Improvement of High Affinity and Selectivity on Biosensors Using Genetically Engineered Phage by Binding Isotherm Screening
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
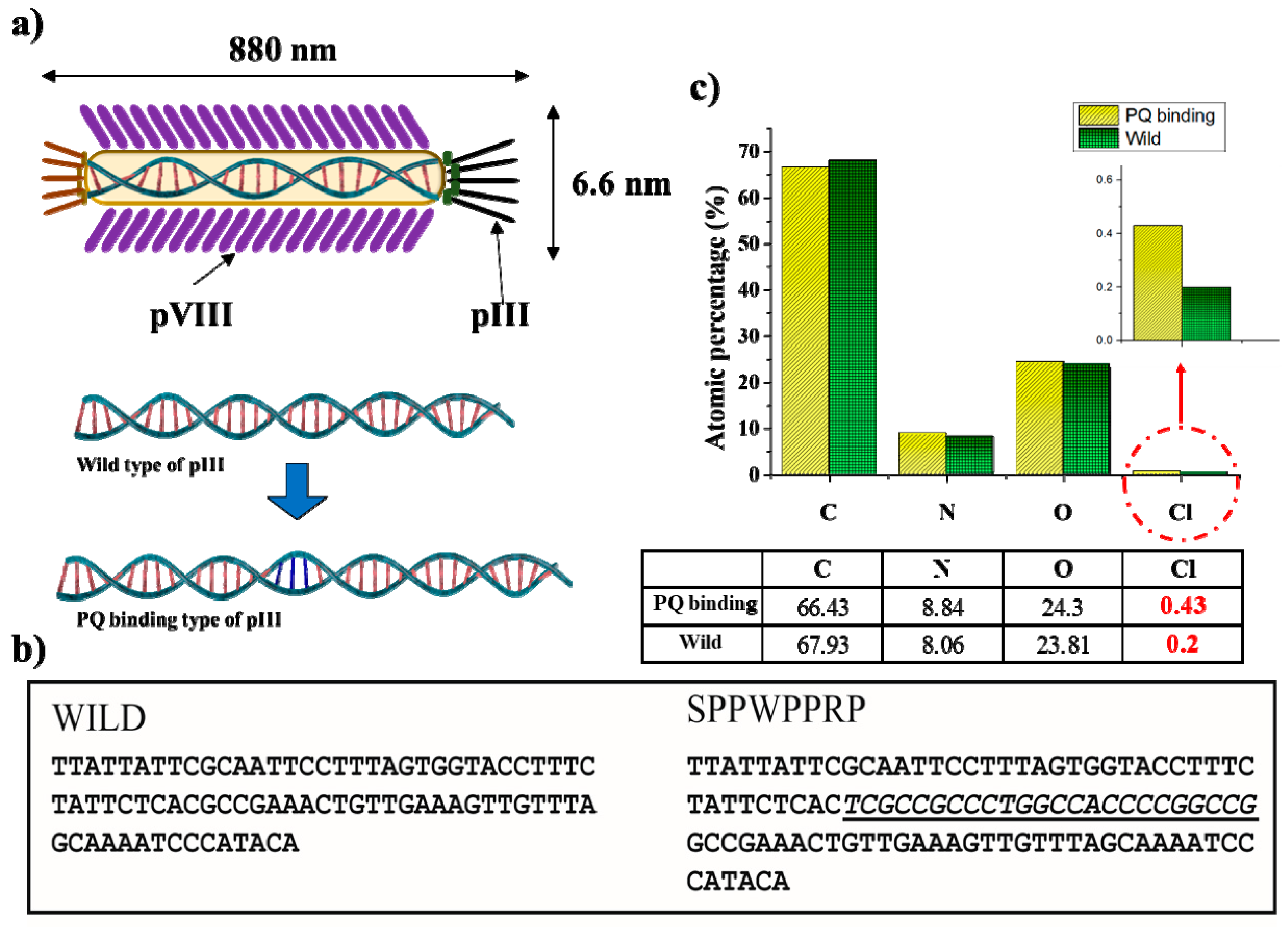
2.2. Genetic Engineering
2.3. Fabrication of Phage Biofilter
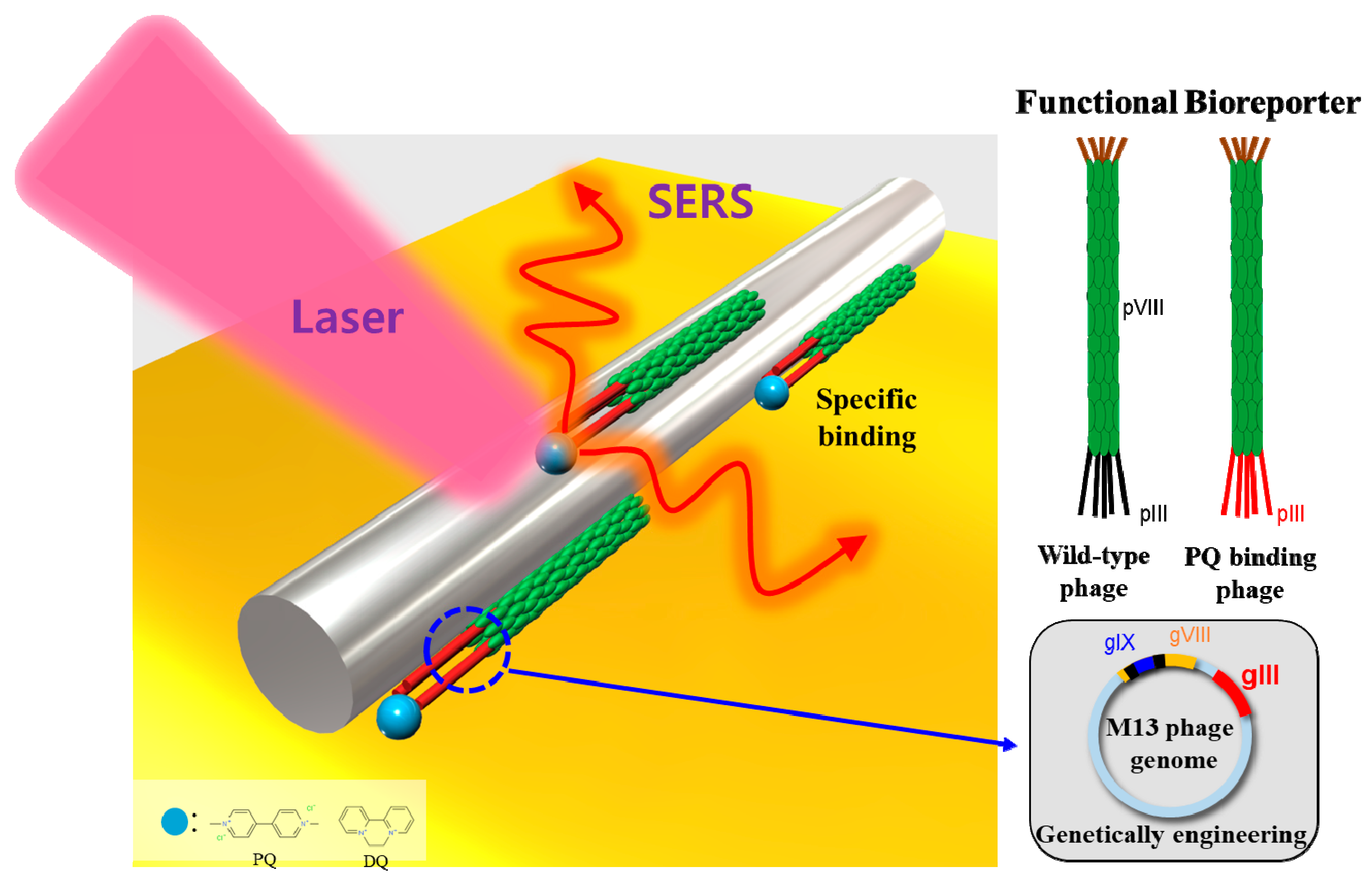
2.4. Preparation of SERS Sensor Platform Functionalized by M13 Phage
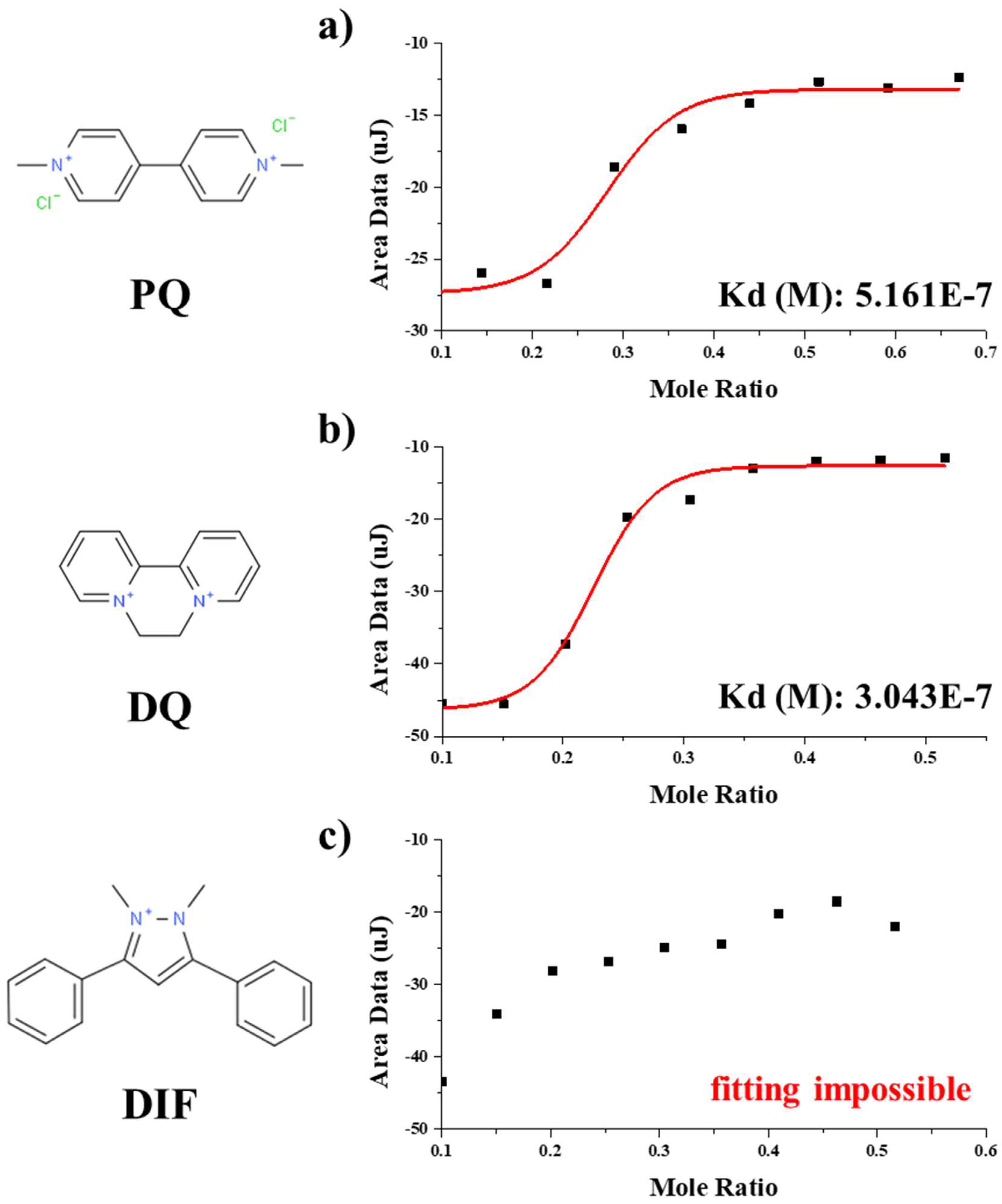
2.5. ITC Measurement
2.6. Analysis of Adsorbed Ion on Phage Biofilter Structures
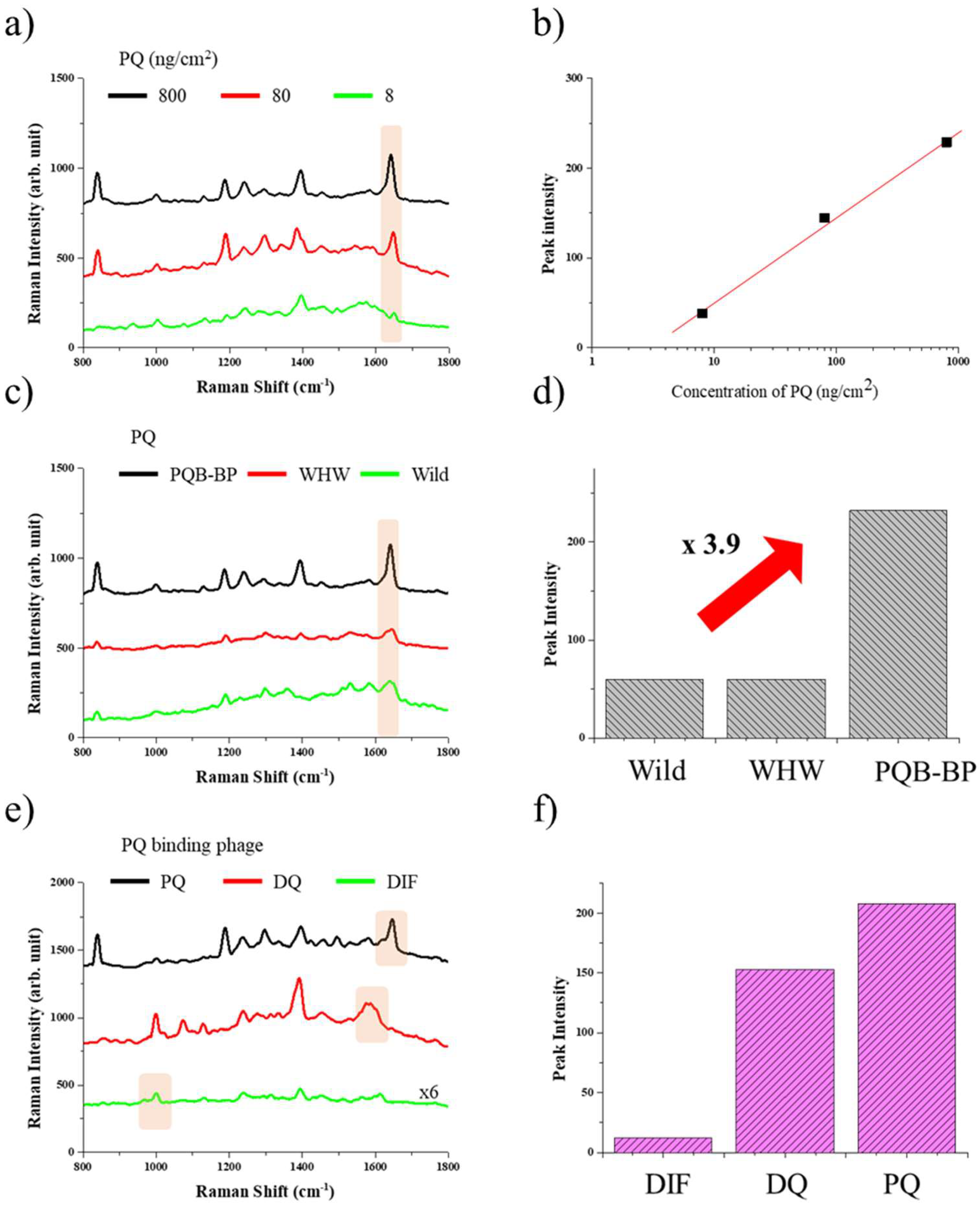
2.7. SERS Measurement
3. Results and Discussion
3.1. Binding Characteristics of the PQ-Binding Peptide
3.2. Phage Biofilter Experiments
3.3. SERS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Sun, B.; Horvat, J.; Kim, H.S.; Kim, W.-S.; Ahn, J.; Wang, G. Synthesis of Mesoporous α-Fe2O3 Nanostructures for Highly Sensitive Gas Sensors and High Capacity Anode Materials in Lithium Ion Batteries. J. Phys. Chem. C 2010, 114, 18753–18761. [Google Scholar] [CrossRef]
- Xing, Z.; Tian, J.; Asiri, A.M.; Qusti, A.H.; Al-Youbi, A.O.; Sun, X. Two-dimensional hybrid mesoporous Fe2O3-graphene nanostructures: A highly active and reusable peroxidase mimetic toward rapid, highly sensitive optical detection of glucose. Biosens. Bioelectron. 2014, 52, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Dijkstra, R.J.; Scheenen, W.J.J.M.; Dam, N.; Roubos, E.W.; ter Meulen, J.J. Monitoring neurotransmitter release using surface-enhanced Raman spectroscopy. J. Neurosci. Methods 2007, 159, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Huang, D.; Wang, H.; Li, H.; Xu, S.; Chang, Y.; Li, H.; Yang, Y.-W.; Liang, C.; Xu, W. In situ surface-enhanced Raman scattering spectroscopy exploring molecular changes of drug-treated cancer cell nucleus. Anal. Chem. 2015, 87, 2504–2510. [Google Scholar] [CrossRef] [PubMed]
- Halvorson, R.A.; Vikesland, P.J. Surface-Enhanced Raman Spectroscopy (SERS) for Environmental Analyses. Environ. Sci. Technol. 2010, 44, 7749–7755. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Li, P.; Liu, H.; Tang, X.; Liu, J. A dynamic surface enhanced Raman spectroscopy method for ultra-sensitive detection: From the wet state to the dry state. Chem. Soc. Rev. 2015, 44, 2837–2848. [Google Scholar] [CrossRef]
- Kim, M.-K.; Sim, H.; Yoon, S.J.; Gong, S.-H.; Ahn, C.W.; Cho, Y.-H.; Lee, Y.-H. Squeezing Photons into a Point-Like Space. Nano Lett. 2015, 15, 4102–4107. [Google Scholar] [CrossRef]
- Kinkhabwala, A.; Yu, Z.; Fan, S.; Avlasevich, Y.; Müllen, K.; Moerner, W.E. Large single-molecule fluorescence enhancements produced by a bowtie nanoantenna. Nat. Photonics 2009, 3, 654–657. [Google Scholar] [CrossRef]
- Kollmann, H.; Piao, X.; Esmann, M.; Becker, S.F.; Hou, D.; Huynh, C.; Kautschor, L.-O.; Bösker, G.; Vieker, H.; Beyer, A.; et al. Toward Plasmonics with Nanometer Precision: Nonlinear Optics of Helium-Ion Milled Gold Nanoantennas. Nano Lett. 2014, 14, 4778–4784. [Google Scholar] [CrossRef]
- Shin, Y.; Song, J.; Kim, D.; Kang, T. Facile Preparation of Ultrasmall Void Metallic Nanogap from Self-Assembled Gold–Silica Core–Shell Nanoparticles Monolayer via Kinetic Control. Adv. Mater. 2015, 27, 4344–4350. [Google Scholar] [CrossRef]
- Tabakman, S.M.; Chen, Z.; Casalongue, H.S.; Wang, H.; Dai, H. A New Approach to Solution-Phase Gold Seeding for SERS Substrates. Small 2011, 7, 499–505. [Google Scholar] [CrossRef]
- Li, J.F.; Huang, Y.F.; Ding, Y.; Yang, Z.L.; Li, S.B.; Zhou, X.S.; Fan, F.R.; Zhang, W.; Zhou, Z.Y.; Wu, D.Y.; et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395. [Google Scholar] [CrossRef] [PubMed]
- Nagy-Simon, T.; Tatar, A.-S.; Craciun, A.-M.; Vulpoi, A.; Jurj, M.-A.; Florea, A.; Tomuleasa, C.; Berindan-Neagoe, I.; Astilean, S.; Boca, S. Antibody Conjugated, Raman Tagged Hollow Gold–Silver Nanospheres for Specific Targeting and Multimodal Dark-Field/SERS/Two Photon-FLIM Imaging of CD19(+) B Lymphoblasts. ACS Appl. Mater. Interfaces 2017, 9, 21155–21168. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Wang, W.; Sun, K.; Chen, L. Reporter-Embedded SERS Tags from Gold Nanorod Seeds: Selective Immobilization of Reporter Molecules at the Tip of Nanorods. ACS Appl. Mater. Interfaces 2016, 8, 28105–28115. [Google Scholar] [CrossRef] [PubMed]
- Kasera, S.; Herrmann, L.O.; del Barrio, J.; Baumberg, J.J.; Scherman, O.A. Quantitative multiplexing with nano-self-assemblies in SERS. Sci. Rep. 2014, 4, 6785. [Google Scholar] [CrossRef] [PubMed]
- Koh, E.H.; Mun, C.; Kim, C.; Park, S.-G.; Choi, E.J.; Kim, S.H.; Dang, J.; Choo, J.; Oh, J.-W.; Kim, D.-H.; et al. M13 Bacteriophage/Silver Nanowire Surface-Enhanced Raman Scattering Sensor for Sensitive and Selective Pesticide Detection. ACS Appl. Mater. Interfaces 2018, 10, 10388–10397. [Google Scholar] [CrossRef] [PubMed]
- Farjami, E.; Campos, R.; Nielsen, J.S.; Gothelf, K.V.; Kjems, J.; Ferapontova, E.E. RNA Aptamer-Based Electrochemical Biosensor for Selective and Label-Free Analysis of Dopamine. Anal. Chem. 2013, 85, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Byrne, B.; Stack, E.; Gilmartin, N.; O’Kennedy, R. Antibody-Based Sensors: Principles, Problems and Potential for Detection of Pathogens and Associated Toxins. Sensors 2009, 9, 4407–4445. [Google Scholar] [CrossRef] [PubMed]
- Smith, G.P.; Petrenko, V.A. Phage Display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef]
- Moon, J.-S.; Kim, W.-G.; Kim, C.; Park, G.-T.; Heo, J.; Yoo, S.Y.; Oh, J.-W. M13 Bacteriophage-Based Self-Assembly Structures and Their Functional Capabilities. Mini-Rev. Org. Chem. 2015, 12, 271–281. [Google Scholar] [CrossRef] [PubMed]
- Sidhu, S.S. Engineering M13 for phage display. Biomol. Eng. 2001, 18, 57–63. [Google Scholar] [CrossRef]
- Chung, W.-J.; Oh, J.-W.; Kwak, K.; Lee, B.Y.; Meyer, J.; Wang, E.; Hexemer, A.; Lee, S.-W. Biomimetic self-templating supramolecular structures. Nature 2011, 478, 364–368. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.-S.; Kim, W.-G.; Shin, D.-M.; Lee, S.-Y.; Kim, C.; Lee, Y.; Han, J.; Kim, K.; Yoo, S.Y.; Oh, J.-W. Bioinspired M-13 bacteriophage-based photonic nose for differential cell recognition. Chem. Sci. 2017, 8, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Yang, M.; Zhu, Y.; Wang, L.; Tomsia, A.P.; Mao, C. Phage nanofibers induce vascularized osteogenesis in 3D printed bone scaffolds. Adv. Mater. Weinheim 2014, 26, 4961–4966. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.M.; Qi, J.; Oh, D.; Belcher, A.; Kong, J. M13 Virus Aerogels as a Scaffold for Functional Inorganic Materials. Adv. Funct. Mater. 2017, 27, 1603203. [Google Scholar] [CrossRef]
- Ghosh, D.; Lee, Y.; Thomas, S.; Kohli, A.G.; Yun, D.S.; Belcher, A.M.; Kelly, K.A. M13-templated magnetic nanoparticles for targeted in vivo imaging of prostate cancer. Nat. Nanotechnol. 2012, 7, 677–682. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.Y.; Zhang, J.; Zueger, C.; Chung, W.-J.; Yoo, S.Y.; Wang, E.; Meyer, J.; Ramesh, R.; Lee, S.-W. Virus-based piezoelectric energy generation. Nat. Nanotechnol. 2012, 7, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Dang, X.; Klug, M.T.; Qi, J.; Dorval Courchesne, N.-M.; Burpo, F.J.; Fang, N.; Hammond, P.T.; Belcher, A.M. Versatile Three-Dimensional Virus-Based Template for Dye-Sensitized Solar Cells with Improved Electron Transport and Light Harvesting. ACS Nano 2013, 7, 6563–6574. [Google Scholar] [CrossRef]
- Shin, D.-M.; Han, H.J.; Kim, W.-G.; Kim, E.; Kim, C.; Hong, S.W.; Kim, H.K.; Oh, J.-W.; Hwang, Y.-H. Bioinspired piezoelectric nanogenerators based on vertically aligned phage nanopillars. Energy Environ. Sci. 2015, 8, 3198–3203. [Google Scholar] [CrossRef]
- Lee, Y.J.; Lee, Y.; Oh, D.; Chen, T.; Ceder, G.; Belcher, A.M. Biologically Activated Noble Metal Alloys at the Nanoscale: For Lithium Ion Battery Anodes. Nano Lett. 2010, 10, 2433–2440. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, Y.; Huai, Y.; Wang, C.; Yi, W.; Mao, C. Evolutionary selection of personalized melanoma cell/tissue dual-homing peptides for guiding bionanofibers to malignant tumors. Chem. Commun. 2018, 54, 1631–1634. [Google Scholar] [CrossRef] [PubMed]
- Ma, K.; Wang, D.-D.; Lin, Y.; Wang, J.; Petrenko, V.; Mao, C. Synergetic Targeted Delivery of Sleeping-Beauty Transposon System to Mesenchymal Stem Cells Using LPD Nanoparticles Modified with a Phage-Displayed Targeting Peptide. Adv. Funct. Mater. 2013, 23, 1172–1181. [Google Scholar] [CrossRef] [PubMed]
- United States Environment Protection Agency. Office of Pesticide Programs, EPA 738-F-96-018. 1997. Available online: http://www.epa.gov/pesticides/reregistration/status.htm/ (accessed on 12 March 2019).
- Smith, L.L. Mechanism of Paraquat Toxicity in Lung and its Relevance to Treatment. Hum. Toxicol. 1987, 6, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Food and Agriculture Organization/World Health Organization. Pesticide Residues in Food—Joint FAO/WHO Meeting on Pesticide Residues: Paraquat. 2003. Available online: http://www.inchem.org/documents/jmpr/jmpmono/v2003pr08.htm (accessed on 12 March 2019).
- Yao, Z.; Hu, X.; Ma, W.; Chen, X.; Zhang, L.; Yu, J.; Zhao, Y.; Wu, H.-C. Colorimetric and fluorescent dual detection of paraquat and diquat based on an anionic polythiophene derivative. Analyst 2013, 138, 5572–5575. [Google Scholar] [CrossRef] [PubMed]
- Devaraj, V.; Han, J.; Kim, C.; Kang, Y.-C.; Oh, J.-W. Self-Assembled Nanoporous Biofilms from Functionalized Nanofibrous M13 Bacteriophage. Viruses 2018, 10, 322. [Google Scholar] [CrossRef]
- Yoon, I.; Kang, T.; Choi, W.; Kim, J.; Yoo, Y.; Joo, S.-W.; Park, Q.-H.; Ihee, H.; Kim, B. Single Nanowire on a Film as an Efficient SERS-Active Platform. J. Am. Chem. Soc. 2009, 131, 758–762. [Google Scholar] [CrossRef] [PubMed]
- Hua, X.; Liu, X.; Shi, H.; Wang, Y.; Kim, H.J.; Gee, S.J.; Wang, M.; Liu, F.; Hammock, B.D. Development of a heterologous enzyme-linked immunosorbent assay for organophosphorus pesticides with phage-borne peptide. RSC Adv. 2014, 4, 42445–42453. [Google Scholar] [CrossRef]
- Jaworski, J.W.; Raorane, D.; Huh, J.H.; Majumdar, A.; Lee, S.-W. Evolutionary Screening of Biomimetic Coatings for Selective Detection of Explosives. Langmuir 2008, 24, 4938–4943. [Google Scholar] [CrossRef]
- Xu, L.; Yan, W.; Ma, W.; Kuang, H.; Wu, X.; Liu, L.; Zhao, Y.; Wang, L.; Xu, C. SERS Encoded Silver Pyramids for Attomolar Detection of Multiplexed Disease Biomarkers. Adv. Mater. 2015, 27, 1706–1711. [Google Scholar] [CrossRef]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, J.-M.; Choi, E.J.; Park, J.; Devaraj, V.; Kim, C.; Han, J.; Kim, W.-G.; Kim, K.; Kang, Y.-C.; Kim, K.H.; et al. Improvement of High Affinity and Selectivity on Biosensors Using Genetically Engineered Phage by Binding Isotherm Screening. Viruses 2019, 11, 248. https://doi.org/10.3390/v11030248
Lee J-M, Choi EJ, Park J, Devaraj V, Kim C, Han J, Kim W-G, Kim K, Kang Y-C, Kim KH, et al. Improvement of High Affinity and Selectivity on Biosensors Using Genetically Engineered Phage by Binding Isotherm Screening. Viruses. 2019; 11(3):248. https://doi.org/10.3390/v11030248
Chicago/Turabian StyleLee, Jong-Min, Eun Jung Choi, Juyun Park, Vasanthan Devaraj, ChunTae Kim, Jiye Han, Won-Geun Kim, Kyujung Kim, Yong-Cheol Kang, Kwang Ho Kim, and et al. 2019. "Improvement of High Affinity and Selectivity on Biosensors Using Genetically Engineered Phage by Binding Isotherm Screening" Viruses 11, no. 3: 248. https://doi.org/10.3390/v11030248
APA StyleLee, J.-M., Choi, E. J., Park, J., Devaraj, V., Kim, C., Han, J., Kim, W.-G., Kim, K., Kang, Y.-C., Kim, K. H., & Oh, J.-W. (2019). Improvement of High Affinity and Selectivity on Biosensors Using Genetically Engineered Phage by Binding Isotherm Screening. Viruses, 11(3), 248. https://doi.org/10.3390/v11030248





