Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance
Abstract
1. Introduction
2. How to Differentiate Mechanical from Biological Vectors?
3. Culicoides Biting Midges: Classification, Morphological Characteristics, and Distribution
4. Public Health and Veterinary Impact of Culicoides Biting Midges
5. The Genus Orthobunyavirus
5.1. The Simbu Serogroup
5.1.1. AKAV
5.1.2. SBV
5.1.3. SHUV
5.1.4. OROV
5.1.5. Further Members of the Simbu Serogroup
5.2. Further Orthobunyaviruses of Public Health Importance
6. Responses to Culicoides-borne Arbovirus Incursions
7. Concluding Remarks
Author Contributions
Funding
Conflicts of Interest
References
- Baud, D.; Gubler, D.J.; Schaub, B.; Lanteri, M.C.; Musso, D. An update on Zika virus infection. Lancet 2017, 390, 2099–2109. [Google Scholar] [CrossRef]
- Petersen, L.R.; Brault, A.C.; Nasci, R.S. West Nile virus: Review of the literature. JAMA 2013, 310, 308–315. [Google Scholar] [CrossRef]
- Young, P.R. Arboviruses: A family on the move. Adv. Exp. Med. Biol. 2018, 1062, 1–10. [Google Scholar] [PubMed]
- Althouse, B.M.; Hanley, K.A. The tortoise or the hare? Impacts of within-host dynamics on transmission success of arthropod-borne viruses. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2015, 370, 20140299. [Google Scholar] [CrossRef] [PubMed]
- Medlock, J.M.; Leach, S.A. Effect of climate change on vector-borne disease risk in the UK. Lancet Infect. Dis. 2015, 15, 721–730. [Google Scholar] [CrossRef]
- Hubálek, Z.; Rudolf, I.; Nowotny, N. Arboviruses pathogenic for domestic and wild animals. Adv. Virus Res. 2014, 89, 201–275. [Google Scholar]
- ICTV. Virus Taxonomy: The Classification and Nomenclature of Viruses. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/ (accessed on 25 March 2019).
- Beerntsen, B.T.; James, A.A.; Christensen, B.M. Genetics of mosquito vector competence. Microbiol. Mol. Biol. Rev. 2000, 64, 115–137. [Google Scholar] [CrossRef] [PubMed]
- Möhlmann, T.W.R.; Oymans, J.; Wichgers Schreur, P.J.; Koenraadt, C.J.M.; Kortekaas, J.; Vogels, C.B.F. Vector competence of biting midges and mosquitoes for Shuni virus. PLoS Negl. Trop. Dis. 2018, 12, e0006993. [Google Scholar] [CrossRef]
- Borkent, A. Numbers of Extant and Fossil Species of Ceratopogonidae. 6 July 2016. Available online: https://www.inhs.illinois.edu/files/4014/6785/5847/WorldCatalogtaxa.pdf (accessed on 25 March 2019).
- Mellor, P.S.; Boorman, J.; Baylis, M. Culicoides biting midges: Their role as arbovirus vectors. Annu. Rev. Entomol. 2000, 45, 307–340. [Google Scholar] [CrossRef]
- Mathieu, B.; Cêtre-Sossah, C.; Garros, C.; Chavernac, D.; Balenghien, T.; Carpenter, S.; Setier-Rio, M.L.; Vignes-Lebbe, R.; Ung, V.; Candolfi, E.; et al. Development and validation of IIKC: An interactive identification key for Culicoides (Diptera: Ceratopogonidae) females from the western Palaearctic region. Parasit. Vectors 2012, 5, 137. [Google Scholar] [CrossRef]
- Pages, N.; Sarto, I.M.V. Differentiation of Culicoides obsoletus and Culicoides scoticus (Diptera: Ceratopogonidae) based on mitochondrial cytochrome oxidase subunit I. J. Med. Entomol. 2005, 42, 1026–1034. [Google Scholar] [CrossRef]
- Dallas, J.F.; Cruickshank, R.H.; Linton, Y.M.; Nolan, D.V.; Patakakis, M.; Braverman, Y.; Capela, R.; Capela, M.; Pena, I.; Meiswinkel, R.; et al. Phylogenetic status and matrilineal structure of the biting midge, Culicoides imicola, in Portugal, Rhodes and Israel. Med. Vet. Entomol. 2003, 17, 379–387. [Google Scholar] [CrossRef]
- Cêtre-Sossah, C.; Mathieu, B.; Setier-Rio, M.L.; Grillet, C.; Baldet, T.; Delécolle, J.C.; Albina, E. Development and evaluation of a real-time quantitative PCR assay for Culicoides imicola, one of the main vectors of bluetongue (BT) and African horse sickness (AHS) in Africa and Europe. Res. Vet. Sci. 2008, 85, 372–382. [Google Scholar] [CrossRef][Green Version]
- Wenk, C.E.; Kaufmann, C.; Schaffner, F.; Mathis, A. Molecular characterization of Swiss Ceratopogonidae (Diptera) and evaluation of real-time PCR assays for the identification of Culicoides biting midges. Vet. Parasitol. 2012, 184, 258–266. [Google Scholar] [CrossRef]
- Deblauwe, I.; de Witte, J.C.; de Deken, G.; de Deken, R.; Madder, M.; van Erk, S.; Hoza, F.A.; Lathouwers, D.; Geysen, D. A new tool for the molecular identification of Culicoides species of the Obsoletus group: The glass slide microarray approach. Med. Vet. Entomol. 2012, 26, 83–91. [Google Scholar] [CrossRef]
- Uhlmann, K.R.; Gibb, S.; Kalkhof, S.; Arroyo-Abad, U.; Schulz, C.; Hoffmann, B.; Stubbins, F.; Carpenter, S.; Beer, M.; von Bergen, M.; et al. Species determination of Culicoides biting midges via peptide profiling using matrix-assisted laser desorption ionization mass spectrometry. Parasit. Vectors 2014, 7, 392. [Google Scholar] [CrossRef]
- Wirth, W.W.; Hubert, A.A. The Culicoides of Southeast Asia (Diptera: Ceratopogonidae). Am. Entomol. Inst. (USA) 1989, 44, 514. [Google Scholar]
- Kline, D.L.; Axtell, R.C. Salt-marsh Culicoides (Diptera—Ceratopogonidae)—Species, seasonal abundance and comparisons of trapping methods. Mosq. News 1976, 36, 1–10. [Google Scholar]
- Van der Rijt, R.; van den Boom, R.; Jongema, Y.; van Oldruitenborgh-Oosterbaan, M.M. Culicoides species attracted to horses with and without insect hypersensitivity. Vet. J. 2008, 178, 91–97. [Google Scholar] [CrossRef]
- Carpenter, S.; Groschup, M.H.; Garros, C.; Felippe-Bauer, M.L.; Purse, B.V. Culicoides biting midges, arboviruses and public health in Europe. Antiviral Res. 2013, 100, 102–113. [Google Scholar] [CrossRef]
- Veiga, J.; Martinez-de la Puente, J.; Vaclav, R.; Figuerola, J.; Valera, F. Culicoides paolae and C. circumscriptus as potential vectors of avian haemosporidians in an arid ecosystem. Parasit. Vectors 2018, 11, 524. [Google Scholar] [CrossRef]
- Chagas, C.R.F.; Bukauskaite, D.; Ilgunas, M.; Iezhova, T.; Valkiunas, G. A new blood parasite of leaf warblers: Molecular characterization, phylogenetic relationships, description and identification of vectors. Parasit. Vectors 2018, 11, 538. [Google Scholar] [CrossRef]
- Yates, J.A.; Lowrie, R.C., Jr.; Eberhard, M. Development of Tetrapetalonema llewellyni to the infective stage in Culicoides hollensis. J. Parasitol. 1982, 68, 293–296. [Google Scholar] [CrossRef]
- Lowrie, R.C., Jr.; Eberhard, M.L.; Orihel, T.C. Development of Tetrapetalonema marmosetae to the infective stage in Culicoides hollensis and C. furens. J. Parasitol. 1978, 64, 1003–1007. [Google Scholar] [CrossRef]
- Linley, J.R. Biting midges (Diptera: Ceratopogonidae) as vectors of nonviral animal pathogens. J. Med. Entomol. 1985, 22, 589–599. [Google Scholar] [CrossRef]
- Meiswinkel, R.; Nevill, E.M.; Venter, G.J. Vectors: Culicoides spp. In Infectious Diseases of Livestock with Special Reference to Southern Africa; Coetzer, J.A.W., Thomson, G.R., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 1994; Volume 1, pp. 68–89. [Google Scholar]
- Carpenter, S.; Veronesi, E.; Mullens, B.; Venter, G. Vector competence of Culicoides for arboviruses: Three major periods of research, their influence on current studies and future directions. Rev. Sci. Tech. 2015, 34, 97–112. [Google Scholar] [CrossRef]
- Jennings, M.; Platt, G.S.; Bowen, E.T. The susceptibility of Culicoides variipennis Coq. (Diptera: Ceratopogonidae) to laboratory infection with Rift Valley fever virus. Trans. R. Soc. Trop. Med. Hyg. 1982, 76, 587–589. [Google Scholar] [CrossRef]
- Carpenter, S.; Szmaragd, C.; Barber, J.; Labuschagne, K.; Gubbins, S.; Mellor, P. An assessment of Culicoides surveillance techniques in northern Europe: Have we underestimated a potential bluetongue virus vector? J. Appl. Ecol. 2008, 45, 1237–1245. [Google Scholar]
- Murray, M.D. Local dispersal of the biting midge Culicoides brevitarsis Kieffer (Diptera, Ceratopogonidae) in Southeastern Australia. Austr. J. Zool. 1987, 35, 559–573. [Google Scholar] [CrossRef]
- Murray, M.D.; Nix, H.A. Southern limits of distribution and abundance of the biting-midge Culicoides brevitarsis Kieffer (Diptera, Ceratopogonidae) in Southeastern Australia—An application of the Growest Model. Austr. J. Zool. 1987, 35, 575–585. [Google Scholar] [CrossRef]
- Kirkland, P.D. Akabane virus infection. Rev. Sci. Tech. 2015, 34, 403–410. [Google Scholar] [CrossRef]
- Sellers, R.F. Weather, host and vector—Their interplay in the spread of insect-borne animal virus diseases. J. Hyg. (London) 1980, 85, 65–102. [Google Scholar] [CrossRef]
- Sellers, R.F.; Maarouf, A.R. Possible introduction of epizootic hemorrhagic disease of deer virus (serotype 2) and bluetongue virus (serotype 11) into British Columbia in 1987 and 1988 by infected Culicoides carried on the wind. Can. J. Vet. Res. 1991, 55, 367–370. [Google Scholar]
- Sellers, R.F.; Pedgley, D.E. Possible windborne spread to western Turkey of bluetongue virus in 1977 and of Akabane virus in 1979. J. Hyg. (London) 1985, 95, 149–158. [Google Scholar] [CrossRef]
- Braverman, Y.; Chechik, F. Air streams and the introduction of animal diseases borne on Culicoides (Diptera, Ceratopogonidae) into Israel. Rev. Sci. Tech. 1996, 15, 1037–1052. [Google Scholar] [CrossRef]
- Hunt, G.J. A Procedural Manual for the Large-Scale Rearing of the Biting Midge, Culicoides variipennis (Diptera: Ceratopogonidae); U.S. Department of Agriculture, National Agricultural Library (USA): Beltsville, MD, USA, 1994.
- Carpenter, S.; Mordue, A.J.; Mordue, W. Oviposition in Culicoides impunctatus under laboratory conditions. Entomol. Exp. Appl. 2001, 101, 123–129. [Google Scholar] [CrossRef]
- Jones, R.H.; Foster, N.M. Relevance of laboratory colonies of the vector in arbovirus research—Culicoides variipennis and bluetongue. Am. J. Trop. Med. Hyg. 1978, 27, 168–177. [Google Scholar] [CrossRef]
- Maes, P.; Alkhovsky, S.V.; Bao, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: update 2018. Arch. Virol. 2018, 163, 2295–2310. [Google Scholar] [CrossRef]
- Barr, J.N.; Walter, C.T. Recent advances in the molecular and cellular biology of bunyaviruses. J. Gen. Virol. 2011, 92, 2467–2484. [Google Scholar]
- Shi, X.; Lappin, D.F.; Elliott, R.M. Mapping the Golgi targeting and retention signal of Bunyamwera virus glycoproteins. J. Virol. 2004, 78, 10793–10802. [Google Scholar] [CrossRef]
- Shi, X.; van Mierlo, J.T.; French, A.; Elliott, R.M. Visualizing the replication cycle of Bunyamwera orthobunyavirus expressing fluorescent protein-tagged Gc glycoprotein. J. Virol. 2010, 84, 8460–8469. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Goli, J.; Clark, G.; Brauburger, K.; Elliott, R.M. Functional analysis of the Bunyamwera orthobunyavirus Gc glycoprotein. J. Gen. Virol. 2009, 90, 2483–2492. [Google Scholar] [CrossRef]
- Elliott, R.M. Orthobunyaviruses: Recent genetic and structural insights. Nat. Rev. Microbiol. 2014, 12, 673–685. [Google Scholar] [CrossRef] [PubMed]
- Elliott, R.M.; Blakqori, G. Molecular biology of orthobunyaviruses. In Bunyaviridae. Molecular and Cellular Biology; Plyusnin, A., Elliott, R.M., Eds.; Caister Academic Press: Norfolk, UK, 2011; pp. 1–39. [Google Scholar]
- Shi, X.; Kohl, A.; Léonard, V.H.J.; Li, P.; McLees, A.; Elliott, R.M. Requirement of the N-terminal region of Orthobunyavirus nonstructural protein NSm for virus assembly and morphogenesis. J. Virol. 2006, 80, 8089. [Google Scholar] [CrossRef]
- Wernike, K.; Beer, M. Schmallenberg virus: A novel virus of veterinary importance. Adv. Virus Res. 2017, 99, 39–60. [Google Scholar] [PubMed]
- Kato, T.; Shirafuji, H.; Tanaka, S.; Sato, M.; Yamakawa, M.; Tsuda, T.; Yanase, T. Bovine arboviruses in Culicoides biting midges and sentinel cattle in southern Japan from 2003 to 2013. Transbound. Emerg. Dis. 2016, 63, e160–e172. [Google Scholar] [CrossRef]
- Kato, T.; Yanase, T.; Suzuki, M.; Katagiri, Y.; Ikemiyagi, K.; Takayoshi, K.; Shirafuji, H.; Ohashi, S.; Yoshida, K.; Yamakawa, M.; et al. Monitoring for bovine arboviruses in the most southwestern islands in Japan between 1994 and 2014. BMC Vet. Res. 2016, 12, 125. [Google Scholar] [CrossRef]
- Hayama, Y.; Yanase, T.; Suzuki, M.; Unten, K.; Tomochi, H.; Kakehi, M.; Shono, Y.; Yamamoto, T.; Kobayashi, S.; Murai, K.; et al. Meteorological factors affecting seroconversion of Akabane disease in sentinel calves in the subtropical Okinawa Islands of Japan. Trop. Anim. Health Prod. 2018, 50, 209–215. [Google Scholar] [CrossRef]
- Wernike, K.; Holsteg, M.; Szillat, K.P.; Beer, M. Development of within-herd immunity and long-term persistence of antibodies against Schmallenberg virus in naturally infected cattle. BMC Vet. Res. 2018, 14, 368. [Google Scholar] [CrossRef]
- Geoghegan, J.L.; Walker, P.J.; Duchemin, J.B.; Jeanne, I.; Holmes, E.C. Seasonal drivers of the epidemiology of arthropod-borne viruses in Australia. PLoS Negl. Trop. Dis. 2014, 8, e3325. [Google Scholar] [CrossRef]
- Wernike, K.; Elbers, A.; Beer, M. Schmallenberg virus infection. Rev. Sci. Tech. 2015, 34, 363–373. [Google Scholar] [CrossRef]
- Saeed, M.F.; Li, L.; Wang, H.; Weaver, S.C.; Barrett, A.D. Phylogeny of the Simbu serogroup of the genus Bunyavirus. J. Gen. Virol. 2001, 82, 2173–2181. [Google Scholar] [CrossRef]
- Oya, A.; Okuno, T.; Ogata, T.; Kobayashii; Matsuyama, T. Akabane, a new arbovirus isolated in Japan. Jpn. J. Med. Sci. Biol. 1961, 14, 101–108. [Google Scholar] [CrossRef]
- Cybinski, D.H. Douglas and Tinaroo viruses: Two Simbu group arboviruses infecting Culicoides brevitarsis and livestock in Australia. Aust. J. Biol. Sci. 1984, 37, 91–97. [Google Scholar] [CrossRef]
- Causey, O.R.; Kemp, G.E.; Causey, C.E.; Lee, V.H. Isolations of Simbu-group viruses in Ibadan, Nigeria 1964-69, including the new types Sango, Shamonda, Sabo and Shuni. Ann. Trop. Med. Parasitol. 1972, 66, 357–362. [Google Scholar] [CrossRef]
- Takahashi, K.; Oya, A.; Okazda, T.; Matsuo, R.; Kuma, M. Aino virus, a new member of Simbu group of arbovirus from mosquitoes in Japan. Jpn. J. Med. Sci. Biol. 1968, 21, 95–101. [Google Scholar] [CrossRef]
- Reeves, W.C.; Scrivani, R.P.; Hardy, J.L.; Roberts, D.R.; Nelson, R.L. Buttonwillow virus, a new Arbovirus isolated from mammals and Culicoides midges in Kern County, California. Am. J. Trop. Med. Hyg. 1970, 19, 544–551. [Google Scholar] [CrossRef]
- Bryant, J.E.; Crabtree, M.B.; Nam, V.S.; Yen, N.T.; Duc, H.M.; Miller, B.R. Isolation of arboviruses from mosquitoes collected in northern Vietnam. Am. J. Trop. Med. Hyg. 2005, 73, 470–473. [Google Scholar] [CrossRef]
- Kono, Y.; Yusnita, Y.; Mohd Ali, A.R.; Maizan, M.; Sharifah, S.H.; Fauzia, O.; Kubo, M.; Aziz, A.J. Characterization and identification of Oya virus, a Simbu serogroup virus of the genus Bunyavirus, isolated from a pig suspected of Nipah virus infection. Arch. Virol. 2002, 147, 1623–1630. [Google Scholar] [CrossRef]
- Doherty, R.L.; Carley, J.G.; Kay, B.H.; Filippich, C.; Marks, E.N.; Frazier, C.L. Isolation of virus strains from mosquitoes collected in Queensland, 1972–1976. Aust. J. Exp. Biol. Med. Sci. 1979, 57, 509–520. [Google Scholar] [CrossRef]
- McIntosh, B.M.; McGillivray, G.M.; Dickinson, D.B. Ingwavuma virus: An arbovirus isolated in South Africa. S. Afr. J. Med. Sci. 1965, 30, 67–70. [Google Scholar]
- Figueiredo, L.T.; Da Rosa, A.P. Jatobal virus antigenic characterization by ELISA and neutralization test using EIA as indicator, on tissue culture. Mem. Inst. Oswaldo Cruz 1988, 83, 161–164. [Google Scholar] [CrossRef][Green Version]
- Doherty, R.L.; Carley, J.G.; Filippich, C.; Kay, B.H.; Gorman, B.M.; Rajapaksa, N. Isolation of Sindbis (alphavirus) and Leanyer viruses from mosquitoes collected in the Northern Territory of Australia, 1974. Aust. J. Exp. Biol. Med. Sci. 1977, 55, 485–489. [Google Scholar] [CrossRef]
- Anderson, C.R.; Spence, L.P.; Downs, W.G.; Aitken, T.H. Manzanilla virus: A new virus isolated from the blood of a howler monkey in Trinidad, W.I. Am. J. Trop. Med. Hyg. 1960, 9, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Pajot, F.X. Diseases transmitted by insects in French Guaiana. Bol. Oficina Sanit. Panam. 1980, 88, 218–227. (In Spanish) [Google Scholar] [PubMed]
- Calisher, C.H.; Kokernot, R.H.; De Moore, J.F.; Boyd, K.R.; Hayes, J.; Chappell, W.A. Arbovirus studies in the Ohio-Mississippi Basin, 1964–1967. VI. Mermet: A Simbu-group arbovirus. Am. J. Trop. Med. Hyg. 1969, 18, 779–788. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.V.; Barrett, A.D.; Saeed, M.F.; Watts, D.M.; Russell, K.; Guevara, C.; Ampuero, J.S.; Suarez, L.; Cespedes, M.; Montgomery, J.M.; et al. Iquitos virus: A novel reassortant Orthobunyavirus associated with human illness in Peru. PLoS Negl. Trop. Dis. 2011, 5, e1315. [Google Scholar] [CrossRef] [PubMed]
- Ladner, J.T.; Savji, N.; Lofts, L.; Travassos da Rosa, A.; Wiley, M.R.; Gestole, M.C.; Rosen, G.E.; Guzman, H.; Vasconcelos, P.F.; Nunes, M.R.; et al. Genomic and phylogenetic characterization of viruses included in the Manzanilla and Oropouche species complexes of the genus Orthobunyavirus, family Bunyaviridae. J. Gen. Virol. 2014, 95, 1055–1066. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Spence, L.; Downs, W.G.; Aitken, T.H. Oropouche virus: A new human disease agent from Trinidad, West Indies. Am. J. Trop. Med. Hyg. 1961, 10, 574–578. [Google Scholar] [CrossRef]
- Tilston-Lunel, N.L.; Hughes, J.; Acrani, G.O.; da Silva, D.E.; Azevedo, R.S.; Rodrigues, S.G.; Vasconcelos, P.F.; Nunes, M.R.; Elliott, R.M. Genetic analysis of members of the species Oropouche virus and identification of a novel M segment sequence. J. Gen. Virol. 2015, 96, 1636–1650. [Google Scholar] [CrossRef]
- Seymour, C.; Peralta, P.H.; Montgomery, G.G. Viruses isolated from Panamanian sloths. Am. J. Trop. Med. Hyg. 1983, 32, 1435–1444. [Google Scholar] [CrossRef] [PubMed]
- St George, T.D.; Standfast, H.A.; Cybinski, D.H.; Filippich, C.; Carley, J.G. Peaton virus: A new Simbu group arbovirus isolated from cattle and Culicoides brevitarsis in Australia. Aust. J. Biol. Sci. 1980, 33, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Dandawate, C.N.; Rajagopalan, P.K.; Pavri, K.M.; Work, T.H. Virus isolations from mosquitoes collected in North Arcot district, Madras state, and Chittoor district, Andhra Pradesh between November 1955 and October 1957. Indian J. Exp. Biol. 1969, 57, 1420–1426. [Google Scholar]
- Hoffmann, B.; Scheuch, M.; Höper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, F.M.; Singh, P.B.; Dandawate, C.N.; Soman, R.S.; Bhatt, P.N. Kaikalur virus—A new arthropod-borne virus belonging to the Simbu group isolated in India from Culex tritaeniorhynchus (Giles). Indian J. Med. Res. 1977, 66, 719–725. [Google Scholar]
- Mitchell, C.J.; Monath, T.P.; Sabattini, M.S.; Cropp, C.B.; Daffner, J.F.; Calisher, C.H.; Jakob, W.L.; Christensen, H.A. Arbovirus investigations in Argentina, 1977–1980. II. Arthropod collections and virus isolations from Argentine mosquitoes. Am. J. Trop. Med. Hyg. 1985, 34, 945–955. [Google Scholar] [CrossRef] [PubMed]
- Weinbren, M.P.; Heymann, C.S.; Kokernot, R.H.; Paterson, H.E. Studies on arthropod-borne viruses of Tongaland. VII. Simbu virus, a hitherto unknown agent isolated from Aedes (Banksinella) circumluteolus Theo. S. Afr. J. Med. Sci. 1957, 22, 93–102. [Google Scholar] [PubMed]
- Carey, D.E.; Reuben, R.; George, S.; Shope, R.E.; Myers, R.M. Kammavanpettai, Kannamangalam, Sembalam and Thimiri viruses: Four unrelated new agents isolated from birds in India. Indian J. Med. Res. 1971, 59, 1708–1711. [Google Scholar]
- Shope, R.E.; Paes de Andrade, A.H.; Bensabath, G. The serological response of animals to virus infection in Utinga Forest, Belem, Brazil. Atas Simp 1967, 243, 225–230. [Google Scholar]
- Oğuzoğlu, T.C.; Toplu, N.; Koc, B.T.; Dogan, F.; Epikmen, E.T.; Ipek, E.; Akkoc, A.N. First molecular detection and characterization of Akabane virus in small ruminants in Turkey. Arch. Virol. 2015, 160, 2623–2627. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.Y.; Erster, O.; Beer, M.; Khinich, Y.; Wernike, K. Development and validation of a universal S-segment-based real-time RT-PCR assay for the detection of Simbu serogroup viruses. J. Virol. Methods 2018, 261, 80–85. [Google Scholar] [CrossRef]
- Oem, J.K.; Lee, K.H.; Kim, H.R.; Bae, Y.C.; Chung, J.Y.; Lee, O.S.; Roh, I.S. Bovine epizootic encephalomyelitis caused by Akabane virus infection in Korea. J. Comp. Pathol. 2012, 147, 101–105. [Google Scholar] [CrossRef]
- Oem, J.K.; Yoon, H.J.; Kim, H.R.; Roh, I.S.; Lee, K.H.; Lee, O.S.; Bae, Y.C. Genetic and pathogenic characterization of Akabane viruses isolated from cattle with encephalomyelitis in Korea. Vet. Microbiol. 2012, 158, 259–266. [Google Scholar] [CrossRef]
- Miyazato, S.; Miura, Y.; Hase, M.; Kubo, M.; Goto, Y.; Kono, Y. Encephalitis of cattle caused by Iriki isolate, a new strain belonging to Akabane virus. Nihon Juigaku Zasshi 1989, 51, 128–136. [Google Scholar] [CrossRef]
- Charles, J.A. Akabane virus. Vet. Clin. North Am. Food Anim. Pract. 1994, 10, 525–546. [Google Scholar] [CrossRef]
- St George, T.D.; Standfast, H.A.; Cybinski, D.H. Isolations of Akabane virus from sentinel cattle and Culicoides brevitarsis. Aust. Vet. J. 1978, 54, 558–561. [Google Scholar] [CrossRef]
- Cybinski, D.H.; St George, T.D.; Paull, N.I. Antibodies to Akabane virus in Australia. Aust. Vet. J. 1978, 54, 1–3. [Google Scholar] [CrossRef]
- St George, T.D.; Standfast, H.A. Diseases caused by Akabane and related Simbu-group viruses. In Infectious Diseases of Livestock with Special Reference to Southern Africa; Coetzer, J.A.W., Thomson, G.R., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 1994; Volume 1, pp. 681–687. [Google Scholar]
- Cybinski, D.H.; Muller, M.J. Isolation of arboviruses from cattle and insects at two sentinel sites in Queensland, Australia, 1979–1985. Austr. J. Zool. 1990, 38, 25–32. [Google Scholar] [CrossRef]
- Kurogi, H.; Akiba, K.; Inaba, Y.; Matumoto, M. Isolation of Akabane virus from the biting midge Culicoides oxystoma in Japan. Vet. Microbiol. 1987, 15, 243–248. [Google Scholar] [CrossRef]
- Stram, Y.; Brenner, J.; Braverman, Y.; Banet-Noach, C.; Kuznetzova, L.; Ginni, M. Akabane virus in Israel: A new virus lineage. Virus Res. 2004, 104, 93–97. [Google Scholar] [CrossRef]
- Al-Busaidy, S.M.; Mellor, P.S. Isolation and identification of arboviruses from the Sultanate of Oman. Epidemiol. Infect. 1991, 106, 403–413. [Google Scholar] [CrossRef]
- Blackburn, N.K.; Searle, L.; Phelps, R.J. Viruses isolated from Culicoides (Diptera: Ceratopogonidae) caught at the veterinary research farm, Mazowe, Zimbabwe. J. Entomol. Soc. South. Afr. 1958, 48, 331–336. [Google Scholar]
- Theodoridis, A.; Nevill, E.M.; Els, H.J.; Boshoff, S.T. Viruses isolated from Culicoides midges in South Africa during unsuccessful attempts to isolate bovine ephemeral fever virus. Onderstepoort J. Vet. Res. 1979, 46, 191–198. [Google Scholar]
- Metselaar, D.; Robin, Y. Akabane virus isolated in Kenya. Vet. Rec. 1976, 99, 86. [Google Scholar] [CrossRef] [PubMed]
- Jennings, M.; Mellor, P.S. Culicoides: Biological vectors of Akabane virus. Vet. Microbiol. 1989, 21, 125–131. [Google Scholar] [CrossRef]
- EFSA. “Schmallenberg” Virus: Analysis of the Epidemiological Data (May 2013). EFSA Supporting Publications 2013. EN-3429. Available online: http://www.efsa.europa.eu/de/supporting/doc/429e.pdf (accessed on 15 July 2013).
- EFSA. Schmallenberg virus: State of Art. EFSA J. 2014, 12, 54. [Google Scholar]
- De Regge, N.; Deblauwe, I.; De Deken, R.; Vantieghem, P.; Madder, M.; Geysen, D.; Smeets, F.; Losson, B.; van den Berg, T.; Cay, A.B. Detection of Schmallenberg virus in different Culicoides spp. by real-time RT-PCR. Transbound. Emerg. Dis. 2012, 59, 471–475. [Google Scholar] [CrossRef]
- Rasmussen, L.D.; Kristensen, B.; Kirkeby, C.; Rasmussen, T.B.; Belsham, G.J.; Bodker, R.; Bøtner, A. Culicoids as vectors of Schmallenberg virus. Emerg. Infect. Dis. 2012, 18, 1204–1206. [Google Scholar] [CrossRef]
- Goffredo, M.; Monaco, F.; Capelli, G.; Quaglia, M.; Federici, V.; Catalani, M.; Montarsi, F.; Polci, A.; Pinoni, C.; Calistri, P.; et al. Schmallenberg virus in Italy: A retrospective survey in Culicoides stored during the bluetongue Italian surveillance program. Prev. Vet. Med. 2013, 111, 230–236. [Google Scholar] [CrossRef]
- Elbers, A.R.; Meiswinkel, R.; van Weezep, E.; Sloet van Oldruitenborgh-Oosterbaan, M.M.; Kooi, E.A. Schmallenberg virus in Culicoides spp. biting midges, the Netherlands, 2011. Emerg. Infect. Dis. 2013, 19, 106–109. [Google Scholar] [CrossRef]
- Larska, M.; Polak, M.P.; Grochowska, M.; Lechowski, L.; Zwiazek, J.S.; Zmudzinski, J.F. First report of Schmallenberg virus infection in cattle and midges in Poland. Transbound. Emerg. Dis. 2013, 60, 97–101. [Google Scholar] [CrossRef] [PubMed]
- Wernike, K.; Jöst, H.; Becker, N.; Schmidt-Chanasit, J.; Beer, M. Lack of evidence for the presence of Schmallenberg virus in mosquitoes in Germany, 2011. Parasit. Vectors 2014, 7, 402. [Google Scholar] [CrossRef] [PubMed]
- Manley, R.; Harrup, L.E.; Veronesi, E.; Stubbins, F.; Stoner, J.; Gubbins, S.; Wilson, A.; Batten, C.; Koenraadt, C.J.; Henstock, M.; et al. Testing of UK populations of Culex pipiens L. for Schmallenberg virus vector competence and their colonization. PLoS ONE 2015, 10, e0134453. [Google Scholar] [CrossRef] [PubMed]
- Scholte, E.J.; Mars, M.H.; Braks, M.; Den Hartog, W.; Ibanez-Justicia, A.; Koopmans, M.; Koenraadt, C.J.; De Vries, A.; Reusken, C. No evidence for the persistence of Schmallenberg virus in overwintering mosquitoes. Med. Vet. Entomol. 2014, 28, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Balenghien, T.; Pagès, N.; Goffredo, M.; Carpenter, S.; Augot, D.; Jacquier, E.; Talavera, S.; Monaco, F.; Depaquit, J.; Grillet, C.; et al. The emergence of Schmallenberg virus across Culicoides communities and ecosystems in Europe. Prev. Vet. Med. 2014, 116, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Segard, A.; Gardes, L.; Jacquier, E.; Grillet, C.; Mathieu, B.; Rakotoarivony, I.; Setier-Rio, M.L.; Chavernac, D.; Cetre-Sossah, C.; Balenghien, T.; et al. Schmallenberg virus in Culicoides Latreille (Diptera: Ceratopogonidae) populations in France during 2011–2012 outbreak. Transbound. Emerg. Dis. 2018, 65, e94–e103. [Google Scholar] [CrossRef] [PubMed]
- Elbers, A.R.; Meiswinkel, R.; van Weezep, E.; Kooi, E.A.; van der Poel, W.H. Schmallenberg virus in Culicoides biting midges in the Netherlands in 2012. Transbound. Emerg. Dis. 2015, 62, 339–342. [Google Scholar] [CrossRef] [PubMed]
- Veronesi, E.; Henstock, M.; Gubbins, S.; Batten, C.; Manley, R.; Barber, J.; Hoffmann, B.; Beer, M.; Attoui, H.; Mertens, P.P.; et al. Implicating Culicoides biting midges as vectors of Schmallenberg virus using semi-quantitative RT-PCR. PLoS ONE 2013, 8, e57747. [Google Scholar] [CrossRef]
- Pagès, N.; Talavera, S.; Verdún, M.; Pujol, N.; Valle, M.; Bensaid, A.; Pujols, J. Schmallenberg virus detection in Culicoides biting midges in Spain: First laboratory evidence for highly efficient infection of Culicoides of the Obsoletus complex and Culicoides imicola. Transbound. Emerg. Dis. 2018, 65, e1–e6. [Google Scholar] [CrossRef]
- McIntosh, B.M.; Jupp, P.G.; De Sousa, J. Further isolations of the arboviruses from mosquitoes collected in Tongaland, South Africa, 1960–1968. J. Med. Entomol. 1972, 9, 155–159. [Google Scholar] [CrossRef]
- Golender, N.; Brenner, J.; Valdman, M.; Khinich, Y.; Bumbarov, V.; Panshin, A.; Edery, N.; Pismanik, S.; Behar, A. Malformations caused by Shuni virus in ruminants, Israel, 2014–2015. Emerg. Infect. Dis. 2015, 21, 2267–2268. [Google Scholar] [CrossRef]
- Van Eeden, C.; Williams, J.H.; Gerdes, T.G.; van Wilpe, E.; Viljoen, A.; Swanepoel, R.; Venter, M. Shuni virus as cause of neurologic disease in horses. Emerg. Infect. Dis. 2012, 18, 318–321. [Google Scholar] [CrossRef]
- Golender, N.; Bumbarov, V.; Assis, I.; Beer, M.; Khinich, Y.; Koren, O.; Edery, N.; Eldar, A.; Wernike, K. Shuni virus in Israel: Neurological disease and fatalities in cattle. Transbound. Emerg. Dis. 2019, in press. [Google Scholar] [CrossRef] [PubMed]
- Moore, D.L.; Causey, O.R.; Carey, D.E.; Reddy, S.; Cooke, A.R.; Akinkugbe, F.M.; David-West, T.S.; Kemp, G.E. Arthropod-borne viral infections of man in Nigeria, 1964–1970. Ann. Trop. Med. Parasitol. 1975, 69, 49–64. [Google Scholar] [CrossRef]
- Van Eeden, C.; Swanepoel, R.; Venter, M. Antibodies against West Nile and Shuni viruses in veterinarians, South Africa. Emerg. Infect. Dis. 2014, 20, 1409–1411. [Google Scholar] [CrossRef] [PubMed]
- Romero-Alvarez, D.; Escobar, L.E. Oropouche fever, an emergent disease from the Americas. Microbes Infect. 2018, 20, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Travassos da Rosa, J.F.; de Souza, W.M.; Pinheiro, F.P.; Figueiredo, M.L.; Cardoso, J.F.; Acrani, G.O.; Nunes, M.R.T. Oropouche virus: Clinical, epidemiological, and molecular aspects of a neglected Orthobunyavirus. Am. J. Trop. Med. Hyg. 2017, 96, 1019–1030. [Google Scholar] [PubMed]
- Pinheiro, F.P.; Travassos da Rosa, A.P.; Travassos da Rosa, J.F.; Ishak, R.; Freitas, R.B.; Gomes, M.L.; LeDuc, J.W.; Oliva, O.F. Oropouche virus. I. A review of clinical, epidemiological, and ecological findings. Am. J. Trop. Med. Hyg. 1981, 30, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, F.; Travassos da Rosa, A.; Vasconcelos, P.F. Oropouche fever. In Textbook of Pediatric Infectious Diseases; Feigin, R.D., Ed.; Saunders: Philadelphia, PA, USA, 2004; pp. 2418–2423. [Google Scholar]
- Hoch, A.L.; Pinheiro, F.P.; Roberts, D.R.; Gomes, M.L. Laboratory transmission of Oropouche virus by Culex quinquefasciatus Say. Bull. Pan. Am. Health Organ. 1987, 21, 55–61. [Google Scholar]
- Cardoso, B.F.; Serra, O.P.; Heinen, L.B.; Zuchi, N.; Souza, V.C.; Naveca, F.G.; Santos, M.A.; Slhessarenko, R.D. Detection of Oropouche virus segment S in patients and in Culex quinquefasciatus in the state of Mato Grosso, Brazil. Mem. Inst. Oswaldo Cruz 2015, 110, 745–754. [Google Scholar] [CrossRef]
- Behar, A.; Leibovich, B.B.; Edery, N.; Yanase, T.; Brenner, J. First genomic detection of Peaton virus in a calf with hydranencephaly in Israel. Vet. Med. Sci. 2018, 5, 87–92. [Google Scholar] [CrossRef]
- Hubalek, Z. Mosquito-borne viruses in Europe. Parasitol. Res. 2008, 103, 29–43. [Google Scholar] [CrossRef]
- Harding, S.; Greig, J.; Mascarenhas, M.; Young, I.; Waddell, L.A. La Crosse virus: A scoping review of the global evidence. Epidemiol. Infect. 2018, 1–13. [Google Scholar] [CrossRef]
- Anonymous. La Cross Encephalitis. Available online: https://www.cdc.gov/lac/index.html (accessed on 12 March 2019).
- Kappus, K.D.; Calisher, C.H.; Baron, R.C.; Davenport, J.; Francy, D.B.; Williams, R.M. La Crosse virus infection and disease in western North Carolina. Am. J. Trop. Med. Hyg. 1982, 31, 556–560. [Google Scholar] [CrossRef]
- Szumlas, D.E.; Apperson, C.S.; Powell, E.E.; Hartig, P.; Francy, D.B.; Karabotsos, N. Relative abundance and species composition of mosquito populations (Diptera: Culicidae) in a La Crosse virus-endemic area in western North Carolina. J. Med. Entomol. 1996, 33, 598–607. [Google Scholar] [CrossRef]
- Miller, B.R.; DeFoliart, G.R.; Yuill, T.M. Vertical transmission of La Crosse virus (California encephalitis group): Transovarial and filial infection rates in Aedes triseriatus (Diptera: Culicidae). J. Med. Entomol. 1977, 14, 437–440. [Google Scholar] [CrossRef]
- Gerhardt, R.R.; Gottfried, K.L.; Apperson, C.S.; Davis, B.S.; Erwin, P.C.; Smith, A.B.; Panella, N.A.; Powell, E.E.; Nasci, R.S. First isolation of La Crosse virus from naturally infected Aedes albopictus. Emerg. Infect. Dis. 2001, 7, 807–811. [Google Scholar] [CrossRef]
- Lambert, A.J.; Blair, C.D.; D’Anton, M.; Ewing, W.; Harborth, M.; Seiferth, R.; Xiang, J.; Lanciotti, R.S. La Crosse virus in Aedes albopictus mosquitoes, Texas, USA, 2009. Emerg. Infect. Dis. 2010, 16, 856–858. [Google Scholar] [CrossRef]
- Westby, K.M.; Fritzen, C.; Paulsen, D.; Poindexter, S.; Moncayo, A.C. La Crosse Encephalitis virus infection in field-collected Aedes albopictus, Aedes japonicus, and Aedes triseriatus in Tennessee. J. Am. Mosq. Control Assoc. 2015, 31, 233–241. [Google Scholar] [CrossRef]
- Harris, M.C.; Dotseth, E.J.; Jackson, B.T.; Zink, S.D.; Marek, P.E.; Kramer, L.D.; Paulson, S.L.; Hawley, D.M. La Crosse virus in Aedes japonicus japonicus mosquitoes in the Appalachian region, United States. Emerg. Infect. Dis. 2015, 21, 646–649. [Google Scholar] [CrossRef]
- Grimstad, P.R.; Kobayashi, J.F.; Zhang, M.B.; Craig, G.B., Jr. Recently introduced Aedes albopictus in the United States: potential vector of La Crosse virus (Bunyaviridae: California serogroup). J. Am. Mosq. Control Assoc. 1989, 5, 422–427. [Google Scholar] [PubMed]
- Sardelis, M.R.; Turell, M.J.; Andre, R.G. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae). J. Med. Entomol. 2002, 39, 635–639. [Google Scholar] [CrossRef] [PubMed]
- Grimstad, P.R. Jamestown Canyon virus. In Encyclopedia of Arthropod-Transmitted Infections of Man and Domestic Animals; Service, M.W., Ashford, R.W., Eds.; CABI Publishing: New York, NY, USA, 2001; pp. 235–239. [Google Scholar]
- Grimstad, P.R. California group virus disease. In The Arboviruses: Epidemiology and Ecology; Monath, T.P., Ed.; CRC Press: Boca Raton, FL, USA, 1988; pp. 99–136. [Google Scholar]
- Whitman, L.; Wallis, R.C.; Leventhal, E.A. Isolation of a California-group arbovirus from Aedes abserratus (Felt and Young) in Simsbury, Connecticut. Am. J. Trop. Med. Hyg. 1968, 17, 449–450. [Google Scholar] [CrossRef] [PubMed]
- Andreadis, T.G.; Anderson, J.F.; Armstrong, P.M.; Main, A.J. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: A ten-year analysis, 1997–2006. Vector Borne Zoonotic Dis. 2008, 8, 175–188. [Google Scholar] [CrossRef]
- Boromisa, R.D.; Grayson, M.A. Incrimination of Aedes provocans as a vector of Jamestown Canyon virus in an enzootic focus of northeastern New York. J. Am. Mosq. Control Assoc. 1990, 6, 504–509. [Google Scholar]
- Heard, P.B.; Zhang, M.B.; Grimstad, P.R. Isolation of Jamestown Canyon virus (California serogroup) from Aedes mosquitoes in an enzootic focus in Michigan. J. Am. Mosq. Control Assoc. 1990, 6, 461–468. [Google Scholar] [PubMed]
- Walker, E.D.; Grayson, M.A.; Edman, J.D. Isolation of Jamestown Canyon and snowshoe hare viruses (California serogroup) from Aedes mosquitoes in western Massachusetts. J. Am. Mosq. Control Assoc. 1993, 9, 131–134. [Google Scholar] [PubMed]
- Boromisa, R.D.; Grimstad, P.R. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am. J. Trop. Med. Hyg. 1986, 35, 1285–1295. [Google Scholar] [CrossRef]
- Anderson, J.F.; Main, A.J.; Armstrong, P.M.; Andreadis, T.G.; Ferrandino, F.J. Arboviruses in North Dakota, 2003–2006. Am. J. Trop. Med. Hyg. 2015, 92, 377–393. [Google Scholar] [CrossRef]
- Bond, J.O.; Hammon, W.M.; Lewis, A.L.; Sather, G.E.; Taylor, D.J. California group arboviruses in Florida and report of a new strain, Keystone virus. Public Health Rep. 1966, 81, 607–613. [Google Scholar] [CrossRef]
- LeDuc, J.W.; Burger, J.F.; Eldridge, B.F.; Russell, P.K. Ecology of Keystone virus, a transovarially maintained arbovirus. Ann. N. Y. Acad. Sci. 1975, 266, 144–151. [Google Scholar] [CrossRef]
- Issel, C.J.; Hoff, G.L.; Trainer, D.O. Serologic evidence of infection of white-tailed deer in Texas with three California group arboviruses, (Jamestown Canyon, San Angelo, and Keystone). J. Wildl. Dis. 1973, 9, 245–248. [Google Scholar] [CrossRef][Green Version]
- Watts, D.M.; LeDuc, J.W.; Bailey, C.L.; Dalrymple, J.M.; Gargan, T.P., 2nd. Serologic evidence of Jamestown Canyon and Keystone virus infection in vertebrates of the DelMarVa Peninsula. Am. J. Trop. Med. Hyg. 1982, 31, 1245–1251. [Google Scholar] [CrossRef]
- Watts, D.M.; Bailey, C.L.; Roberts, N.T.; RF, T.A.; Dalrymple, J.M.; Clark, G.C. Maintenance and transmission of Keystone virus by Aedes atlanticus (Diptera: Culicidae) and the gray squirrel in the Pocomoke Cypress Swamp, Maryland. J. Med. Entomol. 1988, 25, 493–500. [Google Scholar] [CrossRef]
- Sudia, W.D.; Newhouse, V.F.; Calisher, C.H.; Chamberlain, R.W. California group arbo-viruses: Isolations from mosquitoes in North America. Mosq. News 1971, 31, 576–600. [Google Scholar]
- Parkin, W.E.; Hammon, W.M.; Sather, G.E. Review of current epidemiological literature on viruses of the California arbovirus group. Am. J. Trop. Med. Hyg. 1972, 21, 964–978. [Google Scholar] [CrossRef]
- Lednicky, J.A.; White, S.K.; Stephenson, C.J.; Cherabuddi, K.; Loeb, J.C.; Moussatche, N.; Lednicky, A.; Morris, J.G., Jr. Keystone virus isolated from a Florida teenager with rash and subjective fever: Another endemic arbovirus in the Southeastern United States? Clin. Infect. Dis. 2019, 68, 143–145. [Google Scholar] [CrossRef]
- Bardos, V.; Medek, M.; Kania, V.; Hubalek, Z. Isolation of Tahyna virus from the blood of sick children. Acta Virol. 1975, 19, 447. [Google Scholar]
- Rodl, P.; Bardos, V.; Ryba, J. Experimental transmission of Tahyna virus (California group) to wild rabbits (Oryctolagus cuniculus) by mosquitoes. Folia Parasitol. (Praha) 1979, 26, 61–64. [Google Scholar]
- Aspöck, H.; Kunz, C. Felduntersuchungen über die Bedeutung des Igels (Erinaceus europaeus roumanicus Barrett-Hamilton) im Zyklus des Tahyna-virus. Zentralbl Bakteriol. 1970, 213, 304–310. [Google Scholar]
- Bardos, V.; Danielova, V. The Tahyna virus—A virus isolated from mosquitoes in Czechoslovakia. J. Hyg. Epidemiol. Microbiol. Immunol. 1959, 3, 264–276. [Google Scholar]
- Danielová, V.; Hájková, Z.; Kolman, J.M.; Málková, D.; Minár, J.; Smetana, A. Results of virological examination of mosquitoes in Southern Moravia in 1962–1964. Cesk. Epidemiol. Mikrobiol. Imunol. 1966, 15, 178–184. [Google Scholar] [PubMed]
- Kolman, J.M.; Malkova, D.; Nemec, A.; Smetana, A.; Hajkova, Z.; Minar, J. The isolation of the Tahyna virus from the mosquito Aedes vexans in Southern Moravia. J. Hyg. Epidemiol. Microbiol. Immunol. 1964, 8, 380–386. [Google Scholar]
- Hannoun, C.; Panther, R.; Corniou, B. Isolation of Tahyna virus in the South of France. Acta Virol. 1966, 10, 362–364. [Google Scholar]
- Balducci, M.; Verani, P.; Lopes, M.C.; Sacca, G.; Gregorig, B. Isolation of Tahyña virus from Aedes mosquitoes in Northern Italy (Gorizia Province). Acta Virol. 1968, 12, 457–459. [Google Scholar]
- Traavik, T.; Mehl, R.; Wiger, R. California encephalitis group viruses isolated from mosquitoes collected in Southern and Arctic Norway. Acta Pathol. Microbiol. Scand. B 1978, 86B, 335–341. [Google Scholar] [CrossRef]
- Arcan, P.; Topciu, V.; Rošiu, N.; Csaky, N. Isolation of Ťahyňa virus from Culex pipiens mosquitoes in Romania. Acta Virol. 1974, 18, 175. [Google Scholar]
- Hubalek, Z.; Sebesta, O.; Pesko, J.; Betasova, L.; Blazejova, H.; Venclikova, K.; Rudolf, I. Isolation of Tahyna virus (California encephalitis group) from Anopheles hyrcanus (Diptera, Culicidae), a mosquito species new to, and expanding in, Central Europe. J. Med. Entomol. 2014, 51, 1264–1267. [Google Scholar] [CrossRef]
- Anonymous. VMD authorises SBV vaccine for use in the UK. Vet. Rec. 2013, 172, 543. [Google Scholar]
- Anonymous. Schmallenberg virus vaccine. Vet. Rec. 2015, 177, 321. [Google Scholar]
- EMA. Zulvac SBV. 2015. Available online: https://www.ema.europa.eu/medicines/veterinary/EPAR/zulvac-sbv (accessed on 25 March 2019).
- Kim, Y.H.; Kweon, C.H.; Tark, D.S.; Lim, S.I.; Yang, D.K.; Hyun, B.H.; Song, J.Y.; Hur, W.; Park, S.C. Development of inactivated trivalent vaccine for the teratogenic Aino, Akabane and Chuzan viruses. Biologicals 2011, 39, 152–157. [Google Scholar] [CrossRef]
- Patta, C.; Giovannini, A.; Rolesu, S.; Nannini, D.; Savini, G.; Calistri, P.; Santucci, U.; Caporale, V. Bluetongue vaccination in Europe: The Italian experience. Vet. Ital. 2004, 40, 601–610. [Google Scholar]
- Zientara, S.; MacLachlan, N.J.; Calistri, P.; Sanchez-Vizcaino, J.M.; Savini, G. Bluetongue vaccination in Europe. Expert Rev. Vaccines 2010, 9, 989–991. [Google Scholar] [CrossRef] [PubMed]
- Sakkas, H.; Bozidis, P.; Franks, A.; Papadopoulou, C. Oropouche fever: A review. Viruses 2018, 10, 175. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, S.; Mellor, P.S.; Torr, S.J. Control techniques for Culicoides biting midges and their application in the U.K. and northwestern Palaearctic. Med. Vet. Entomol. 2008, 22, 175–187. [Google Scholar] [CrossRef]
- Wernike, K.; Conraths, F.; Zanella, G.; Granzow, H.; Gache, K.; Schirrmeier, H.; Valas, S.; Staubach, C.; Marianneau, P.; Kraatz, F.; et al. Schmallenberg virus—Two years of experiences. Prev. Vet. Med. 2014, 116, 423–434. [Google Scholar] [CrossRef]
- Corbel, V.; Stankiewicz, M.; Pennetier, C.; Fournier, D.; Stojan, J.; Girard, E.; Dimitrov, M.; Molgo, J.; Hougard, J.M.; Lapied, B. Evidence for inhibition of cholinesterases in insect and mammalian nervous systems by the insect repellent DEET. BMC Biol. 2009, 7, 47. [Google Scholar] [CrossRef]
- Mayer, S.V.; Tesh, R.B.; Vasilakis, N. The emergence of arthropod-borne viral diseases: A global prospective on dengue, chikungunya and zika fevers. Acta Trop. 2017, 166, 155–163. [Google Scholar] [CrossRef] [PubMed]
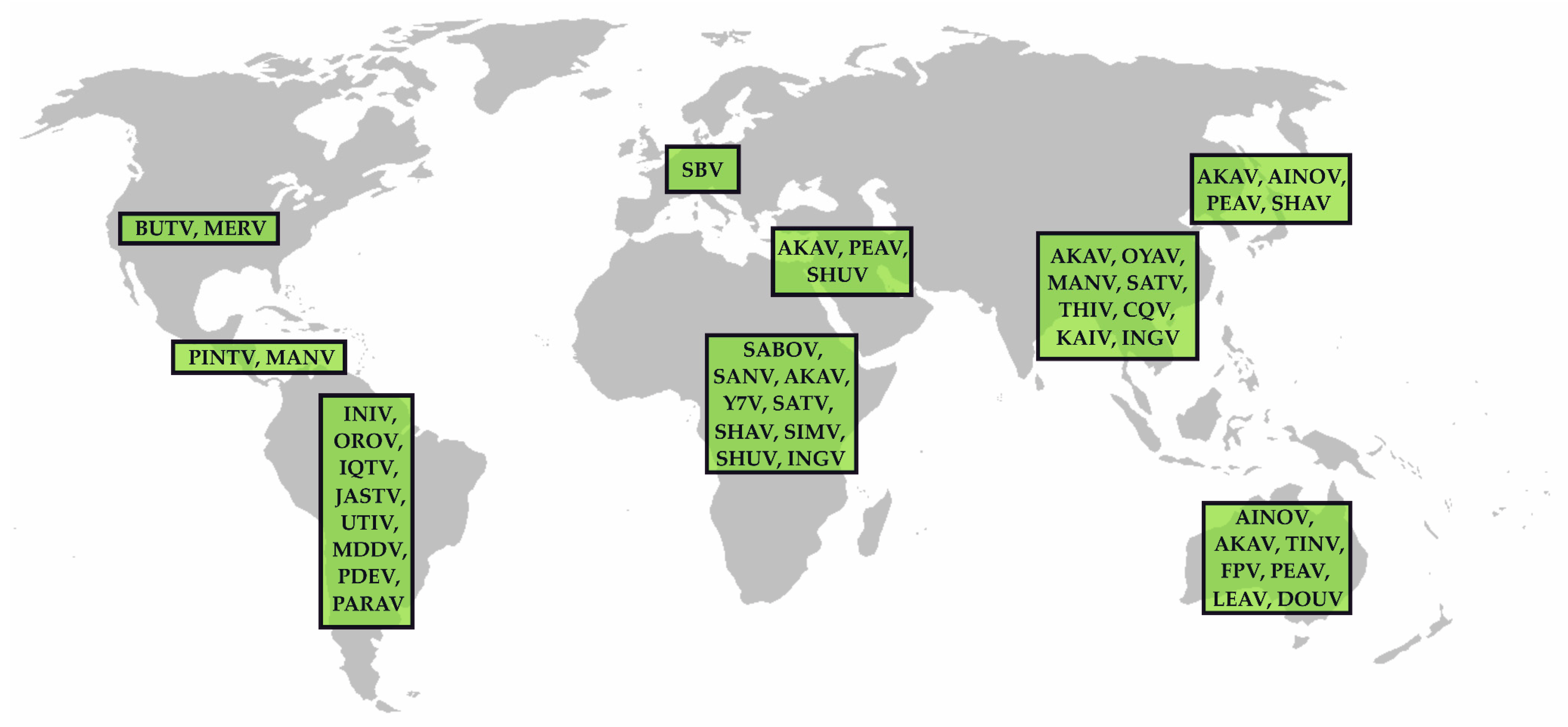
| Virus Species | Virus (Abbreviation) | First Isolation | Insect Vector | Distribution | |||||
|---|---|---|---|---|---|---|---|---|---|
| Year | Country | Organism | Putative Vectors | Demonstrated Vectors | Continent | Animal Hosts (Virus Detection) | Reference (1st Description) | ||
| Akabane orthobunyavirus | Akabane virus (AKAV) | 1959 | Japan | mosquitoes | biting midges, mosquitoes | C. brevitarsis, C. variipennis1 | Asia, Africa, Australia | ruminants, swine | [58] |
| Tinaroo virus (TINV) | 1978 | Australia | Culicoides midges | biting midges (C. brevitarsis) | Australia | ruminants | [59] | ||
| Yaba-7 virus (Y7V) | 1963 | Nigeria | mosquitoes | mosquitoes | Africa | [60] | |||
| Aino orthobunyavirus | Aino virus (AINOV) | 1964 | Japan | mosquitoes | mosquitoes, biting midges (C. brevitarsis) | Asia, Australia | ruminants | [61] | |
| Buttonwillow orthobunyavirus | Buttonwillow virus (BUTV) | 1962 | USA | Cottontail rabbit | midges (C. variipennis) | North America | leproids | [62] | |
| Cat Que orthobunyavirus | Cát Quế virus (CQV) | 2004 | Vietnam | mosquitoes | mosquitoes (Culex spp., Anopheles spp., Mansonia spp.) | Asia | swine, birds | [63] | |
| Oya virus (OYAV) | 1999 | Malaysia | swine | mosquitoes | Asia | swine, humans | [64] | ||
| Faceys paddock orthobunyavirus | Facey’s paddock virus (FPV) | 1974 | Australia | mosquitoes | mosquitoes (Culex annulirostris), midges | Australia | [65] | ||
| Ingwavuma orthobunyavirus | Ingwavuma virus (INGV) | 1959 | South Africa | spectacled weaver | mosquitoes (Culex spp., Monsonia spp.), biting midges | Africa, Asia | swine, dogs, birds | [66] | |
| Jatobal orthobunyavirus | Jatobal virus (JASTV) | 1985 | Brazil | coati | mosquitoes, biting midges | South America | coati | [67] | |
| Leanyer orthobunyavirus | Leanyer virus (LEAV) | 1974 | Australia | mosquitoes | mosquitoes (Anopheles meraukensis) | Australia | cattle, wallabies, dogs | [68] | |
| Manzanilla orthobunyavirus | Manzanilla virus (MANV) | 1954 | Trinidad | Howler monkey | mosquitoes (Culex tritaeniorhynchus), biting midges | Central America, Asia | [69] | ||
| Inini virus (INIV) | 1973 | French Guaiana | Aracari bird | South America | birds | [70] | |||
| Mermet orthobunyavirus | Mermet virus (MERV) | 1964 | USA | purple martin | mosquitoes (Culex spp.) | North America | birds | [71] | |
| Oropouche orthobunyavirus | Iquitos virus (IQTV) | 1999 | Peru | human | midges | South America | humans | [72] | |
| Madre de Dios virus (MDDV) | 2007 | Peru | human | mosquitoes, biting midges | South America | humans, monkeys | [73] | ||
| Oropouche virus (OROV) | 1955 | Trinidad | human | mosquitoes (Aedes spp., Coquillettidia venezuelensis, Culex quinquefasciatus), biting midges | C. paraensis | Central/South America | sloths, non-human primates, rodents, birds, humans | [74] | |
| Perdões virus (PDEV) | 2012 | Brazil | black-tufted marmoset | South America | non-human primates | [75] | |||
| Pintupo virus (PINTV) | Panama | sloth | biting midges | Central America | sloths | [76] | |||
| Peaton orthobunyavirus | Peaton virus (PEAV) | 1976 | Australia | Culicoides spp. | biting midges (C. brevitarsis, C. imicola, C. jacobsoni) | Australia, Asia | ruminants, horses | [77] | |
| Sabo orthobunyavirus | Sabo virus (SABOV) | 1966 | Nigeria | goat | biting midges | Africa | goat | [60] | |
| Sango orthobunyavirus | Sango virus (SANV) | 1965 | Nigeria | cattle | mosquitoes, biting midges | Africa | cattle | [60] | |
| Schmallenberg orthobunyavirus | Douglas virus (DOUV) | 1978 | Australia | cattle | biting midges (C. brevitarsis) | Australia/Oceania | ruminants | [59] | |
| Sathuperi virus (SATV) | 1957 | India | mosquitoes | mosquitoes (Culex vishnui), biting midges (C. oxystoma) | Asia, Africa | ruminants | [78] | ||
| Schmallenberg virus (SBV) | 2011 | Germany | cattle | biting midges | biting midges (C. obsoletus, C. scoticus, C. chiopterus, C. imicola, C. sonorensis 1) | Europe | ruminants | [79] | |
| Shamonda virus (SHAV) | 1965 | Nigeria | cattle | biting midges | Africa, Asia | ruminants | [60] | ||
| Shuni orthobunyavirus | Kaikalur virus (KAIV) | 1971 | India | mosquitoes | mosquitoes | Asia | [80] | ||
| Shuni virus (SHUV) | 1966 | Nigeria | cattle | mosquitoes (Culex theileri), biting midges | biting midges (C. nubeculosus 1, C. sonorensis 1) | Africa, Asia | ruminants, horse, (humans?) | [60] | |
| Simbu orthobunyavirus | Para virus (PARAV) | Argentina | mosquitoes | mosquitoes | South America | [81] | |||
| Simbu virus (SIMV) | 1955 | South Africa | mosquitoes | mosquitoes (Aedes spp.), biting midges | Africa | [82] | |||
| Thimiri orthobunyavirus | Thimiri virus (THIV) | 1963 | India | Indian Pond Heron | biting midges (C. histrio) | Asia | birds | [83] | |
| Utinga orthobunyavirus | Utinga virus (UTIV) | 1965 | Brazil | brown-throated sloth | ? | South America | sloths | [84] | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sick, F.; Beer, M.; Kampen, H.; Wernike, K. Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses 2019, 11, 376. https://doi.org/10.3390/v11040376
Sick F, Beer M, Kampen H, Wernike K. Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses. 2019; 11(4):376. https://doi.org/10.3390/v11040376
Chicago/Turabian StyleSick, Franziska, Martin Beer, Helge Kampen, and Kerstin Wernike. 2019. "Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance" Viruses 11, no. 4: 376. https://doi.org/10.3390/v11040376
APA StyleSick, F., Beer, M., Kampen, H., & Wernike, K. (2019). Culicoides Biting Midges—Underestimated Vectors for Arboviruses of Public Health and Veterinary Importance. Viruses, 11(4), 376. https://doi.org/10.3390/v11040376





