A Single-Cycle Adenovirus Type 7 Vaccine for Prevention of Acute Respiratory Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Ad7 Virus and DNA Purification
2.3. Development of an Ad7 Fiber Expressing Cell Line
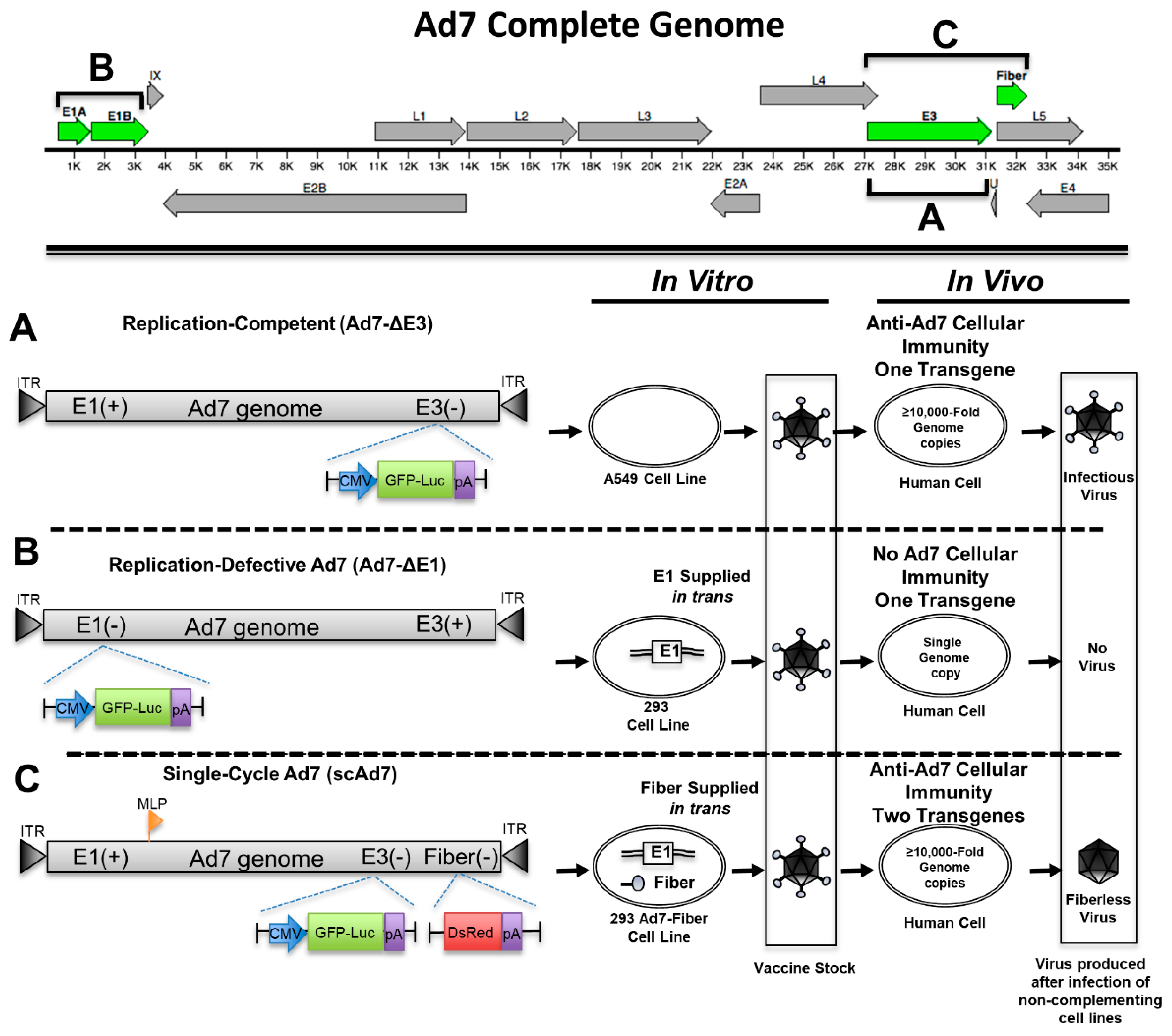
2.4. Recombinant Ad7 Plasmid Construction
2.5. Recombinant Ad7 Rescue and Purification
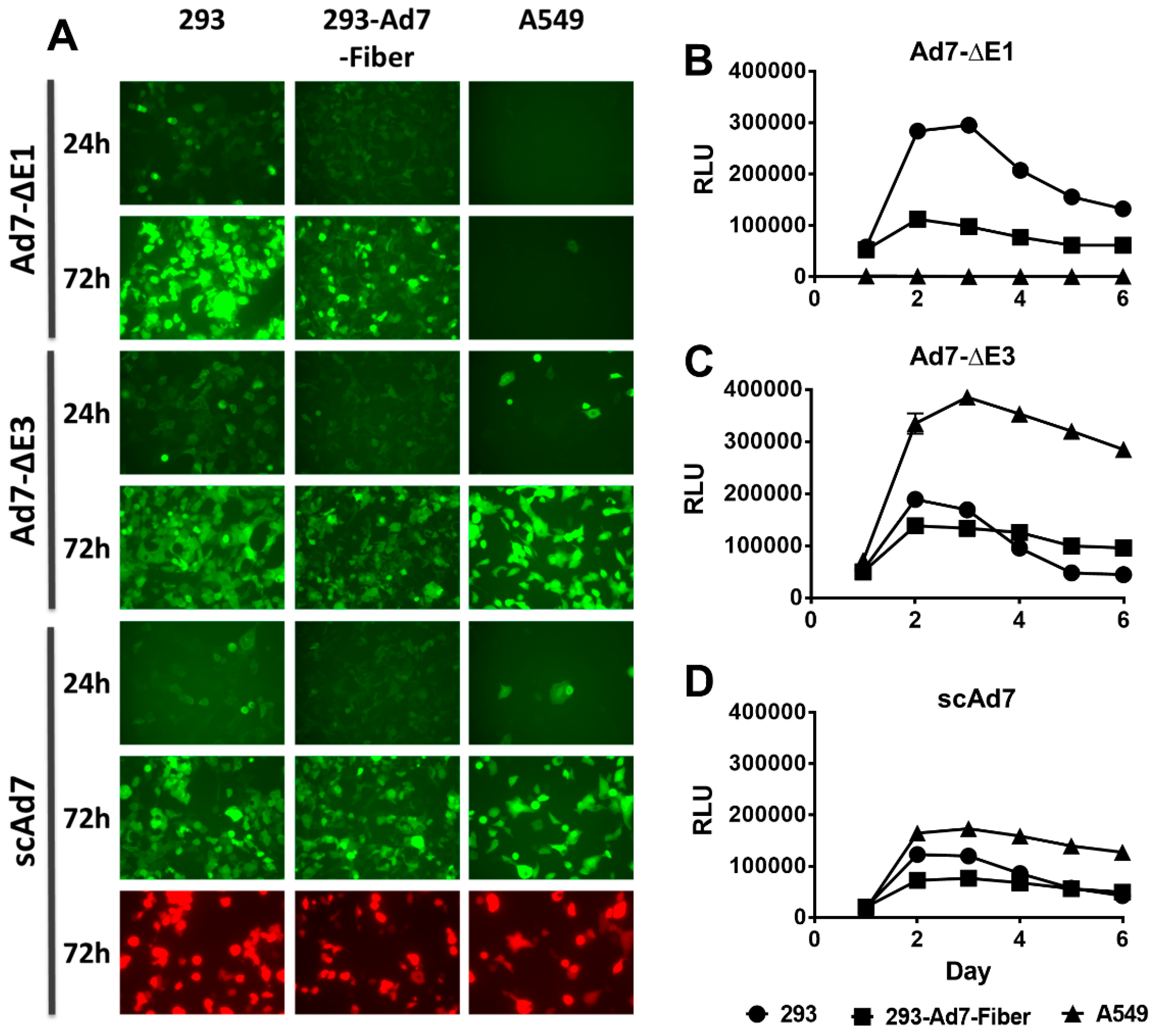
2.6. In Vitro Reporter Gene Expression and Luciferase Assay
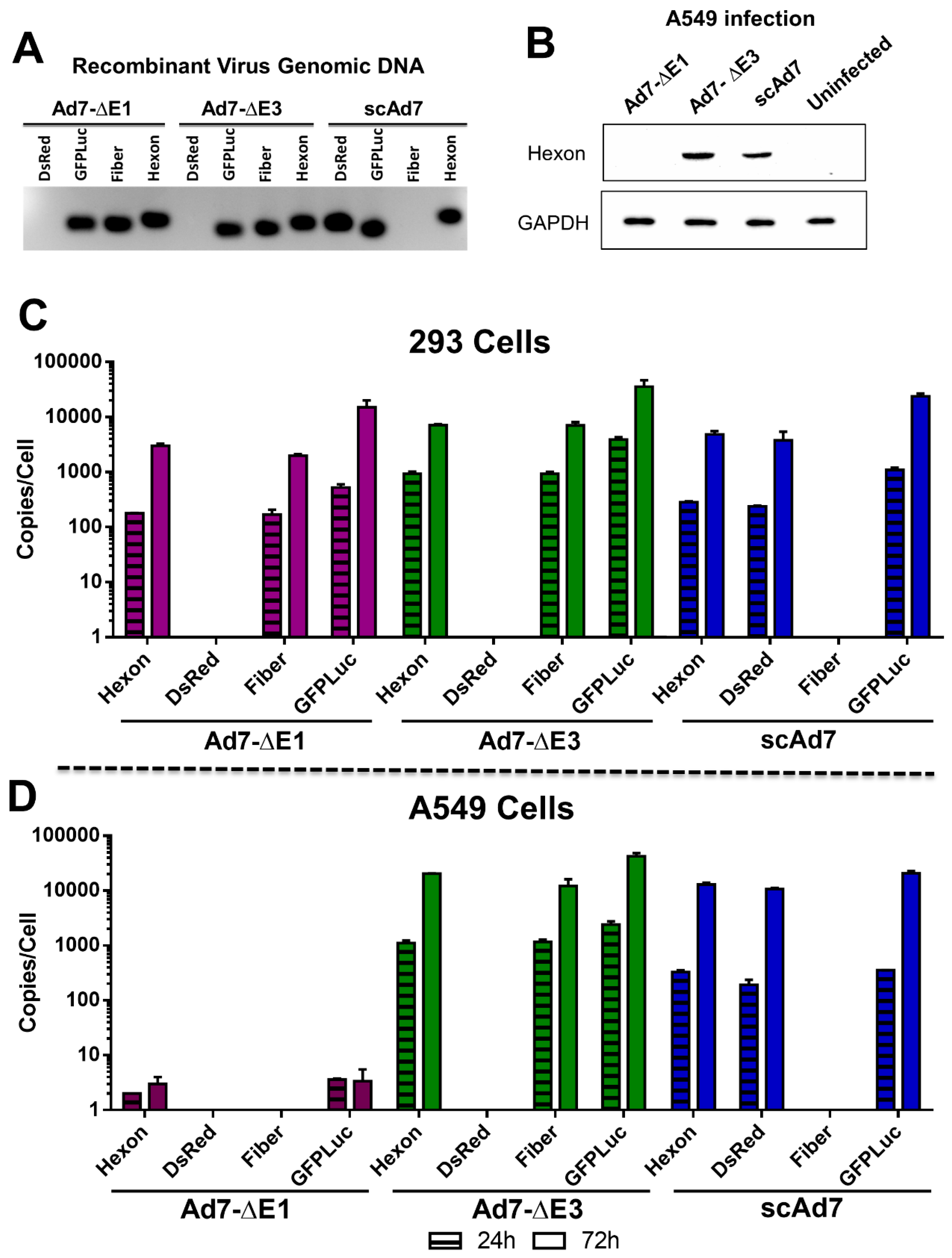
2.7. In Vitro qPCR of Viruses
2.8. Western Blot
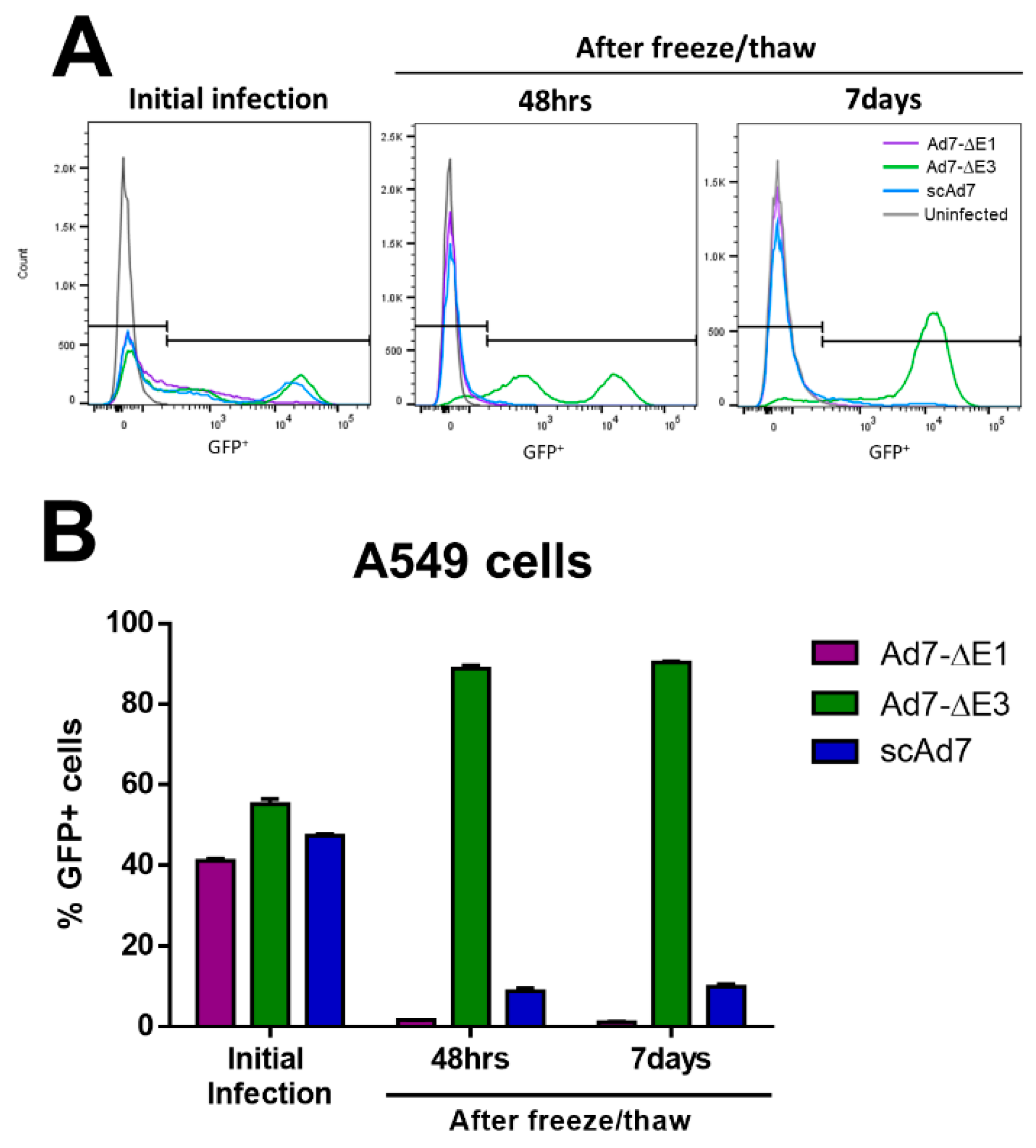
2.9. Virus Progeny Assay
2.10. Anti-Ad7 Neutralization Titer
2.11. Elipsot
3. Results
3.1. Development of a Single-Cycle Ad7 Virus
3.2. In Vitro GFP Expression and Luciferase Activity
3.3. Ad7 Hexon Expression and gDNA Replication In Vitro
3.4. scAd7 Virus Infection Produces Virus Progeny with Impaired Infectivity
3.5. In Vivo Immunogenicity of the Ad7 Vaccine Constructs
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lynch, J.P.; Kajon, A.E. Adenovirus: Epidemiology, global spread of novel serotypes, and advances in treatment and prevention. Semin. Respir. Crit Care Med. 2016, 37, 586–602. [Google Scholar]
- Lynch, J.P.; Fishbein, M.; Echavarria, M. Adenovirus. Semin. Respir. Crit. Care Med. 2011, 32, 494–511. [Google Scholar] [CrossRef]
- Hoke, C.H., Jr.; Snyder, C.E., Jr. History of the restoration of adenovirus type 4 and type 7 vaccine, live oral (adenovirus vaccine) in the context of the department of defense acquisition system. Vaccine 2013, 31, 1623–1632. [Google Scholar] [CrossRef]
- Hyer, R.N.; Howell, M.R.; Ryan, M.A.K.; Gaydos, J.C. Cost-effectiveness analysis of reacquiring and using adenovirus types 4 and 7 vaccines in naval recruits. Am. J. Trop. Med. Hyg. 2000, 62, 613–618. [Google Scholar] [CrossRef]
- Lyons, A.; Longfield, J.; Kuschner, R.; Straight, T.; Binn, L.; Seriwatana, J.; Reitstetter, R.; Froh, I.B.; Craft, D.; McNabb, K.; et al. A double-blind, placebo-controlled study of the safety and immunogenicity of live, oral type 4 and type 7 adenovirus vaccines in adults. Vaccine 2008, 26, 2890–2898. [Google Scholar] [CrossRef]
- Adenovirus Type 4 and Type 7 Vaccine, Live, Oral Enteric Coated Tablets for Oral Administration [package insert]. Available online: https://www.fda.gov/media/80211/download (accessed on 2 April 2019).
- NJDOH Confirms 11th Pediatric Death of Wanaque Patient. Available online: https://nj.gov/health/news/2018/approved/20181116a.shtml (accessed on 2 April 2019).
- Adenovirus: Director’s Letter to Campus. Available online: https://health.umd.edu/adenovirus-directors-letter-campus (accessed on 2 April 2019).
- Zhao, S.; Wan, C.; Ke, C. Re-emergent human adenovirus genome type 7d caused an acute respiratory disease outbreak in southern china after a twenty-one year absence. Sci Rep. 2014, 4, 7365. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, D.; Okamoto, M.; Lupisan, S. Impact of human adenovirus serotype 7 in hospitalized children with severe fatal pneumonia in the philippines. Jpn. J. Infect. Dis 2014, 67, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Jeon, K.; Kang, C.I.; Yoon, C.H. High isolation rate of adenovirus serotype 7 from south korean military recruits with mild acute respiratory disease. Eur. J. Clin. Microbiol. Infect. Dis. 2007, 26, 481–483. [Google Scholar] [CrossRef] [PubMed]
- Noda, M.; Yoshida, T.; Sakaguchi, T.; Ikeda, Y.; Yamaoka, K.; Ogino, T. Molecular and epidemiological analyses of human adenovirus type 7 strains isolated from the 1995 nationwide outbreak in japan. J. Clin. Microbiol. 2002, 40, 140–145. [Google Scholar] [CrossRef]
- Hong, J.-Y.; Lee, H.-J.; Piedra, P.A.; Choi, E.-H.; Park, K.-H.; Koh, Y.-Y.; Kim, W.-S. Lower respiratory tract infections due to adenovirus in hospitalized korean children: Epidemiology, clinical features, and prognosis. Clin. Infect. Dis. 2001, 32, 1423–1429. [Google Scholar] [CrossRef]
- Weaver, E.A.; Nehete, P.N.; Buchl, S.S.; Senac, J.S.; Palmer, D.; Ng, P.; Sastry, K.J.; Barry, M.A. Comparison of replication-competent, first generation, and helper-dependent adenoviral vaccines. PLoS ONE 2009, 4, e5059. [Google Scholar] [CrossRef]
- Legrand, V.; Spehner, D.; Schlesinger, Y.; Settelen, N.; Pavirani, A.; Mehtali, M. Fiberless recombinant adenoviruses: Virus maturation and infectivity in the absence of fiber. J. Virol. 1999, 73, 907–919. [Google Scholar]
- Von Seggern, D.J.; Chiu, C.Y.; Fleck, S.K.; Stewart, P.L.; Nemerow, G.R. A helper-independent adenovirus vector with e1, e3, and fiber deleted: Structure and infectivity of fiberless particles. J. Virol. 1999, 73, 1601–1608. [Google Scholar]
- Wang, H.; Beyer, I.; Persson, J.; Song, H.; Li, Z.; Richter, M.; Cao, H.; van Rensburg, R.; Yao, X.; Hudkins, K.; et al. A new human dsg2-transgenic mouse model for studying the tropism and pathology of human adenoviruses. J. Virol. 2012, 86, 6286–6302. [Google Scholar] [CrossRef] [PubMed]
- Berge, T.O.; England, B.; Mauris, C.; Shuey, H.E.; Lennette, E.H. Etiology of acute respiratory disease among service personnel at fort ord, california. Am. J. Hyg. 1955, 62, 283–294. [Google Scholar] [PubMed]
- Von Seggern, D.J.; Huang, S.; Fleck, S.K.; Stevenson, S.C.; Nemerow, G.R. Adenovirus vector pseudotyping in fiber-expressing cell lines: Improved transduction of epstein-barr virus-transformed b cells. J. Virol. 2000, 74, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Camacho, Z.T.; Turner, M.A.; Barry, M.A.; Weaver, E.A. Cd46-mediated transduction of a species d adenovirus vaccine improves mucosal vaccine efficacy. Hum. Gene Ther. 2014, 25, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Weaver, E.A.; Rubrum, A.M.; Webby, R.J.; Barry, M.A. Protection against divergent influenza h1n1 virus by a centralized influenza hemagglutinin. PLoS ONE 2011, 6, e18314. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, Z.; Yu, R.; Zhang, J.; Liu, Y.; Song, X.; Yi, S.; Liu, J.; Chen, J.; Yin, Y.; et al. Intramuscular delivery of adenovirus serotype 5 vector expressing humanized protective antigen induces rapid protection against anthrax that may bypass intranasally originated preexisting adenovirus immunity. Clin. Vaccine Immunol. CVI 2014, 21, 156–164. [Google Scholar] [CrossRef]
- Wang, H.; Li, Z.-Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.-B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2010, 17, 96–104. [Google Scholar] [CrossRef]
- Jogler, C.; Hoffmann, D.; Theegarten, D.; Grunwald, T.; Uberla, K.; Wildner, O. Replication properties of human adenovirus in vivo and in cultures of primary cells from different animal species. J. Virol. 2006, 80, 3549–3558. [Google Scholar] [CrossRef] [PubMed]
- Havenga, M.; Vogels, R.; Zuijdgeest, D.; Radosevic, K.; Mueller, S.; Sieuwerts, M.; Weichold, F.; Damen, I.; Kaspers, J.; Lemckert, A.; et al. Novel replication-incompetent adenoviral b-group vectors: High vector stability and yield in per.C6 cells. J. Gen. Virol. 2006, 87, 2135–2143. [Google Scholar] [CrossRef] [PubMed]
- Saha, B.; Parks, R.J. Human adenovirus type 5 vectors deleted of early region 1 (e1) undergo limited expression of early replicative e2 proteins and DNA replication in non-permissive cells. PLoS ONE 2017, 12, e0181012. [Google Scholar] [CrossRef]
- Crosby, C.M.; Matchett, W.E.; Anguiano-Zarate, S.S.; Parks, C.A.; Weaver, E.A.; Pease, L.R.; Webby, R.J.; Barry, M.A. Replicating single-cycle adenovirus vectors generate amplified influenza vaccine responses. J. Virol. 2017, 91. [Google Scholar] [CrossRef]
- Anguiano-Zarate, S.S.; Matchett, W.E.; Nehete, P.; Sastry, K.J.; Marzi, A.; Barry, M.A. A replicating single-cycle adenovirus vaccine against ebola virus. J. Infect. Dis. 2018, 218, 1883–1889. [Google Scholar] [CrossRef] [PubMed]

| A. Primers for Ad7 Fiber Deletion/Gene Replacement Shuttle Plasmids. | |||
| Ad7-Fib-Ins-F | GTTTAAACGAACGCGTGACCAAAGAGCTCAGAG | ||
| Ad7-Fib-Ins-OL-R | AATAAACAAGTTAAACTTTATTTTGTGGCGCGCCCTGGGAAGAAAGACATGAAGATTGTG | ||
| Ad7-Fib-Ins-OL-F | CACAATCTTCATGTCTTTCTTCCCAGGGCGCGCCACAAAATAAAGTTTAACTTGTTTATT | ||
| Ad7-Fib-Ins-R | GTTTAAACTAATCTAAGTGAAATCAGAATGCGT | ||
| B. Primers for DsRed. | |||
| AscI-DsRed-F | GGCGCGCCATGGCCTCCTCCGAGAACGTCATCA | ||
| DsRed-LBL-R | AAGGGCGAATTCGGAGCCTGCTTTTTTCTACAGGAACAGGTGGTGGCGGCCCTCGGTG | ||
| DsRed-LBL-F | CACCGAGGGCCGCCACCACCTGTTCCTGTAGAAAAAAGCAGGCTCCGAATTCGCCCTT | ||
| AscI-LBL-R | GGCGCGCCCAAGAAAGCTGGGTCGAATTCGCCC | ||
| C. qPCR Primers | |||
| DsRed-F | CACTACCTGGTGGAGTTCAAG | DsRed-R | GATGGTGTAGTCCTCGTTGTG |
| Ad7 Fiber-F | GATTCCTTCAACCCTGTCTACC | Ad7 Fiber-R | CCGTCTGGGCTTTGTGTAA |
| Ad7 Hexon-F | GGAACCTTACCCAGCCAATTA | Ad7 Hexon-R | AGTTGCTGGAGAAGGGAATG |
| ACTB gDNA-F | GGCCTTGGAGTGTGTATTAAGT | ACTB gDNA-R | GGACATGCAGAAAGTGCAAAG |
| eGFPLuc-F | CGGAAAGACGATGACGGAAA | eGFPLuc-R | CGGTACTTCGTCCACAAACA |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bullard, B.L.; Corder, B.N.; Weaver, E.A. A Single-Cycle Adenovirus Type 7 Vaccine for Prevention of Acute Respiratory Disease. Viruses 2019, 11, 413. https://doi.org/10.3390/v11050413
Bullard BL, Corder BN, Weaver EA. A Single-Cycle Adenovirus Type 7 Vaccine for Prevention of Acute Respiratory Disease. Viruses. 2019; 11(5):413. https://doi.org/10.3390/v11050413
Chicago/Turabian StyleBullard, Brianna L., Brigette N. Corder, and Eric A. Weaver. 2019. "A Single-Cycle Adenovirus Type 7 Vaccine for Prevention of Acute Respiratory Disease" Viruses 11, no. 5: 413. https://doi.org/10.3390/v11050413
APA StyleBullard, B. L., Corder, B. N., & Weaver, E. A. (2019). A Single-Cycle Adenovirus Type 7 Vaccine for Prevention of Acute Respiratory Disease. Viruses, 11(5), 413. https://doi.org/10.3390/v11050413






