Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments
Abstract
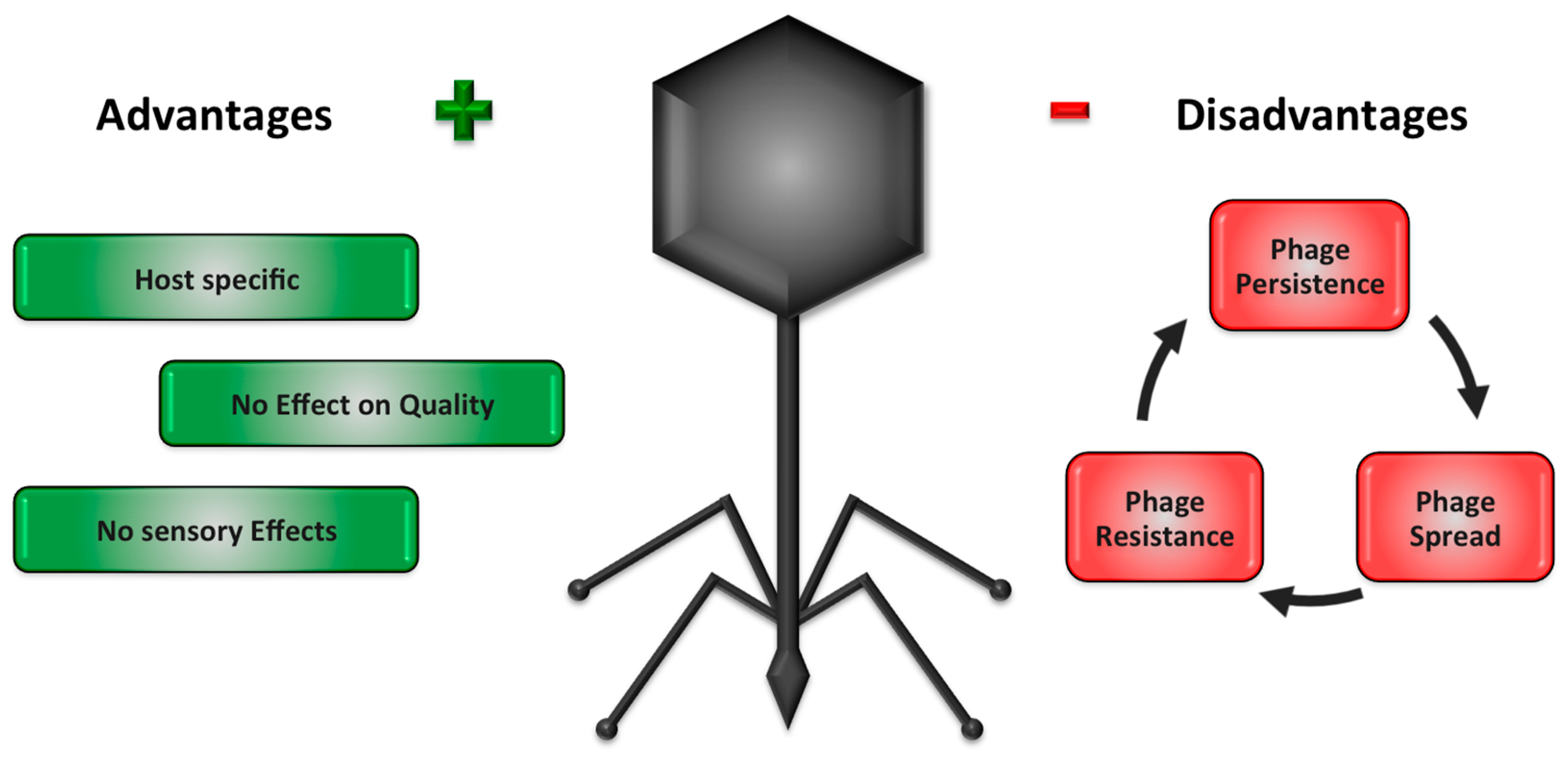
1. Introduction—Advantages and Disadvantages of Commercially Available Phage Products Used in Food Environments
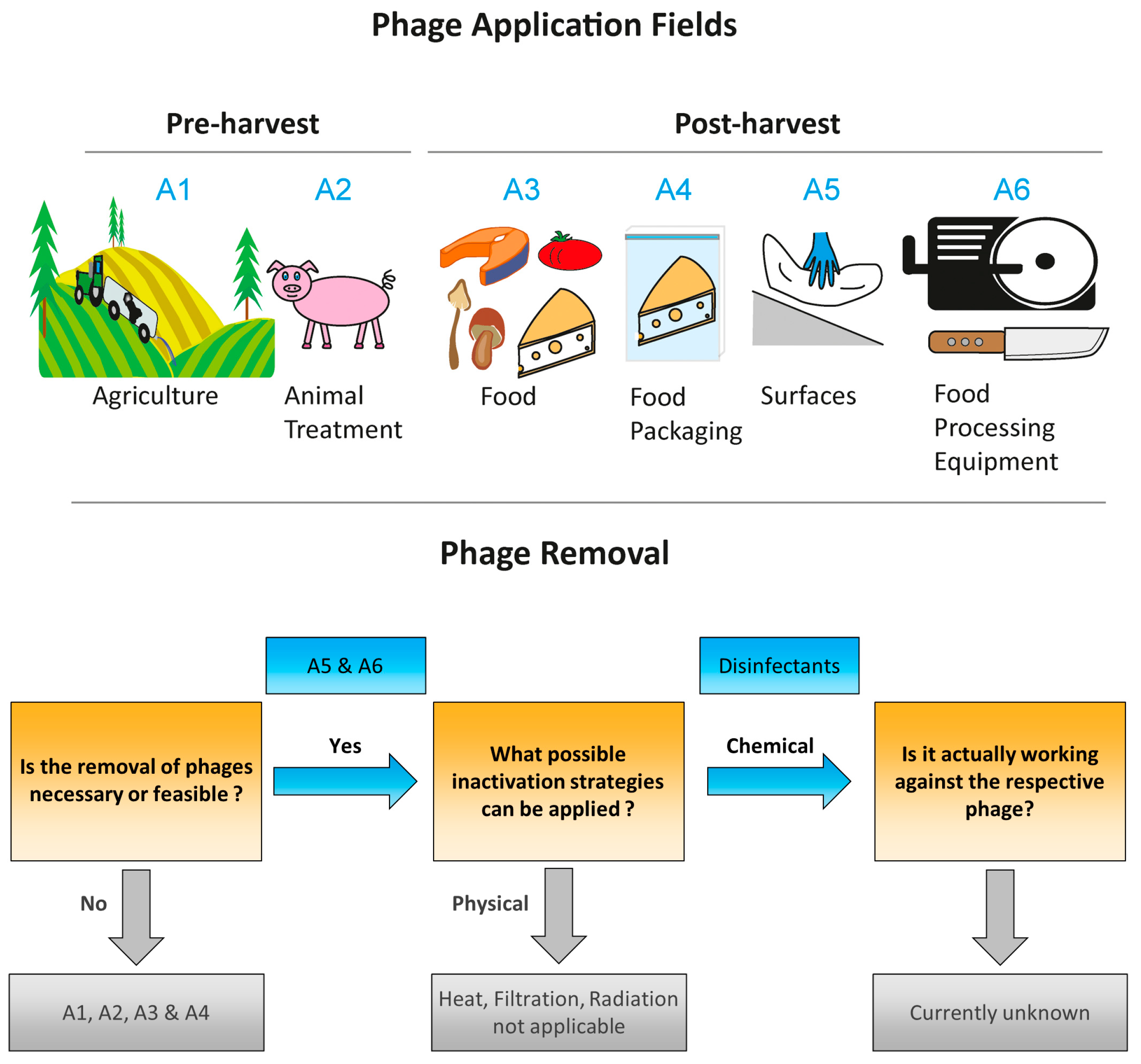
2. Commercial Phage Products, Their Application Field and Legal Framework
3. Legal Regulation of Disinfectants Currently Used in Food and Feed Processing Industry
3.1. Alcohols
3.2. Aldehydes
3.3. Acids and Bases
3.4. Chlorine and Chlorine Releasing Agents
3.5. Peroxides
3.6. Virucides Currently not Approved or Awaiting Approval
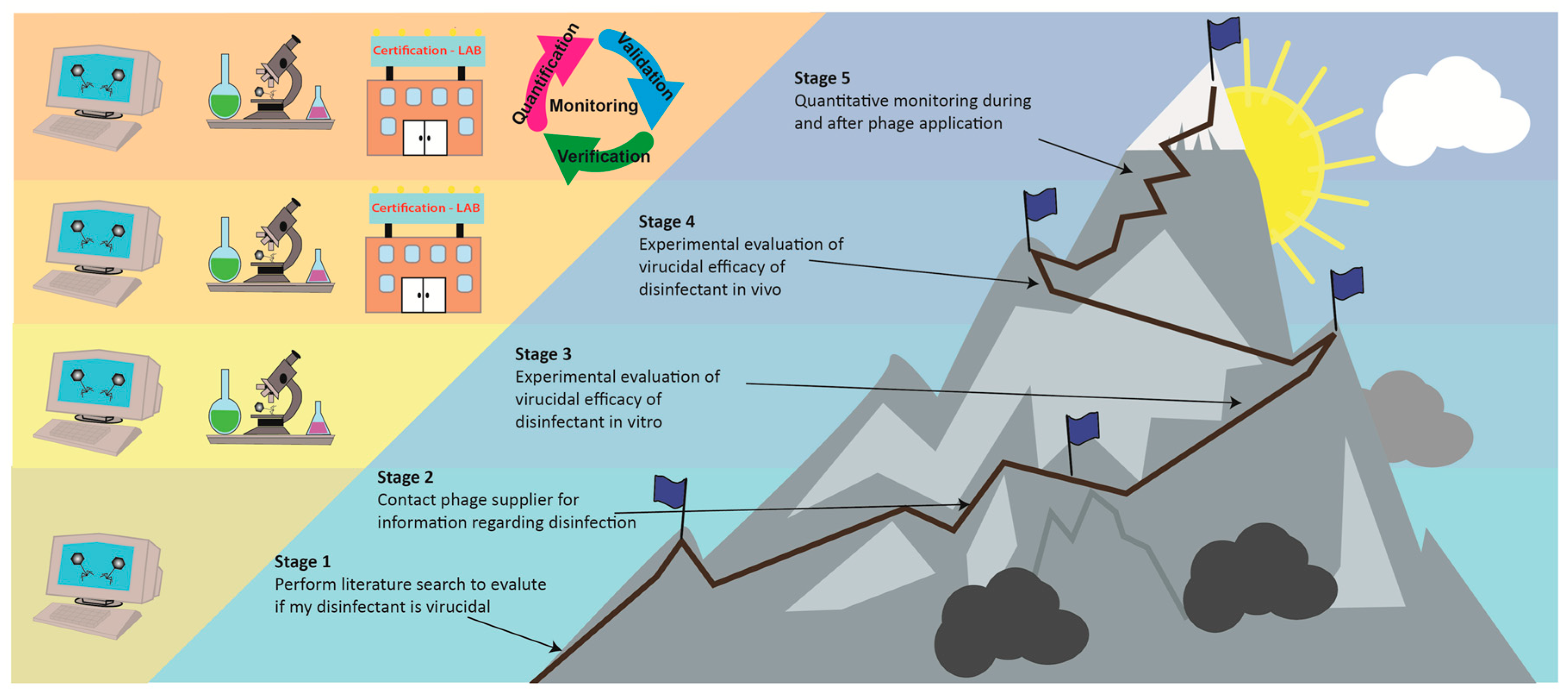
4. Assessing the Virucidal Activity of Disinfectants against Phages
4.1. Phages as Models in Disinfectant Testing
4.2. Efficacy of Common Disinfectants against Naturally Occurring Phages
4.3. Efficacy of Common Disinfectants against Commercial Phages
5. Future Perspectives
6. Conclusions
Supplementary Materials
Funding
Acknowledgments
Conflicts of Interest
References
- Jacobsen, K.H. Globalization and the changing epidemiology of hepatitis a virus. CSH Perspect. Med. 2018, 8. [Google Scholar] [CrossRef]
- Depoux, A.; Philibert, A.; Rabier, S.; Philippe, H.-J.; Fontanet, A.; Flahault, A. A multi-faceted pandemic: A review of the state of knowledge on the zika virus. Public Health Rev. 2018, 39, 10. [Google Scholar] [CrossRef] [PubMed]
- Carroll, D.; Watson, B.; Togami, E.; Daszak, P.; Mazet, J.A.; Chrisman, C.J.; Rubin, E.M.; Wolfe, N.; Morel, C.M.; Gao, G.F.; et al. Building a global atlas of zoonotic viruses. Bull. World Health Organ. 2018, 96, 292–294. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The european union summary report on trends and sources of zoonoses, zoonotic agents and food-borne outbreaks in 2017. EFSA J. 2018, 16, e05500. [Google Scholar]
- Bosch, A.; Gkogka, E.; Le Guyader, F.S.; Loisy-Hamon, F.; Lee, A.; van Lieshout, L.; Marthi, B.; Myrmel, M.; Sansom, A.; Schultz, A.C.; et al. Foodborne viruses: Detection, risk assessment, and control options in food processing. Int. J. Food Microbiol. 2018, 285, 110–128. [Google Scholar] [CrossRef] [PubMed]
- Verhoef, L.; Hewitt, J.; Barclay, L.; Ahmed, S.M.; Lake, R.; Hall, A.J.; Lopman, B.; Kroneman, A.; Vennema, H.; Vinjé, J.; et al. Norovirus genotype profiles associated with foodborne transmission, 1999–2012. Emerg. Infect. Dis. 2015, 21, 592–599. [Google Scholar] [CrossRef]
- De Graaf, M.; van Beek, J.; Koopmans, M.P. Human norovirus transmission and evolution in a changing world. Nat. Rev. Microbiol. 2016, 14, 421–433. [Google Scholar] [CrossRef] [PubMed]
- De Melo, A.G.; Levesque, S.; Moineau, S. Phages as friends and enemies in food processing. Curr. Opin. Biotechnol. 2018, 49, 185–190. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, L.; Bolton, D.; McAuliffe, O.; Coffey, A. Bacteriophages in food applications: From foe to friend. Annu. Rev. Food Sci. Technol. 2019, 10, 151–172. [Google Scholar] [CrossRef]
- Mahony, J.; Murphy, J.; van Sinderen, D. Lactococcal 936-type phages and dairy fermentation problems: From detection to evolution and prevention. Front. Microbiol. 2012, 3, 335. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, K.; Kang, H.S.; Hyun, W.B.; Kim, K.-P. High prevalence of bacillus subtilis-infecting bacteriophages in soybean-based fermented foods and its detrimental effects on the process and quality of cheonggukjang. Food Microbiol. 2018, 76, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Hayes, S.; Murphy, J.; Mahony, J.; Lugli, G.A.; Ventura, M.; Noben, J.-P.; Franz, C.M.A.P.; Neve, H.; Nauta, A.; Van Sinderen, D. Biocidal inactivation of Lactococcus lactis bacteriophages: Efficacy and targets of commonly used sanitizers. Front. Microbiol. 2017, 8, 107. [Google Scholar] [CrossRef]
- Los, M.; Czyz, A.; Sell, E.; Wegrzyn, A.; Neubauer, P.; Wegrzyn, G. Bacteriophage contamination: Is there a simple method to reduce its deleterious effects in laboratory cultures and biotechnological factories? J. Appl. Genet. 2004, 45, 111–120. [Google Scholar] [PubMed]
- Jung, Y.; Matthews, K.R. Chapter 17—Development and application of novel antimicrobials in food and food processing. In Antimicrobial Resistance and Food Safety; Chen, C.-Y., Yan, X., Jackson, C.R., Eds.; Academic Press: San Diego, CA, USA, 2015; pp. 347–364. [Google Scholar]
- Greer, G.G. Bacteriophage control of foodborne bacteriat. J. Food Prot. 2005, 68, 1102–1111. [Google Scholar] [CrossRef]
- Intralytix. Ecoshieldtm. Available online: http://www.intralytix.com/files/prod/07EP/07EP-Desc.pdf (accessed on 25 January 2019).
- FINK-TEC-GmbH. Escherichia coli-Specific Phage Preparation. Available online: http://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM597733.pdf (accessed on 13 February 2019).
- Micreos-Food-Safety-BV. Phageguard e Reduces E. coli on Beef Carcass, Parts and Trim. Available online: http://www.phageguard.com/wp-content/uploads/2018/09/PhageGuard-E-ADS-beef.pdf (accessed on 13 February 2019).
- Intralytix. Listshieldtm. Available online: http://www.intralytix.com/files/prod/01LP/01LP-Desc.pdf (accessed on 25 January2019).
- Micreos-Food-Safety-BV. Phageguard Listex Application on (Food Contact) Surface Areas. Available online: http://www.phageguard.com/wp-content/uploads/2018/09/PhageGuard-Listex-on-food-contact-surface-areas-EU.pdf (accessed on 25 January2019).
- Intralytix. Salmofreshtm. Available online: http://www.intralytix.com/files/prod/02SP/02SP-Desc.pdf (accessed on 25 January2019).
- Phagelux-Inc. Gras Notification: Salmopro®. Available online: http://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/UCM624100.pdf (accessed on 25 January 2019).
- Micreos-Food-Safety-BV. Phageguard s Reduces Salmonella on Fresh Poultry. Available online: http://www.phageguard.com/wp-content/uploads/2018/03/PhageGuard-ADS-Post-Harvest-Poultry-v82.pdf (accessed on 25 January2019).
- Moye, Z.D.; Woolston, J.; Sulakvelidze, A. Bacteriophage applications for food production and processing. Viruses 2018, 10, 205. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Obeso, J.M.; Rodríguez, A. Bacteriophages and their application in food safety. Lett. Appl. Microbiol. 2008, 47, 479–485. [Google Scholar] [CrossRef]
- Karthik, K.; Selvaraj Muneeswaran, N.; Manjunathachar, H.V.; Gopi, M.; Appavoo, E.; Semmannan, K. Bacteriophages: Effective alternative to antibiotics. Adv. Anim. Vet. Sci. 2014, 2, 1–7. [Google Scholar] [CrossRef]
- Sillankorva, S.M.; Oliveira, H.; Azeredo, J. Bacteriophages and their role in food safety. Int. J. Microbiol. 2012, 2012, 863945. [Google Scholar] [CrossRef]
- Loc-Carrillo, C.; Abedon, S.T. Pros and cons of phage therapy. Bacteriophage 2011, 1, 111–114. [Google Scholar] [CrossRef]
- Endersen, L.; O’Mahony, J.; Hill, C.; Paul Ross, R.; McAuliffe, O.; Coffey, A. Phage therapy in the food industry. Annu. Rev. Food Sci. Technol. 2014, 5, 327–349. [Google Scholar] [CrossRef]
- Teng-Hern, T.L.; Kok-Gan, C.; Han, L.L. Application of bacteriophage in biocontrol of major foodborne bacterial pathogens. J. Mol. Biol. Mol. Imaging 2014, 1, 9. [Google Scholar]
- Fister, S.; Mester, P.; Witte, A.K.; Sommer, J.; Schoder, D.; Rossmanith, P. Part of the problem or the solution? Indiscriminate use of bacteriophages in the food industry can reduce their potential and impair growth-based detection methods. Trends Food Sci. Technol. 2019. [Google Scholar] [CrossRef]
- Mateus, L.; Costa, L.; Silva, Y.J.; Pereira, C.; Cunha, A.; Almeida, A. Efficiency of phage cocktails in the inactivation of vibrio in aquaculture. Aquaculture 2014, 424–425, 167–173. [Google Scholar] [CrossRef]
- Zago, M.; Orru, L.; Rossetti, L.; Lamontanara, A.; Fornasari, M.E.; Bonvini, B.; Meucci, A.; Carminati, D.; Cattivelli, L.; Giraffa, G. Survey on the phage resistance mechanisms displayed by a dairy lactobacillus helveticus strain. Food Microbiol. 2017, 66, 110–116. [Google Scholar] [CrossRef] [PubMed]
- Chan, B.K.; Abedon, S.T. Chapter 1—Phage therapy pharmacology: Phage cocktails. In Advances in Applied Microbiology; Laskin, A.I., Sariaslani, S., Gadd, G.M., Eds.; Academic Press: Cambridge, MA, USA, 2012; Volume 78, pp. 1–23. [Google Scholar]
- Fister, S.; Fuchs, S.; Stessl, B.; Schoder, D.; Wagner, M.; Rossmanith, P. Screening and characterisation of bacteriophage p100 insensitive listeria monocytogenes isolates in austrian dairy plants. Food Control 2016, 59, 108–117. [Google Scholar] [CrossRef]
- Hosseinidoust, Z.; Tufenkji, N.; van de Ven, T.G. Formation of biofilms under phage predation: Considerations concerning a biofilm increase. Biofouling 2013, 29, 457–468. [Google Scholar] [CrossRef]
- Christiansen, R.H.; Madsen, L.; Dalsgaard, I.; Castillo, D.; Kalatzis, P.G.; Middelboe, M. Effect of bacteriophages on the growth of flavobacterium psychrophilum and development of phage-resistant strains. Microb. Ecol. 2016, 71, 845–859. [Google Scholar] [CrossRef]
- Guenther, S.; Herzig, O.; Fieseler, L.; Klumpp, J.; Loessner, M.J. Biocontrol of salmonella typhimurium in rte foods with the virulent bacteriophage fo1-e2. Int. J. Food. Microbiol. 2012, 154, 66–72. [Google Scholar] [CrossRef]
- Valerio, N.; Oliveira, C.; Jesus, V.; Branco, T.; Pereira, C.; Moreirinha, C.; Almeida, A. Effects of single and combined use of bacteriophages and antibiotics to inactivate escherichia coli. Virus Res. 2017, 240, 8–17. [Google Scholar] [CrossRef]
- Lopes, A.; Pereira, C.; Almeida, A. Sequential combined effect of phages and antibiotics on the inactivation of escherichia coli. Microorganisms 2018, 6, 125. [Google Scholar] [CrossRef]
- Agun, S.; Fernandez, L.; Gonzalez-Menendez, E.; Martinez, B.; Rodriguez, A.; Garcia, P. Study of the interactions between bacteriophage phiipla-rodi and four chemical disinfectants for the elimination of staphylococcus aureus contamination. Viruses 2018, 10, 103. [Google Scholar] [CrossRef]
- Sulakvelidze, A. Using lytic bacteriophages to eliminate or significantly reduce contamination of food by foodborne bacterial pathogens. J. Sci. Food Agric. 2013, 93, 3137–3146. [Google Scholar] [CrossRef] [PubMed]
- Brown-Jaque, M.; Muniesa, M.; Navarro, F. Bacteriophages in clinical samples can interfere with microbiological diagnostic tools. Sci. Rep. 2016, 6, 33000. [Google Scholar] [CrossRef] [PubMed]
- Muniesa, M.; Blanch, A.R.; Lucena, F.; Jofre, J. Bacteriophages may bias outcome of bacterial enrichment cultures. Appl. Environ. Microbiol. 2005, 71, 4269–4275. [Google Scholar] [CrossRef] [PubMed]
- Micreos-Food-Safety-BV. Phageguard e Reduces E. coli on Leafy Green Vegetables. Available online: http://www.phageguard.com/wp-content/uploads/2018/09/PhageGuard-E-ADS-on-leafy-greens.pdf (accessed on 25 January2019).
- U.S. Senate Committee on Health, Education, Labor, and Pensions. S. Hrg. 109–834—Food Safety: Current Challenges and New Ideas to Safeguard Consumers; U.S. Government Printing Office: Washington, DC, USA, 2006.
- Ly-Chatain, M.H. The factors affecting effectiveness of treatment in phages therapy. Front. Microbiol. 2014, 5, 51. [Google Scholar] [CrossRef]
- Fister, S.; Robben, C.; Witte, A.K.; Schoder, D.; Wagner, M.; Rossmanith, P. Influence of environmental factors on phage-bacteria interaction and on the efficacy and infectivity of phage p100. Front. Microbiol. 2016, 7, 1152. [Google Scholar] [CrossRef]
- Meaden, S.; Koskella, B. Exploring the risks of phage application in the environment. Front. Microbiol. 2013, 4, 358. [Google Scholar] [CrossRef] [PubMed]
- Allue-Guardia, A.; Jofre, J.; Muniesa, M. Stability and infectivity of cytolethal distending toxin type v gene-carrying bacteriophages in a water mesocosm and under different inactivation conditions. Appl. Environ. Microbiol. 2012, 78, 5818–5823. [Google Scholar] [CrossRef]
- Jończyk, E.; Kłak, M.; Międzybrodzki, R.; Górski, A. The influence of external factors on bacteriophages--review. Folia Microbiol. 2011, 56, 191–200. [Google Scholar] [CrossRef]
- Ramirez, K.; Cazarez-Montoya, C.; Lopez-Moreno, H.S.; Castro-del Campo, N. Bacteriophage cocktail for biocontrol of escherichia coli o157:H7: Stability and potential allergenicity study. PLoS ONE 2018, 13, e0195023. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Premaratne, A.; On, S.L. Inactivation of escherichia coli o157:H7 using ultraviolet light-treated bacteriophages. Food Sci. Technol. Int. 2016, 22, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Aquino de Carvalho, N.; Stachler, E.N.; Cimabue, N.; Bibby, K. Evaluation of phi6 persistence and suitability as an enveloped virus surrogate. Environ. Sci. Technol. 2017, 51, 8692–8700. [Google Scholar] [CrossRef] [PubMed]
- Vasickova, P.; Kovarcik, K. 9—Natural persistence of food-and waterborne viruses. In Viruses in Food and Water; Cook, N., Ed.; Woodhead Publishing: Sawston, UK, 2013; pp. 179–204. [Google Scholar]
- Eterpi, M.; McDonnell, G.; Thomas, V. Disinfection efficacy against parvoviruses compared with reference viruses. J. Sci. Food Agric. 2009, 73, 64–70. [Google Scholar] [CrossRef]
- Ly-Chatain, M.H.; Moussaoui, S.; Vera, A.; Rigobello, V.; Demarigny, Y. Antiviral effect of cationic compounds on bacteriophages. Front. Microbiol. 2013, 4, 46. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Sattar, S.; Pinto, F. Virucidal Activity of Microbicides; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 178–207. [Google Scholar]
- Jurczak-Kurek, A.; Gasior, T.; Nejman-Falenczyk, B.; Bloch, S.; Dydecka, A.; Topka, G.; Necel, A.; Jakubowska-Deredas, M.; Narajczyk, M.; Richert, M.; et al. Biodiversity of bacteriophages: Morphological and biological properties of a large group of phages isolated from urban sewage. Sci. Rep. 2016, 6, 34338. [Google Scholar] [CrossRef]
- Allué-Guardia, A.; Martínez-Castillo, A.; Muniesa, M. Persistence of infectious shiga toxin-encoding bacteriophages after disinfection treatments. Appl. Environ. Microbiol. 2014, 80, 2142–2149. [Google Scholar] [CrossRef]
- Komora, N.; Bruschi, C.; Ferreira, V.; Maciel, C.; Brandao, T.R.S.; Fernandes, R.; Saraiva, J.A.; Castro, S.M.; Teixeira, P. The protective effect of food matrices on listeria lytic bacteriophage p100 application towards high pressure processing. Food Microbiol. 2018, 76, 416–425. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Ku, H.-J.; Lee, D.-H.; Kim, Y.-T.; Shin, H.; Ryu, S.; Lee, J.-H. Characterization and genomic study of the novel bacteriophage hy01 infecting both escherichia coli o157:H7 and shigella flexneri: Potential as a biocontrol agent in food. PLoS ONE 2016, 11, e0168985. [Google Scholar] [CrossRef]
- Ahmadi, H.; Radford, D.; Kropinski, A.M.; Lim, L.T.; Balamurugan, S. Thermal-stability and reconstitution ability of listeria phages p100 and a511. Front. Microbiol. 2017, 8, 2375. [Google Scholar] [CrossRef]
- Shen, J.; Zhou, J.; Fu, H.; Mu, Y.; Sun, Y.; Xu, Y.; Xiu, Z. A Klebsiella pneumoniae bacteriophage and its effect on 1,3-propanediol fermentation. In Process Biochemistry; Boudrant, J., Zhong, J.J., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; Volume 51, pp. 1323–1330. [Google Scholar]
- Sun, W.-J.; Liu, C.-F.; Yu, L.; Cui, F.-J.; Zhou, Q.; Yu, S.-L.; Sun, L. A novel bacteriophage ksl-1 of 2-keto-gluconic acid producer pseudomonas fluorescens k1005: Isolation, characterization and its remedial action. BMC Microbiol. 2012, 12, 127. [Google Scholar] [CrossRef]
- Xu, Y.; Ma, Y.; Yao, S.; Jiang, Z.; Pei, J.; Cheng, C. Characterization, genome sequence, and analysis of Escherichia phage cicc 80001, a bacteriophage infecting an efficient l-aspartic acid producing Escherichia coli. Food Environ. Virol. 2016, 8, 18–26. [Google Scholar] [CrossRef]
- Jones, D.T.; Shirley, M.; Wu, X.; Keis, S. Bacteriophage infections in the industrial acetone butanol (ab) fermentation process. J. Mol. Microbiol. Biotechnol. 2000, 2, 21–26. [Google Scholar] [PubMed]
- Cooper, C.J.; Khan Mirzaei, M.; Nilsson, A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016, 7, 1209. [Google Scholar] [CrossRef] [PubMed]
- Kingwell, K. Bacteriophage therapies re-enter clinical trials. Nat. Rev. Drug Discov. 2015, 14, 515. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. The strange history of phage therapy. Bacteriophage 2012, 2, 130–133. [Google Scholar] [CrossRef]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212–213, 38–58. [Google Scholar] [CrossRef]
- Hudson, J.A.; Billington, C.; Cornelius, A.J.; Wilson, T.; On, S.L.; Premaratne, A.; King, N.J. Use of a bacteriophage to inactivate Escherichia coli o157:H7 on beef. Food Microbiol. 2013, 36, 14–21. [Google Scholar] [CrossRef]
- Spricigo, D.A.; Bardina, C.; Cortés, P.; Llagostera, M. Use of a bacteriophage cocktail to control Salmonella in food and the food industry. Int. J. Food Microbiol. 2013, 165, 169–174. [Google Scholar] [CrossRef]
- Leverentz, B.; Conway, W.S.; Alavidze, Z.; Janisiewicz, W.J.; Fuchs, Y.; Camp, M.J.; Chighladze, E.; Sulakvelidze, A. Examination of bacteriophage as a biocontrol method for Salmonella on fresh-cut fruit: A model study. J. Food Prot. 2001, 64, 1116–1121. [Google Scholar] [CrossRef]
- Figueiredo, A.C.L.; Almeida, R.C.C. Antibacterial efficacy of nisin, bacteriophage p100 and sodium lactate against Listeria monocytogenes in ready-to-eat sliced pork ham. Braz. J. Microbiol. Publ. Braz. Soc. Microbiol. 2017, 48, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Greer, G.G.; Dilts, B.D. Control of brochothrix thermosphacta spoilage of pork adipose tissue using bacteriophages. J. Food Prot. 2002, 65, 861–863. [Google Scholar] [CrossRef] [PubMed]
- McDonnell, G.E. Antisepsis, Disinfection, and Sterilization; ASM Press: Bel Air, MD, USA, 2017. [Google Scholar]
- Fernández, L.; Gutiérrez, D.; Rodríguez, A.; García, P. Application of bacteriophages in the agro-food sector: A long way toward approval. Front. Cell. Infect. Microbiol. 2018, 8, 296. [Google Scholar] [CrossRef]
- Reuter, G. Disinfection and hygiene in the field of food of animal origin. Int. Biodeterior. Biodegrad. 1998, 41, 209–215. [Google Scholar] [CrossRef]
- Otto, C.; Zahn, S.; Rost, F.; Zahn, P.; Jaros, D.; Rohm, H. Physical methods for cleaning and disinfection of surfaces. Food Eng. Rev. 2011, 3, 171–188. [Google Scholar] [CrossRef]
- Stanga, M. Sanitation: Cleaning and Disinfection in the Food Industry; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2010. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Evaluation of the safety and efficacy of listex™ p100 for reduction of pathogens on different ready-to-eat (rte) food products. EFSA J. 2016, 14, e04565. [Google Scholar]
- EFSA Panel on Biological Hazards (BIOHAZ). Scientific opinion on the evaluation of the safety and efficacy of listex™ p100 for the removal of Listeria monocytogenes surface contamination of raw fish. EFSA J. 2012, 10, 2615. [Google Scholar] [CrossRef]
- Authority, E.F.S. The use and mode of action of bacteriophages in food production—Endorsed for public consultation 22 January 2009—public consultation 30 January—6 March 2009. EFSA J. 2009, 7. [Google Scholar] [CrossRef]
- Phagelux-Inc. Gras Notification: Salmopro®. Available online: http://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm476554.pdf (accessed on 25 January2019).
- Schulz, S. Eine never ending story: Die abgrenzung von zusatzstoffen und verarbeitungshilfsstoffen. ZLR 2017, 1, 4–15. [Google Scholar]
- Jagow, C.V.; Teufer, T. Das große fressen bakteriophagen in der lebensmittelherstellung: Eine rechtliche einordnung. ZLR 2007, 1, 25. [Google Scholar]
- Verbeken, G.; Pirnay, J.P.; De Vos, D.; Jennes, S.; Zizi, M.; Lavigne, R.; Casteels, M.; Huys, I. Optimizing the european regulatory framework for sustainable bacteriophage therapy in human medicine. Arch. Immunol. Ther. Exp. 2012, 60, 161–172. [Google Scholar] [CrossRef]
- Sulakvelidze, A.; Pasternack, G.R. Industrial and regulatory issues in bacteriophage applications in food production and processing. In Bacteriophages in the Control of Food-and Waterborne Pathogens; Sabour, P.M., Griffiths, M.W., Eds.; American Society of Microbiology: Washington, DC, USA, 2010. [Google Scholar]
- Verordnung (eg) nr. 1333/2008 des europäischen parlaments und des rates vom 16. Dezember 2008 über lebensmittelzusatzstoffe. Available online: https://eur-lex.europa.eu/eli/reg/2008/1333/2014–04–14 (accessed on 2 April 2019).
- Narvaez, S.O. Post harvest-einsatz virulenter bakteriophagen gegen campylobacter spp. Und yersinia enterocolitica. In Aus dem Institut für Lebensmittelhygiene des Fachbereichs Veterinärmedizin der Freien Universität Berlin; Mensch & Buch: Berlin, Germany, 2012. [Google Scholar]
- Svircev, A.; Roach, D.; Castle, A. Framing the future with bacteriophages in agriculture. Viruses 2018, 10, 218. [Google Scholar] [CrossRef] [PubMed]
- Cooper, I.R. A review of current methods using bacteriophages in live animals, food and animal products intended for human consumption. J. Microbiol. Methods 2016, 130, 38–47. [Google Scholar] [CrossRef]
- Buttimer, C.; McAuliffe, O.; Ross, R.P.; Hill, C.; O’Mahony, J.; Coffey, A. Bacteriophages and bacterial plant diseases. Front. Microbiol. 2017, 8, 34. [Google Scholar] [CrossRef]
- Jones, J.B.; Jackson, L.E.; Balogh, B.; Obradovic, A.; Iriarte, F.B.; Momol, M.T. Bacteriophages for plant disease control. Annu. Rev. Phytopathol. 2007, 45, 245–262. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.K.; Király, L.; Schwarczinger, I. Phage therapy for plant disease control with a focus on fire blight. Cent. Eur. J.Biol. 2012, 7, 1–12. [Google Scholar] [CrossRef]
- Tolen, N.T.; Xie, Y.; Hairgrove, B.T.; Gill, J.J.; Taylor, M.T. Evaluation of commercial prototype bacteriophage intervention designed for reducing o157 and non-o157 shiga-toxigenic escherichia coli (stec) on beef cattle hide. Foods 2018, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Wernicki, A.; Nowaczek, A.; Urban-Chmiel, R. Bacteriophage therapy to combat bacterial infections in poultry. Virol. J. 2017, 14, 179. [Google Scholar] [CrossRef]
- Klopatek, S.; Callaway, T.R.; Wickersham, T.; Sheridan, T.G.; Nisbet, D. Bacteriophage utilization in animal hygiene. In Bacteriophages, Biology, Technology, Therapy; Harper, D.R., Abedon, S.T., Burrowes, B.H., McConville, M.L., Eds.; Springer: Berlin, Germany, 2018; pp. 1–28. [Google Scholar]
- Born, Y.; Bosshard, L.; Duffy, B.; Loessner, M.J.; Fieseler, L. Protection of erwinia amylovora bacteriophage y2 from uv-induced damage by natural compounds. Bacteriophage 2015, 5, e1074330. [Google Scholar] [CrossRef] [PubMed]
- Loc Carrillo, C.; Atterbury, R.J.; El-Shibiny, A.; Connerton, P.L.; Dillon, E.; Scott, A.; Connerton, I.F. Bacteriophage therapy to reduce Campylobacter jejuni colonization of broiler chickens. Appl. Environ. Microbiol. 2005, 71, 6554. [Google Scholar] [CrossRef]
- Miller, R.W.; Skinner, E.J.; Sulakvelidze, A.; Mathis, G.F.; Hofacre, C.L. Bacteriophage therapy for control of necrotic enteritis of broiler chickens experimentally infected with clostridium perfringens. Avian Dis. 2010, 54, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, R.; Dhama, K.; Kumar, A.; Rahal, A.; Kapoor, S. Bacteriophage therapy for safeguarding animal and human health: A review. PJBS 2014, 17, 301–315. [Google Scholar] [CrossRef]
- Wang, L.; Qu, K.; Li, X.; Cao, Z.; Wang, X.; Li, Z.; Song, Y.; Xu, Y. Use of bacteriophages to control Escherichia coli o157:H7 in domestic ruminants, meat products, and fruits and vegetables. Foodborne Pathog. Dis. 2017, 14, 483–493. [Google Scholar] [CrossRef]
- Labrie, S.J.; Samson, J.E.; Moineau, S. Bacteriophage resistance mechanisms. Nat. Rev. Microbiol. 2010, 8, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Marcó, M.B.; Moineau, S.; Quiberoni, A. Bacteriophages and dairy fermentations. Bacteriophage 2012, 2, 149–158. [Google Scholar] [CrossRef]
- Sabour, P.M.; Griffiths, M.W. Bacteriophages in the Control of Food- and Waterborne Pathogens; ASM Press: Washington, DC, USA, 2010. [Google Scholar]
- Bridier, A.; Sanchez-Vizuete, P.; Guilbaud, M.; Piard, J.C.; Naïtali, M.; Briandet, R. Biofilm-associated persistence of food-borne pathogens. Food Microbiol. 2015, 45, 167–178. [Google Scholar] [CrossRef]
- Banach, L.J.; Sampers, I.; Van Haute, S.; Van der, F.-K. Effect of disinfectants on preventing the cross-contamination of pathogens in fresh produce washing water. Int. J. Environ. Res. Public Health 2015, 12, 8658–8677. [Google Scholar] [CrossRef] [PubMed]
- Gil, M.I.; Selma, M.V.; López-Gálvez, F.; Allende, A. Fresh-cut product sanitation and wash water disinfection: Problems and solutions. Int. J. Food Microbiol. 2009, 134, 37–45. [Google Scholar] [CrossRef]
- Maillard, J.-Y.; Sattar, S.A.; Pinto, F. Virucidal activity of microbicides. In Russell, Hugo & Ayliffe’s: Principles and Practice of Disinfection, Preservation and Sterilization; Wiley-Blackwell: Hoboken, NJ, USA, 2012; pp. 178–207. [Google Scholar]
- Boyd, E.F.; Brüssow, H. Common themes among bacteriophage-encoded virulence factors and diversity among the bacteriophages involved. Trends Microbiol. 2002, 10, 521–529. [Google Scholar] [CrossRef]
- Passport-Food-Safety-Solutions-Inc. Finalyse®: A Novel Pre-Harvest Hide Wash. Available online: http://www.passportfoodsafety.com/assets/pdf/Finalyse Detailer_2017.03.28_LR.pdf (accessed on 13 February 2019).
- Passport-Food-Safety-Solutions-Inc. Finalyse® Overhead Spray System. Available online: http://www.passportfoodsafety.com/assets/pdf/Finalyse Product Insert_2017.03.28_LR.pdf (accessed on 13 February 2019).
- Micreos-Food-Safety-BV. Phageguard Listex Application Data Sheet Cheese. Available online: http://www.phageguard.com/wp-content/uploads/2017/03/PhageGuard-Listex-Application-Data-Sheet-Cheese-Final.pdf (accessed on 25 January 2019).
- Micreos-Food-Safety-BV. Phageguard Listex Application Data Sheet: Salmon. Available online: http://www.phageguard.com/wp-content/uploads/2017/03/PhageGuard-Listex-Aplication-Data-Sheet-Salmon-March-17.pdf (accessed on 25 January 2019).
- Micreos-Food-Safety-BV. Phageguard Listex Application Data Sheet Meat. Available online: http://www.phageguard.com/wp-content/uploads/2017/03/PhageGuard-Listex-Aplication-Data-Sheet-RTE-Meat-FINAL.pdf (accessed on 25 January 2019).
- Mai, V.; Ukhanova, M.; Visone, L.; Abuladze, T.; Sulakvelidze, A. Bacteriophage administration reduces the concentration of Listeria monocytogenes in the gastrointestinal tract and its translocation to spleen and liver in experimentally infected mice. Int. J. Microbiol. 2010, 2010, 624234. [Google Scholar] [CrossRef] [PubMed]
- Intralytix. Veterinary Applications. Available online: http://www.intralytix.com/index.php?page=vet (accessed on 13 February 2019).
- Proteon-Pharmaceuticals. Bafasal®. Available online: http://www.proteonpharma.com/products/bafasal-poultry/ (accessed on 13 February 2019).
- Magnone, J.P.; Marek, P.J.; Sulakvelidze, A.; Senecal, A.G. Additive approach for inactivation of Escherichia coli o157:H7, Salmonella, and Shigella spp. On contaminated fresh fruits and vegetables using bacteriophage cocktail and produce wash. J. Food Prot. 2013, 76, 1336–1341. [Google Scholar] [CrossRef]
- Albino, L.A.A.; Rostagno, M.H.; Húngaro, H.M.; Mendonça, R.C.S. Isolation, characterization, and application of bacteriophages for salmonella spp. Biocontrol in pigs. Foodborne Pathog. Dis. 2014, 11, 602–609. [Google Scholar] [CrossRef]
- Micreos-Food-Safety-BV. Salmonelextm Notification. Available online: http://www.fda.gov/downloads/Food/IngredientsPackagingLabeling/GRAS/NoticeInventory/ucm505161.pdf (accessed on 25 January 2019).
- Alagappan, K.M.; Deivasigamani, B.; Somasundaram, S.T.; Kumaran, S. Occurrence of vibrio parahaemolyticus and its specific phages from shrimp ponds in east coast of India. Curr. Microbiol. 2010, 61, 235–240. [Google Scholar] [CrossRef]
- Letchumanan, V.; Chan, K.-G.; Pusparajah, P.; Saokaew, S.; Duangjai, A.; Goh, B.-H.; Ab Mutalib, N.-S.; Lee, L.-H. Insights into bacteriophage application in controlling Vibrio species. Front. Microbiol. 2016, 7, 1114. [Google Scholar] [CrossRef]
- Phagelux-Inc. Lexia. Available online: http://www.phagelux.com/en/single/menu_84.htm?menuid=84 (accessed on 2 April 2019).
- Zhang, H.; Wang, R.; Bao, H. Phage inactivation of foodborne shigella on ready-to-eat spiced chicken. Poult. Sci. 2013, 92, 211–217. [Google Scholar] [CrossRef]
- Gras Notification of the Bacteriophage Cocktail: Shigashield tm. Available online: http://www.fda.gov/downloads/food/ingredientspackaginglabeling/gras/noticeinventory/ucm529756.pdf (accessed on 25 January 2019).
- OmniLytics. Agriphage. Available online: http://www.agriphage.com/wp-content/uploads/2018/11/67986–1-EPA-Final-Print-Label-AGRIPHAGE-10–18–18.pdf (accessed on 25 January 2019).
- Balogh, B.; Jone, J.B. Improved efficacy of newly formulated bacteriophages for management of bacterial spot on tomato. Plant Dis. 2003, 87, 6. [Google Scholar] [CrossRef]
- Lee, C.-N.; Lin, J.-W.; Weng, S.-F.; Tseng, Y.-H. Genomic characterization of the intron-containing t7-like phage phil7 of xanthomonas campestris. Appl. Environ. Microbiol. 2009, 75, 7828. [Google Scholar] [CrossRef]
- Yu, J.G.; Lim, J.A.; Song, Y.R.; Heu, S.; Kim, G.H.; Koh, Y.J.; Oh, C.S. Isolation and characterization of bacteriophages against Pseudomonas syringae pv. Actinidiae causing bacterial canker disease in kiwifruit. J. Microbiol. Biotechnol. 2016, 26, 385–393. [Google Scholar] [CrossRef]
- Brimrose-Technology-Corporation. Prevention&Treatment-Bacteriophage Product. Available online: http://www.brimrosetechnology.com/prevention-treatment (accessed on 13 February 2019).
- Eliava-Pharmacy. Pyo Bacteriophage. Available online: http://bacteriophagepharmacy.com/product/pyo-bacteriophage/ (accessed on 13 February 2019).
- OmniLytics. Agriphage-cmm. Available online: http://www.agriphage.com/wp-content/uploads/2018/11/67986–6-EPA-Final-Print-Label-AGRIPHAGE-CMM-10–23–18.pdf (accessed on 25 January 2019).
- Wittmann, J.; Gartemann, K.-H.; Eichenlaub, R.; Dreiseikelmann, B. Genomic and molecular analysis of phage cmp1 from clavibacter michiganensis subspecies michiganensis. Bacteriophage 2011, 1, 6–14. [Google Scholar] [CrossRef]
- Eliava-Pharmacy. Intesti Bacteriophage. Available online: http://bacteriophagepharmacy.com/product/intesti-bacteriophage/ (accessed on 13 February 2019).
- OmniLytics. Agriphage-fire Blight. Available online: http://www.agriphage.com/wp-content/uploads/2018/11/EPA-Stamped-Label-67986–8-20180927.pdf (accessed on 25 January 2019).
- Gill, J.J.; Svircev, A.M.; Smith, R.; Castle, A.J. Bacteriophages of Erwinia amylovora. Appl. Environ. Microbiol. 2003, 69, 2133. [Google Scholar] [CrossRef]
- Born, Y.; Fieseler, L.; Marazzi, J.; Lurz, R.; Duffy, B.; Loessner, M.J. Novel virulent and broad-host-range erwinia amylovora bacteriophages reveal a high degree of mosaicism and a relationship to enterobacteriaceae phages. Appl. Environ. Microbiol. 2011, 77, 5945. [Google Scholar] [CrossRef]
- Enviroinvest-Környezetvédelmi-és-Biotechnológiai-Zrt. Erwiphage Plus. Available online: http://www.erwiphage.com (accessed on 13 February 2019).
- Meczker, K.; Domotor, D.; Vass, J.; Rakhely, G.; Schneider, G.; Kovacs, T. The genome of the erwinia amylovora phage phieah1 reveals greater diversity and broadens the applicability of phages for the treatment of fire blight. FEMS Microbiol. Lett. 2014, 350, 25–27. [Google Scholar] [CrossRef]
- Eliava-Pharmacy. Ses Bacteriophage; Eliava Institute: Tbilisi, Georgia, 2019. [Google Scholar]
- Ahmad, A.A.; Ogawa, M.; Kawasaki, T.; Fujie, M.; Yamada, T. Characterization of bacteriophages cp1 and cp2, the strain-typing agents for Xanthomonas axonopodis pv. Citri. Appl. Environ. Microbiol. 2014, 80, 77. [Google Scholar] [CrossRef]
- Eliava-Pharmacy. Enko Bacteriophage. Available online: http://bacteriophagepharmacy.com/product/enko-bacteriophage/ (accessed on 13 February 2019).
- Adriaenssens, E.M.; Van Vaerenbergh, J.; Vandenheuvel, D.; Dunon, V.; Ceyssens, P.-J.; De Proft, M.; Kropinski, A.M.; Noben, J.-P.; Maes, M.; Lavigne, R. T4-related bacteriophage limestone isolates for the control of soft rot on potato caused by ‘Dickeya solani’. PLoS ONE 2012, 7, e33227. [Google Scholar] [CrossRef]
- APS-Biocontrol-Ltd. Biolyse®-pb. Available online: http://www.apsbiocontrol.com/products (accessed on 13 February 2019).
- Eliava-Pharmacy. Fersis Bacteriophage. Available online: http://bacteriophagepharmacy.com/product/fersis-bacteriophage/ (accessed on 13 February 2019).
- Proteon-Pharmaceuticals. Bafador®. Available online: http://www.proteonpharma.com/products/bafador-aquaculture/ (accessed on 13 February 2019).
- Europäischen Union. Verordnung (eg) nr. 2073/2005 der Kommission Vom 15. November 2005 Über Mikrobiologische Kriterien für Lebensmittel; Europäischen Union: Brussels, Belgium, 2005. [Google Scholar]
- Atamer, Z.; Samtlebe, M.; Neve, H.; Heller, K.J.; Hinrichs, J. Review: Elimination of bacteriophages in whey and whey products. Front. Microbiol. 2013, 4, 191. [Google Scholar] [CrossRef]
- Guglielmotti, D.M.; Mercanti, D.J.; Reinheimer, J.A.; Quiberoni Adel, L. Review: Efficiency of physical and chemical treatments on the inactivation of dairy bacteriophages. Front. Microbiol. 2012, 2, 282. [Google Scholar] [CrossRef]
- Sadiq, F.; Guoqing, H.; Sakandar, H.; Li, Y.; Ou, K. Lactococcus lactis phages from the perspective of their diversity, thermal and biocidal resistance. Int. Dairy J. 2019, 90, 28–38. [Google Scholar] [CrossRef]
- Leistner, L.; Gould, G.W. Hurdle Technologies: Combination Treatments for Food Stability, Safety and Quality; Springer: Berlin, Germany, 2002; pp. 1–15. [Google Scholar]
- U.S.-Food-and-Drug-Administration. Available online: http://www.Fda.Gov (accessed on 2 April 2019).
- Health-Canada. Available online: http://www.Canada.Ca/en/health-canada.Html (accessed on 2 April 2019).
- European-Chemicals-Agency. Available online: https://echa.Europa.Eu/home (accessed on 2 April 2019).
- European-Food-Safety-Authority. Available online: http://www.Efsa.Europa.Eu (accessed on 2 April 2019).
- Food-and-Agriculture-Organization-of-the-United-Nations. Available online: http://www.Fao.Org/home/en/ (accessed on 2 April 2019).
- World-Health-Organization. Available online: http://www.Who.Int (accessed on 2 April 2019).
- EU-Pesticides-Database-for-Plants. Available online: http://ec.Europa.Eu/food/plant/pesticides/eu-pesticides-database/public/?Event=homepage&language=en (accessed on 2 April 2019).
- Joint-Committee-on-Food-Additives-and-Contaminants. Available online: http://apps.Who.Int/food-additives-contaminants-jecfa-database/search.Aspx (accessed on 2 April 2019).
- Codex-Alimentarius. Available online: http://www.Fao.Org/fao-who-codexalimentarius/en/ (accessed on 2 April 2019).
- U.S.-Environmental-Protection-Agency. Available online: https://www.Epa.Gov (accessed on 2 April 2019).
- Generally-Recognized-As-Safe. Available online: http://www.Fda.Gov/food/ingredientspackaginglabeling/gras/default.Htm (accessed on 2 April 2019).
- Europäischen Union. Durchführungsverordnung (eu) 2015/1759 der Kommission; Europäischen Union: Brussels, Belgium, 2015. [Google Scholar]
- Sands, J.A. Inactivation and inhibition of replication of the enveloped bacteriophage phi6 by fatty acids. Antimicrob. Agents Chemother. 1977, 12, 523–528. [Google Scholar] [CrossRef]
- Takahashi, E.; Hirotani, H.; Kobayashi, M.; Ohigashi, H.; Koshimizu, K. Inactivation of t5 phage by cis-vaccenic acid, an antivirus substance from Rhodopseudomonas capsulata, and by unsaturated fatty acids and related alcohols. FEMS Microbiol. Lett. 1991, 77, 13–17. [Google Scholar]
- Ebrecht, A.C.; Guglielmotti, D.M.; Tremmel, G.; Reinheimer, J.A.; Suarez, V.B. Temperate and virulent Lactobacillus delbrueckii bacteriophages: Comparison of their thermal and chemical resistance. Food Microbiol. 2010, 27, 515–520. [Google Scholar] [CrossRef]
- D’Souza, D.H.; Su, X. Efficacy of chemical treatments against murine norovirus, feline calicivirus, and ms2 bacteriophage. Foodborne Pathog. Dis. 2010, 7, 319–326. [Google Scholar] [CrossRef]
- Mercanti, D.J.; Guglielmotti, D.M.; Patrignani, F.; Reinheimer, J.A.; Quiberoni, A. Resistance of two temperate lactobacillus paracasei bacteriophages to high pressure homogenization, thermal treatments and chemical biocides of industrial application. Food Microbiol. 2012, 29, 99–104. [Google Scholar] [CrossRef]
- Europäischen Union. Durchführungsverordnung (eu) 2016/672 der Kommission vom 29 April 2016 zur Genehmigung von Peressigsäure als Alten Wirkstoff zur Verwendung in Biozidprodukten der Produktarten 1, 2, 3, 4, 5 und 6; Europäischen Union: Brussels, Belgium, 2016. [Google Scholar]
- Morin, T.; Martin, H.; Soumet, C.; Fresnel, R.; Lamaudiere, S.; Le Sauvage, A.L.; Deleurme, K.; Maris, P. Comparison of the virucidal efficacy of peracetic acid, potassium monopersulphate and sodium hypochlorite on bacteriophages p001 and ms2. J. Appl. Microbiol. 2015, 119, 655–665. [Google Scholar] [CrossRef]
- Cook, A.M.; Brown, W.R.L. Inactivation of a bacteriophage by chemical antibacterial agents. J. Pharm. Pharmacol. 1964, 16, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Dunkin, N.; Weng, S.; Schwab, K.J.; McQuarrie, J.; Bell, K.; Jacangelo, J.G. Comparative inactivation of murine norovirus and ms2 bacteriophage by peracetic acid and monochloramine in municipal secondary wastewater effluent. Environ. Sci. Technol. 2017, 51, 2972–2981. [Google Scholar] [CrossRef] [PubMed]
- Maillard, J.Y.; Beggs, T.S.; Day, M.J.; Hudson, R.A.; Russell, A.D. Effect of biocides on ms2 and k coliphages. Appl. Environ. Microbiol. 1994, 60, 2205–2206. [Google Scholar]
- Fister, S.; Mester, P.; Sommer, J.; Witte, A.K.; Kalb, R.; Wagner, M.; Rossmanith, P. Virucidal influence of ionic liquids on phages p100 and ms2. Front. Microbiol. 2017, 8, 1608. [Google Scholar] [CrossRef] [PubMed]
- Sommer, J.; Fister, S.; Gundolf, T.; Bromberger, B.; Mester, P.-J.; Witte, A.; Kalb, R.; Rossmanith, P. Virucidal or not virucidal? That is the question—Predictability of ionic liquid’s virucidal potential in biological test systems. Int. J. Mol. Sci. 2018, 19, 790. [Google Scholar] [CrossRef]
- Kobayashi, H.; Tsuzuki, M.; Koshimizu, K.; Toyama, H.; Yoshihara, N.; Shikata, T.; Abe, K.; Mizuno, K.; Otomo, N.; Oda, T. Susceptibility of hepatitis b virus to disinfectants or heat. J. Clin. Microbiol. 1984, 20, 214–216. [Google Scholar]
- Thurston-Enriquez, J.A.; Haas, C.N.; Jacangelo, J.; Gerba, C.P. Chlorine inactivation of adenovirus type 40 and feline calicivirus. Appl. Environ. Microbiol. 2003, 69, 3979–3985. [Google Scholar] [CrossRef]
- Zyara, A.M.; Torvinen, E.; Veijalainen, A.M.; Heinonen-Tanski, H. The effect of chlorine and combined chlorine/uv treatment on coliphages in drinking water disinfection. J. Water Health 2016, 14, 640–649. [Google Scholar] [CrossRef]
- Campagna, C.; Villion, M.; Labrie, S.J.; Duchaine, C.; Moineau, S. Inactivation of dairy bacteriophages by commercial sanitizers and disinfectants. Int. J. Food Microbiol. 2014, 171, 41–47. [Google Scholar] [CrossRef]
- Grunert, A.; Frohnert, A.; Selinka, H.C.; Szewzyk, R. A new approach to testing the efficacy of drinking water disinfectants. Int. J. Hyg. Environ. Health 2018, 221, 1124–1132. [Google Scholar] [CrossRef]
- Binetti, A.G.; Reinheimer, J.A. Thermal and chemical inactivation of indigenous streptococcus thermophilus bacteriophages isolated from argentinian dairy plants. J. Food Prot. 2000, 63, 509–515. [Google Scholar] [CrossRef]
- Briggiler Marco, M.; De Antoni, G.L.; Reinheimer, J.A.; Quiberoni, A. Thermal, chemical, and photocatalytic inactivation of Lactobacillus plantarum bacteriophages. J. Food Prot. 2009, 72, 1012–1019. [Google Scholar] [CrossRef]
- Tomat, D.; Balagué, C.; Aquili, V.; Verdini, R.; Quiberoni, A. Resistance of phages lytic to pathogenic Escherichia coli to sanitisers used by the food industry and in home settings. Int. J. Food Sci. Technol. 2018, 53, 533–540. [Google Scholar] [CrossRef]
- Branston, S.D.; Stanley, E.C.; Ward, J.M.; Keshavarz-Moore, E. Determination of the survival of bacteriophage m13 from chemical and physical challenges to assist in its sustainable bioprocessing. Biotechnol. Bioprocess Eng. 2013, 18, 560–566. [Google Scholar] [CrossRef]
- Reinhardt, A.; Cadden, S.; Sands, J.A. Inhibitory effect of fatty acids on the entry of the lipid-containing bacteriophage pr4 into Escherichia coli. J. Virol. 1978, 25, 479–485. [Google Scholar]
- Allwood, P.B.; Malik, Y.S.; Hedberg, C.W.; Goyal, S.M. Effect of temperature and sanitizers on the survival of feline calicivirus, escherichia coli, and f-specific coliphage ms2 on leafy salad vegetables. J. Food Prot. 2004, 67, 1451–1456. [Google Scholar] [CrossRef]
- Association-Française-de-Normalisation. Available online: http://www.Afnor.Org (accessed on 2 April 2019).
- Deutsche-Vereinigung-zur-Bekämpfung-der-Viruskrankheiten. Available online: http://www.Dvv-ev.De (accessed on 2 April 2019).
- Department-of-Environment. Available online: http://www.Gov.Uk/government/organisations/department-for-environment-food-rural-affairs (accessed on 2 April 2019).
- Classification and nomenclature of viruses. Fourth report of the international committee on taxonomy of viruses. Intervirology 1982, 17, 1–199.
- Mayo, M.A.; Pringle, C.R. Virus taxonomy—1997. J. Gen. Virol. 1998, 79, 649–657. [Google Scholar] [CrossRef]
- Nanavutty, S.H. The thermal death-rate of the bacteriophage. J. Pathol. Bacteriol. 1930, 33, 203–214. [Google Scholar] [CrossRef]
- Krueger, A.P. The heat inactivation of antistaphylococcus bacteriophage. J. Gen. Physiol. 1932, 15, 363–368. [Google Scholar] [CrossRef]
- Sharp, D.G.; Hook, A.E.; Taylor, A.R.; Beard, D.; Beard, J.W. Sedimentation characters and ph stability of the t2 bacteriophage of Escherichia coli. J. Biol. Chem. 1946, 165, 259–270. [Google Scholar]
- Kerby, G.P.; Gowdy, R.A.; Dillon, E.S.; Dillon, M.L.; Csâky, T.Z.; Sharp, D.G.; Beard, J.W. Purification ph stability and sedimentation properties of the t7 bacteriophage of Escherichia coli. J. Immunol. 1949, 63, 93–107. [Google Scholar]
- Sauerbrei, A.; Sehr, K.; Brandstadt, A.; Heim, A.; Reimer, K.; Wutzler, P. Sensitivity of human adenoviruses to different groups of chemical biocides. J. Hosp. Infect. 2004, 57, 59–66. [Google Scholar] [CrossRef]
- WoIff, M.H.; Schmitt, J.; Rahaus, M.; König, A. Hepatitis a virus: A test method for virucidal activity. J. Hosp. Infect. 2001, 48, S18–S22. [Google Scholar] [CrossRef]
- Schijven, J.F.; Sadeghi, G.; Hassanizadeh, S.M. Long-term inactivation of bacteriophage prd1 as a function of temperature, ph, sodium and calcium concentration. Water Res. 2016, 103, 66–73. [Google Scholar] [CrossRef]
- Richards, G.P. Critical review of norovirus surrogates in food safety research: Rationale for considering volunteer studies. Food Environ. Virol. 2012, 4, 6–13. [Google Scholar] [CrossRef]
- Charles, K.J.; Shore, J.; Sellwood, J.; Laverick, M.; Hart, A.; Pedley, S. Assessment of the stability of human viruses and coliphage in groundwater by pcr and infectivity methods. J. Appl. Microbiol. 2009, 106, 1827–1837. [Google Scholar] [CrossRef]
- Gibson, K.E.; Crandall, P.G.; Ricke, S.C. Removal and transfer of viruses on food contact surfaces by cleaning cloths. Appl. Environ. Microbiol. 2012, 78, 3037–3044. [Google Scholar] [CrossRef]
- Armstrong, A.M.; Sobsey, M.D.; Casanova, L.M. Disinfection of bacteriophage ms2 by copper in water. Appl. Microbiol. Biotechnol. 2017, 101, 6891–6897. [Google Scholar] [CrossRef]
- Guo, L.; Xu, R.; Gou, L.; Liu, Z.; Zhao, Y.; Liu, D.; Zhang, L.; Chen, H.; Kong, M.G. Mechanism of virus inactivation by cold atmospheric-pressure plasma and plasma-activated water. Appl. Environ. Microbiol. 2018, 84. [Google Scholar] [CrossRef]
- Hornstra, L.M.; Schijven, J.F.; Waade, A.; Prat, G.S.; Smits, F.J.C.; Cirkel, G.; Stuyfzand, P.J.; Medema, G.J. Transport of bacteriophage ms2 and prd1 in saturated dune sand under suboxic conditions. Water Res. 2018, 139, 158–167. [Google Scholar] [CrossRef]
- Stevenson, M.E.; Sommer, R.; Lindner, G.; Farnleitner, A.H.; Toze, S.; Kirschner, A.K.; Blaschke, A.P.; Sidhu, J.P. Attachment and detachment behavior of human adenovirus and surrogates in fine granular limestone aquifer material. J. Environ. Qual. 2015, 44, 1392–1401. [Google Scholar] [CrossRef]
- Catel-Ferreira, M.; Tnani, H.; Hellio, C.; Cosette, P.; Lebrun, L. Antiviral effects of polyphenols: Development of bio-based cleaning wipes and filters. J. Virol. Methods 2015, 212, 1–7. [Google Scholar] [CrossRef]
- Deboosere, N.; Pinon, A.; Caudrelier, Y.; Delobel, A.; Merle, G.; Perelle, S.; Temmam, S.; Loutreul, J.; Morin, T.; Estienney, M.; et al. Adhesion of human pathogenic enteric viruses and surrogate viruses to inert and vegetal food surfaces. Food Microbiol. 2012, 32, 48–56. [Google Scholar] [CrossRef]
- Grabow, W.O.K.; Coubrough, P.; Hilner, C.; Bateman, B.W. Inactivation of hepatitis a virus, other enteric viruses and indicator organisms in water by chlorination. Water Sci. Techol. 1985, 17, 657–664. [Google Scholar] [CrossRef]
- Harakeh, S. The Behavior of Viruses on Disinfection by Chlorine Dioxide and Other Disinfectants in Effluent; Elsevier: Amsterdam, The Netherlands, 1987; Volume 44, pp. 335–341. [Google Scholar]
- Pinto, F.; Maillard, J.-Y.; Denyer, S.P. Effect of surfactants, temperature, and sonication on the virucidal activity of polyhexamethylene biguanide against the bacteriophage ms2. Am. J. Infect. Control 2010, 38, 393–398. [Google Scholar] [CrossRef] [PubMed]
- Shin, G.-A.; Sobsey, M.D. Reduction of norwalk virus, poliovirus 1, and bacteriophage ms2 by ozone disinfection of water. Appl. Environ. Microbiol. 2003, 69, 3975–3978. [Google Scholar] [CrossRef]
- Taylor, G.R.; Butler, M. A comparison of the virucidal properties of chlorine, chlorine dioxide, bromine chloride and iodine. J. Hyg. Camb. 1982, 89, 321–328. [Google Scholar] [CrossRef]
- Harakeh, M.S. Inactivation of enteroviruses, rotaviruses and bacteriophages by peracetic acid in a municipal sewage effluent. FEMS Microbiol. Lett. 1984, 23, 27–30. [Google Scholar] [CrossRef][Green Version]
- Almeida, G.; Gibson, K.E. Evaluation of a recirculating dipper well combined with ozone sanitizer for control of foodborne pathogens in food service operations. J. Food Prot. 2016, 79, 1537–1548. [Google Scholar] [CrossRef]
- Emmoth, E.; Rovira, J.; Rajkovic, A.; Corcuera, E.; Wilches Perez, D.; Dergel, I.; Ottoson, J.R.; Widen, F. Inactivation of viruses and bacteriophages as models for swine hepatitis e virus in food matrices. Food Environ. Virol. 2017, 9, 20–34. [Google Scholar] [CrossRef]
- Gallandat, K.; Lantagne, D. Selection of a biosafety level 1 (bsl-1) surrogate to evaluate surface disinfection efficacy in ebola outbreaks: Comparison of four bacteriophages. PLoS ONE 2017, 12, e0177943. [Google Scholar] [CrossRef] [PubMed]
- Chandler-Bostock, R.; Mellits, K.H. Efficacy of disinfectants against porcine rotavirus in the presence and absence of organic matter. Lett. Appl. Microbiol. 2015, 61, 538–543. [Google Scholar] [CrossRef] [PubMed]
- Deveau, H.; Labrie, S.J.; Chopin, M.-C.; Moineau, S. Biodiversity and classification of lactococcal phages. Appl. Environ. Microbiol. 2006, 72, 4338–4346. [Google Scholar] [CrossRef] [PubMed]
- Capra, M.L.; Quiberoni, A.; Reinheimer, J.A. Thermal and chemical resistance of Lactobacillus casei and Lactobacillus paracasei bacteriophages. Lett. Appl. Microbiol. 2004, 38, 499–504. [Google Scholar] [CrossRef]
- Quiberoni, A.; Guglielmotti, D.M.; Reinheimer, J.A. Inactivation of Lactobacillus delbrueckii bacteriophages by heat and biocides. Int. J. Food Microbiol. 2003, 84, 51–62. [Google Scholar] [CrossRef]
- Murphy, J.; Mahony, J.; Bonestroo, M.; Nauta, A.; Van Sinderen, D.; Sinderen, V. Impact of Thermal and Biocidal Treatments on Lactococcal 936-Type Phages; Elsevier: Amsterdam, The Netherlands, 2013. [Google Scholar]
- Meyer, A.; Greene, M.; Kimmelshue, C.; Cademartiri, R. Stabilization of t4 bacteriophage at acidic and basic ph by adsorption on paper. Colloid Surf. B. 2017, 160, 169–176. [Google Scholar] [CrossRef]
- Ecolab-Inc. Regulatorische Anforderungen Testnormen und Listungen von Desinfektionsmitteln, Medizinprodukten und Kosmetika; Ecolab-Inc.: Saint Paul, MN, USA, 2017. [Google Scholar]
- Rossmanith, P.; Fister, S.; Wagner, M.; Schoder, D. Evaluation of an in-house laboratory for listeria self-monitoring of a dairy production plant. In Proceedings of the European Symposium on Food Safety, Des Moines, IA, USA, 7–9 May 2014; pp. 71–72. [Google Scholar]
- Mester, P.; Witte, A.K.; Robben, C.; Streit, E.; Fister, S.; Schoder, D.; Rossmanith, P. Optimization and evaluation of the qpcr-based pooling strategy dep-pooling in dairy production for the detection of Listeria monocytogenes. Food Control 2017, 82, 298–304. [Google Scholar] [CrossRef]
- Sybesma, W.; Rohde, C.; Bardy, P.; Pirnay, J.P.; Cooper, I.; Caplin, J.; Chanishvili, N.; Coffey, A.; De Vos, D.; Scholz, A.H.; et al. Silk route to the acceptance and re-implementation of bacteriophage therapy-part ii. Antibiotics 2018, 7, 35. [Google Scholar] [CrossRef]
- Abedon, S.T. Information phage therapy research should report. Pharmaceuticals 2017, 10, 43. [Google Scholar] [CrossRef] [PubMed]
| Pre-Harvest | Post-Harvest | |||||||
|---|---|---|---|---|---|---|---|---|
| Target Organisms | Phage Product | Taxonomy | References | Target Organisms | Phage Product | Taxonomy | Application | References |
| Escherichia coli O157:H7 | Ecolicide PX™ Finalyse® | Caudovirales | [112,113,114] | Listeria monocytogenes * | ListShield™ ListPhage™ PhageGuard Listex™ | Caudovirales: Myoviridae | (Pet) Food Safety | [90,115,116,117,118] |
| Salmonella | PLSV-1™ BAFASAL® | / | [119,120] | Escherichia coli O157:H7* | EcoShield™ Ecolicide® PhageGuard E™ Secure Shield E1 | Caudovirales: Myoviridae, Podoviridae | (Pet) Food Safety | [17,18,45,112,121] |
| Clostridium perfringens | INT-401™ | Caudovirales: Myoviridae, Siphoviridae | [102,119] | Salmonella * | SalmoFresh™ SalmoLyse® PhageGuard S™ SalmonelexTM SalmoPro®(2015) SalmoPro®(2018) Biotector® S1 Biotector® S4 | Caudovirales: Myoviridae, Podoviridae, Siphoviridae | (Pet) Food Safety | [22,23,85,121,122,123] |
| Vibrio parahemolyticus | Lexia | Caudovirales: Myoviridae | [124,125,126] | Shigella spp. | ShigaShield™ (ShigaActive™) | Caudovirales: Myoviridae, Siphoviridae | Food Safety | [121,127,128] |
| Xanthomonas campestris pv. vesicatoria and Pseudomonas syringe pv. tomato | Agriphage™ | Caudovirales: Myoviridae | [129,130,131,132] | Staphylococcus, Streptococcus, Escherichia coli, Pseudomonas Aeruginosa, Proteus | Pyo Bacteriophage | / | Pet Food Safety | [133,134] |
| Clavibacter michiganensis subsp. michiganensis | Agriphage™ CMM | Caudovirales: Mycobacteriophage | [135,136] | Shigella, Salmonella, Escherichia coli, Proteus, Staphylococcus, Pseudomonas, Enterococcus | Intesti Bacteriophage | / | Pet Food Safety | [133,137] |
| Erwinia amylovora | Agriphage™ FireBligth Erwiphage PLUS | Caudovirales: Siphoviridae | [138,139,140,141,142] | Staphylococcus, Streptococcus, Escherichia coli | SES Bacteriophage | / | Pet Food Safety | [133,143] |
| Xanthomonas citri subsp. citri | Agriphage™ CitrusCranker | Caudovirales | [112,138,144] | Salmonellae, Shigella, Escherichia coli, Staphylococcus | EnkoPhagum | / | Pet Food Safety | [133,145] |
| specific against soft rot Enterobacteriacea | Biolyse®-PB | Caudovirales: Myoviridae | [146,147] | Staphylococcus Streptococcus | Fersisi Bacteriophage | / | Pet Food Safety | [133,148] |
| Pseudomonas and Aeromonas | BAFADOR® | / | [9,149] | Staphylococcal, Escherichia coli, Streptococcal, Pseudomonas aeruginosa, Proteus | Mono-phage Preparations | / | Pet Food Safety | [133] |
| Substance Class | Substance | CAS reg. | Canada (HC) | U.S. (FDA) | EU (ECHA) | FAO/WHO | References |
|---|---|---|---|---|---|---|---|
| Aldehydes | Glutaraldehyde | 111-30-8 | Not approved | Food additive | Approved | Not approved | [170,176,179] |
| Chlorine/Chlorine releasing agents | Chlorine | 7782-50-5 | Approved | Approved | Not approved * | Approved | [180,181] |
| Chlorine dioxide | 10049-04-4 | Approved | Food additive | Under review * | Approved | [182,183] | |
| Sodium hypochlorite | 7681-52-9 | Approved | Food additive | Approved | Not approved | [169,182,184,185] | |
| Peroxides | Hydrogen peroxide | 7722-84-1 | Food additive | Food additive, GRAS | Approved | Approved | [12,41] |
| Peracetic acid | 79-21-0 | Food additive | Food additive | Approved | Approved | [169,184,185] | |
| Peroctanoic acid | 33734-57-5 | Not approved | Food additive | Approval in progress | Approved | [171,186] | |
| Alcohols | Ethanol | 64-17-5 | Food additive | Food additive, GRAS | Approval in progress | Approved | [169,184,185] |
| Isopropanol | 67-63-0 | Food additive | Food additive | Approved | Approved | [169,184,185] | |
| Acids | Trisodium phosphate | 7601-54-9 | Food additive | Food additive, GRAS | Not approved | Approved | [169,170,171] |
| Sulfuric acid | 7664-93-9 | Approved | Food additive, GRAS | Not approved; preregistered | Approved | [187] | |
| Sodium hypochlorite | 7681-52-9 | Food additive | Food additive | Approved | Not approved | [169,182,184,185] | |
| Different fatty acids | Various—see Supplement | Approved ° | Approved ° | Approved ° | Approved ° | [167,168,188] | |
| Bases | Sodium bicarbonate | 144-55-8 | Food additive | Food additive, GRAS | Not approved; preregistered | Approved | [189] |
| Sodium hydroxide | 1310-73-2 | Food additive | Food additive, GRAS | Not approved | Approved | [187] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sommer, J.; Trautner, C.; Witte, A.K.; Fister, S.; Schoder, D.; Rossmanith, P.; Mester, P.-J. Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses 2019, 11, 468. https://doi.org/10.3390/v11050468
Sommer J, Trautner C, Witte AK, Fister S, Schoder D, Rossmanith P, Mester P-J. Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses. 2019; 11(5):468. https://doi.org/10.3390/v11050468
Chicago/Turabian StyleSommer, Julia, Christoph Trautner, Anna Kristina Witte, Susanne Fister, Dagmar Schoder, Peter Rossmanith, and Patrick-Julian Mester. 2019. "Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments" Viruses 11, no. 5: 468. https://doi.org/10.3390/v11050468
APA StyleSommer, J., Trautner, C., Witte, A. K., Fister, S., Schoder, D., Rossmanith, P., & Mester, P.-J. (2019). Don’t Shut the Stable Door after the Phage Has Bolted—The Importance of Bacteriophage Inactivation in Food Environments. Viruses, 11(5), 468. https://doi.org/10.3390/v11050468





