzi, S.; Gianinazzi-Pearson, V. Arbuscular mycorrhiza affects grapevine fanleaf virus transmission by the nematode vector Xiphinema index. Appl. Soil Ecol. 2018, 129, 107–111. [Google Scholar] [CrossRef]
- Opik, M.; Vanatoa, A.; Vanatoa, E.; Moora, M.; Davison, J.; Kalwij, J.M.; Reier, U.; Zobel, M. The online database MaarjAM reveals global and ecosystemic distribution patterns in arbuscular mycorrhizal fungi (Glomeromycota). New Phytol. 2010, 188, 223–241. [Google Scholar] [CrossRef] [PubMed]
- Gosling, P.; Jones, J.; Bending, G.D. Evidence for functional redundancy in arbuscular mycorrhizal fungi and implications for agroecosystem management. Mycorrhiza 2016, 26, 77–83. [Google Scholar] [CrossRef] [PubMed]
- Gai, J.P.; Feng, G.; Christie, P.; Li, X.L. Screening of arbuscular mycorrhizal fungi for symbiotic efficiency with sweet potato. J. Plant Nutr. 2006, 29, 1085–1094. [Google Scholar] [CrossRef]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Hyakumachi, M. The plant growth-promoting fungus Fusarium equiseti and the arbuscular mycorrhizal fungus Glomus mosseae induce systemic resistance against Cucumber mosaic virus in cucumber plants. Plant Soil 2012, 361, 397–409. [Google Scholar] [CrossRef]
- Pennazio, S. Recovery. An enigmatic and neglected form of plant resistance to viruses. Riv. Biol-Biol. Forum 2010, 103, 51–70. [Google Scholar]
- Morilla, G.; Krenz, B.; Jeske, H.; Bejarano, E.R.; Wege, C. Tete a tete of Tomato yellow leaf curl virus and Tomato yellow leaf curl Sardinia virus in single nuclei. J. Virol. 2004, 78, 10715–10723. [Google Scholar] [CrossRef]
- Johnson, N.C.; Graham, J.H.; Smith, F.A. Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytol. 1997, 135, 575–586. [Google Scholar] [CrossRef]
- Cameron, D.D.; Neal, A.L.; van Wees, S.C.M.; Ton, J. Mycorrhiza-induced resistance: More than the sum of its parts? Trends Plant Sci. 2013, 18, 539–545. [Google Scholar] [CrossRef] [PubMed]
- Powell, J.R.; Rillig, M.C. Biodiversity of arbuscular mycorrhizal fungi and ecosystem function. New Phytol. 2018, 220, 1059–1075. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borer, E.T.; Seabloom, E.W.; Mitchell, C.E.; Power, A.G. Local context drives infection of grasses by vector-borne generalist viruses. Ecol. Lett. 2010, 13, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Harrison, M.J.; Vanbuuren, M.L. A phosphate transporter from the mycorrhizal fungus Glomus versiforme. Nature 1995, 378, 626–629. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P. The future has roots in the past: The ideas and scientists that shaped mycorrhizal research. New Phytol. 2018, 220, 982–995. [Google Scholar] [CrossRef] [PubMed]
- Nagy, R.; Karandashov, V.; Chague, W.; Kalinkevich, K.; Tamasloukht, M.; Xu, G.H.; Jakobsen, I.; Levy, A.A.; Amrhein, N.; Bucher, M. The characterization of novel mycorrhiza-specific phosphate transporters from Lycopersicon esculentum and Solanum tuberosum uncovers functional redundancy in symbiotic phosphate transport in solanaceous species. Plant J. 2005, 42, 236–250. [Google Scholar] [CrossRef] [PubMed]
- Fiorilli, V.; Catoni, M.; Miozzi, L.; Novero, M.; Accotto, G.P.; Lanfranco, L. Global and cell-type gene expression profiles in tomato plants colonized by an arbuscular mycorrhizal fungus. New Phytol. 2009, 184, 975–987. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, P.; Genre, A. Mechanisms underlying beneficial plant-fungus interactions in mycorrhizal symbiosis. Nat. Commun. 2010, 1, 48. [Google Scholar] [CrossRef]
- Kiers, E.T.; Duhamel, M.; Beesetty, Y.; Mensah, J.A.; Franken, O.; Verbruggen, E.; Fellbaum, C.R.; Kowalchuk, G.A.; Hart, M.M.; Bago, A.; et al. Reciprocal rewards stabilize cooperation in the mycorrhizal symbiosis. Science 2011, 333, 880–882. [Google Scholar] [CrossRef]
- Zipfel, C.; Oldroyd, G.E.D. Plant signalling in symbiosis and immunity. Nature 2017, 543, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Bucher, M.; Hause, B.; Krajinski, F.; Kuester, H. Through the doors of perception to function in arbuscular mycorrhizal symbioses. New Phytol. 2014, 204, 833–840. [Google Scholar] [CrossRef] [PubMed]
- Zamioudis, C.; Pieterse, C.M.J. Modulation of host immunity by beneficial microbes. Mol. Plant Microbe In. 2012, 25, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Jaiti, F.; Kassami, M.; Meddich, A.; El Hadrami, I. Effect of Arbuscular Mycorrhization on the accumulation of hydroxycinnamic acid derivatives in date palm seedlings challenged with Fusarium oxysporum f. sp. albedinis. J. Phytopathol. 2008, 156, 641–646. [Google Scholar] [CrossRef]
- Mandadi, K.K.; Scholthof, K.-B.G. Plant immune responses against viruses: How does a virus cause disease? Plant Cell 2013, 25, 1489–1505. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y. New and old roles of plasmodesmata in immunity and parallels to tunneling nanotubes. Plant Sci. 2014, 221, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Vuorinen, A.L.; Kelloniemi, J.; Valkonen, J.P.T. Why do viruses need phloem for systemic invasion of plants? Plant Sci. 2011, 181, 355–363. [Google Scholar] [CrossRef]
- Gallou, A.; Lucero Mosquera, H.P.; Cranenbrouck, S.; Pablo Suarez, J.; Declerck, S. Mycorrhiza induced resistance in potato plantlets challenged by Phytophthora infestans. Physiol. Mol. Plant Pathol. 2011, 76, 20–26. [Google Scholar] [CrossRef]
- Malamy, J.; Carr, J.P.; Klessig, D.F.; Raskin, I. Salicylic-acid - a likely endogenous signal in the resistance response of tobacco to viral-infection. Science 1990, 250, 1002–1004. [Google Scholar] [CrossRef]
- Campos-Soriano, L.; Garcia-Martinez, J.; San Segundo, B. The arbuscular mycorrhizal symbiosis promotes the systemic induction of regulatory defence-related genes in rice leaves and confers resistance to pathogen infection. Mol. Plant Pathol. 2012, 13, 579–592. [Google Scholar] [CrossRef]
- Cordier, C.; Pozo, M.J.; Barea, J.M.; Gianinazzi, S.; Gianinazzi-Pearson, V. Cell defense responses associated with localized and systemic resistance to Phytophthora parasitica induced in tomato by an arbuscular mycorrhizal fungus. Mol. Plant Microbe In. 1998, 11, 1017–1028. [Google Scholar] [CrossRef]
- Liu, J.; Maldonado-Mendoza, I.; Lopez-Meyer, M.; Cheung, F.; Town, C.D.; Harrison, M.J. Arbuscular mycorrhizal symbiosis is accompanied by local and systemic alterations in gene expression and an increase in disease resistance in the shoots. Plant J. 2007, 50, 529–544. [Google Scholar] [CrossRef] [PubMed]
- Hao, Z.; Fayolle, L.; van Tuinen, D.; Chatagnier, O.; Li, X.; Gianinazzi, S.; Gianinazzi-Pearson, V. Local and systemic mycorrhiza-induced protection against the ectoparasitic nematode Xiphinema index involves priming of defence gene responses in grapevine. J. Exp. Bot. 2012, 63, 3657–3672. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, Z.; Hou, H.; Lei, H.; Zhu, X.; Li, X.; He, X.; Tian, C. Arbuscular mycorrhizal fungi-enhanced resistance against Phytophthora sojae infection on soybean leaves is mediated by a network involving hydrogen peroxide, jasmonic acid, and the metabolism of carbon and nitrogen. Acta Physiol. Plant 2013, 35, 3465–3475. [Google Scholar] [CrossRef]
- Pozo, M.J.; Van Loon, L.C.; Pieterse, C.M.J. Jasmonates - Signals in plant-microbe interactions. J. Plant Growth Regul. 2004, 23, 211–222. [Google Scholar]
- Kovac, M.; Mueller, A.; Jarh, D.M.; Milavec, M.; Duechting, P.; Ravnikar, M. Multiple hormone analysis indicates involvement of jasmonate signalling in the early defence of potato to potato virus Y-NTN. Biol. Plantarum 2009, 53, 195–199. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef]
- Saenz, P.; Salvador, B.; Simon-Mateo, C.; Kasschau, K.D.; Carrington, J.C.; Garcia, J.A. Host-specific involvement of the HC protein in the long-distance movement of potyviruses. J. Virol. 2002, 76, 1922–1931. [Google Scholar] [CrossRef]
- Catoni, M.; Miozzi, L.; Fiorilli, V.; Lanfranco, L.; Accotto, G.P. Comparative analysis of expression profiles in shoots and roots of tomato systemically infected by tomato spotted wilt virus reveals organ-specific transcriptional responses. Mol. Plant Microbe In. 2009, 22, 1504–1513. [Google Scholar] [CrossRef]
- Veresoglou, S.D.; Rillig, M.C. Suppression of fungal and nematode plant pathogens through arbuscular mycorrhizal fungi. Biol. Lett. 2012, 8, 214–217. [Google Scholar] [CrossRef]
- Jawhar, J.; Vovlas, N.; Digiaro, M. Occurrence of Xiphinema index in Lebanese vineyards. J. Plant Pathol. 2006, 88, 117–119. [Google Scholar]
- Teliz, D.; Landa, B.B.; Rapoport, H.F.; Perez Camacho, F.; Jimenez-Diaz, R.M.; Castillo, P. Plant-parasitic nematodes infecting grapevine in southern Spain and susceptible reaction to root-knot nematodes of rootstocks reported as moderately resistant. Plant Dis. 2007, 91, 1147–1154. [Google Scholar] [CrossRef] [PubMed]
- Villate, L.; Fievet, V.; Hanse, B.; Delemarre, F.; Plantard, O.; Esmenjaud, D.; van Helden, M. Spatial distribution of the dagger nematode Xiphinema index and its associated Grapevine fanleaf virus in French vineyard. Phytopathology 2008, 98, 942–948. [Google Scholar] [CrossRef] [PubMed]
- Gange, A.C.; West, H.M. Interactions between arbuscular mycorrhizal fungi and foliar-feeding insects in Plantago lanceolata L. New Phytol. 1994, 128, 79–87. [Google Scholar] [CrossRef]
- Hartley, S.E.; Gange, A.C. Impacts of plant symbiotic fungi on insect herbivores: Mutualism in a multitrophic context. Annu. Rev. Entomol. 2009, 54, 323–342. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.L.; Zhu-Salzman, K.; Elzaki, M.E.A.; Huang, Q.Q.; Chen, S.; Ma, Z.H.; Liu, S.W.; Zhang, J.E. Mikania micrantha wilt virus alters insect vector’s host preference to enhance its own spread. Viruses 2019, 11, 336. [Google Scholar] [CrossRef] [PubMed]
- Majewska, M.L.; Blaszkowski, J.; Nobis, M.; Rola, K.; Nobis, A.; Lakomiec, D.; Czachura, P.; Zubek, S. Root-inhabiting fungi in alien plant species in relation to invasion status and soil chemical properties. Symbiosis 2015, 65, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bzdyk, R.M.; Kohler, J.; Olchowik, J.; Aleksandrowicz-Trzcinska, M.; Kirisits, T. Arum-type of arbuscular mycorrhizae, dark septate endophytes and Olpidium spp. in fine roots of container-grown seedlings of Sorbus torminalis (Rosaceae). Acta Soc. Bot. Pol. 2016, 85, 3495. [Google Scholar] [CrossRef]
- Alfaro-Fernandez, A.; del Carmen Cordoba-Selles, M.; Angel Herrera-Vasquez, J.; del Carmen Cebrian, M.; Jorda, C. Transmission of Pepino mosaic virus by the fungal vector Olpidium virulentus. J. Phytopathol. 2010, 158, 217–226. [Google Scholar] [CrossRef]
- Maccarone, L.D.; Barbetti, M.J.; Sivasithamparam, K.; Jones, R.A.C. Molecular genetic characterization of Olpidium virulentus isolates associated with big-vein diseased lettuce plants. Plant Dis. 2010, 94, 563–569. [Google Scholar] [CrossRef]
- Salvioli, A.; Bonfante, P. Systems biology and “omics” tools: A cooperation for next-generation mycorrhizal studies. Plant Sci. 2013, 203, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Ijdo, M.; Cranenbrouck, S.; Declerck, S. Methods for large-scale production of AM fungi: Past, present, and future. Mycorrhiza 2011, 21, 1–16. [Google Scholar] [CrossRef] [PubMed]
| Virus | AM Fungus | Plant | Mycorrhizal Effects on Virus Development | References | ||||
|---|---|---|---|---|---|---|---|---|
| Code | Group | Family | Genus | Species | ||||
| 1 | Group II: ssDNA | Geminiviridae | Begomovirus | Tomato yellow leaf curl Sardinia virus | Funneliformis mosseae (Syn. Glomus mosseae BEG12) | Tomato (Solanum lycopersicum L. (‘Moneymaker’)) | Both root and shoot concentrations of viral DNA are lower in mycorrhizal plants than in control plants. | [25] |
| 2 | Group IV: ssRNA(+) | Bromoviridae | Ilarvirus | Citrus leaf rugose virus (CLRV-2) | Funneliformis geosporum (Syn. Endogone macrocarpa var. Geospora) | Citrus rootstocks (alemow (Citrus macrophylla Wester); grapefruit (Citrus paradisi Macf. ‘Duncan’); sour orange (Citrus aurantium L.)) | The leaf shock symptom development is more severe in mycorrhizal plants than in control plants. | [21] |
| 3 | Group IV: ssRNA(+) | Bromoviridae | Cucumovirus | Cucumber mosaic virus (CMV-Y, yellow strain) | Funneliformis mosseae (Syn. Glomus mosseae) | Cucumber (Cucumis sativus L. cv. Tokiwa Jibai) | No significant difference is observed between mycorrhizal and control treatments. | [30] |
| 4 | Group IV: ssRNA(+) | Closteroviridae | Closterovirus | Citrus tristeza virus (a severe tristeza isolate, T-3; a severe tristeza isolate, T-3) | Claroideoglomus etunicatum (Syn. Glomus etunicatum) | Citrus rootstocks (alemow (Citrus macrophylla Wester); grapefruit (Citrus paradisi Macf. ‘Duncan’); sour orange (Citrus aurantium L.)) | Virus-induced root degeneration is observed in both control and mycorrhizal plants. | [21] |
| 5 | Group IV: ssRNA(+) | Potyviridae | Potyvirus | Potato virus Y | Funneliformis geosporum (Syn. Endogone macrocarpa var. Geospora) | Strawberry (Fragaria × ananassa Duch. var. Talisman) | Virus contents of the mycorrhizal plants are higher than those of corresponding control plants. | [22] |
| 6 | Group IV: ssRNA(+) | Potyviridae | Potyvirus | Potato virus Y | Rhizophagus intraradices (Syn. Glomus intraradices isolate no. OM/95) | Potato (Solanum tuberosum L. cv. ‘Marfona’) | Reproduction of the virus and disease severity are significantly increased in mycorrhizal plants than in control plants. | [23] |
| 7 | Group IV: ssRNA(+) | Secoviridae | Nepovirus | Arabis mosaic virus | Funneliformis geosporum (Syn. Endogone macrocarpa var. Geospora) | Petunia (Petunia hybrida (J. D. Hooker) Vilmorin, var. Rose of Heaven), | Both the leaves and roots of the mycorrhizal plants contain more virus at each harvest than those of control plants. | [22] |
| 8 | Group IV: ssRNA(+) | Secoviridae | Nepovirus | Grapevine fanleaf virus | Rhizophagus intraradices (Syn. Glomus intraradices isolate BEG141) | Grapevine rootstock (Vitis berlandieri×Vitis riparia SO4) | The virus is present in both non-mycorrhizal and mycorrhizal plants at a high abundance of the nematode vector, while, the virus is detected only in non-mycorrhizal roots but absent from mycorrhizal grapevine at a low vector abundance. | [26] |
| 9 | Group IV: ssRNA(+) | Virgaviridae | Tobamovirus | Tomato (aucuba) mosaic virus | Funneliformis geosporum (Syn. Endogone macrocarpa var. Geospora) | Tomato (Lycopersicon esculentum Mill. F1 hybrid, var. Eurocross A) | Control leaves contain more virus at early stage (4 and·7 days), while the rate of virus multiplication was faster in the leaves of mycorrhizal plants, which leaded to more virus accumulation in long-term (14 and 21 days). | [22] |
| 10 | Group IV: ssRNA(+) | Virgaviridae | Tobamovirus | Tobacco mosaic virus (strain U1) | Rhizophagus intraradices (Syn. Glomus intraradices isolate no. OM/95) | Tobacco (Nicotiana tabacum cv. Xanthi-nc) | Leaves of mycorrhizal plants show a higher severity of symptoms than those of control plants. | [20] |
| 11 | Group V: ssRNA(-) | Peribunyaviridae | Tospovirus | Tomato spotted wilt virus (isolate T1012) | Funneliformis mosseae (Syn. Glomus mosseae isolate BEG12) | Tomato (Solanum lycopersicum L. (‘Moneymaker’)) | No differences in symptom severity or virus concentration are observed between mycorrhizal and non-mycorrhizal plants in short time (14 days post-virus inoculation (dpi)), while an increase in virus titer is detected in mycorrhizal plants in a longer period (34 and 56 dpi). | [24] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hao, Z.; Xie, W.; Chen, B. Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation. Viruses 2019, 11, 534. https://doi.org/10.3390/v11060534
Hao Z, Xie W, Chen B. Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation. Viruses. 2019; 11(6):534. https://doi.org/10.3390/v11060534
Chicago/Turabian StyleHao, Zhipeng, Wei Xie, and Baodong Chen. 2019. "Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation" Viruses 11, no. 6: 534. https://doi.org/10.3390/v11060534
APA StyleHao, Z., Xie, W., & Chen, B. (2019). Arbuscular Mycorrhizal Symbiosis Affects Plant Immunity to Viral Infection and Accumulation. Viruses, 11(6), 534. https://doi.org/10.3390/v11060534







