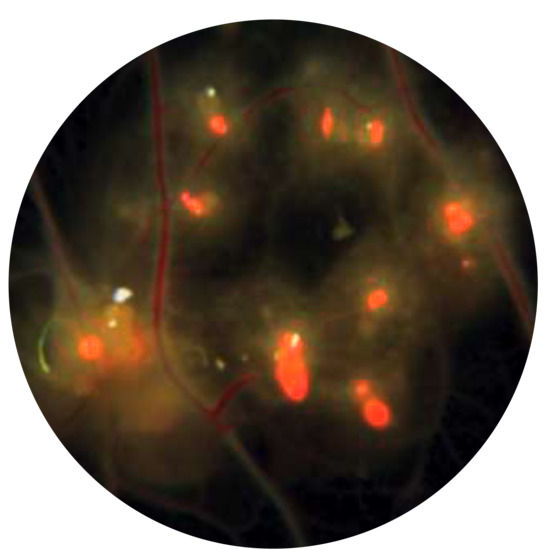
Tracking Modified Vaccinia Virus Ankara in the Chicken Embryo: In Vivo Tropism and Pathogenesis of Egg Infections
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Egg Preparation and CAM Infection
2.3. Necropsy and Imaging
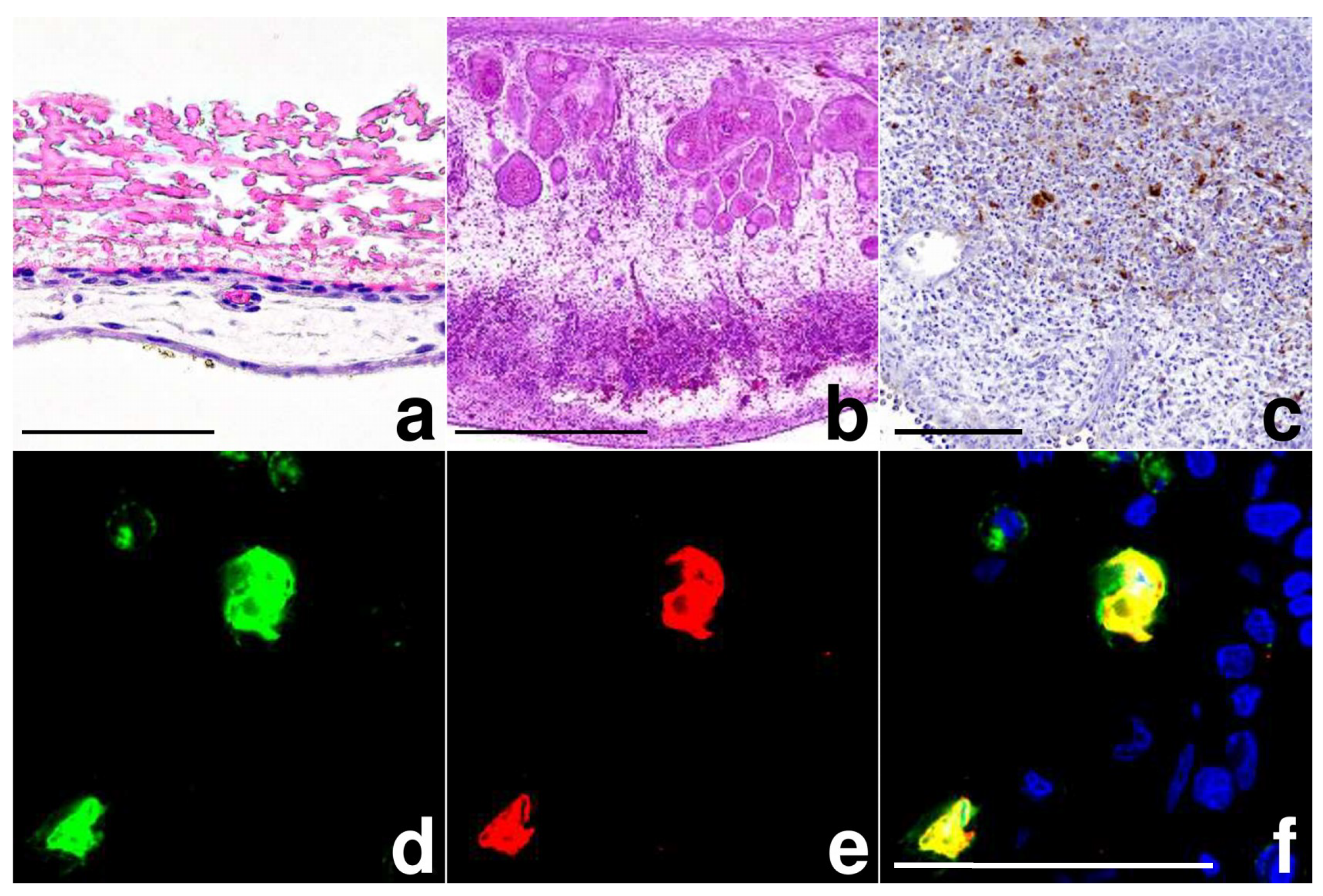
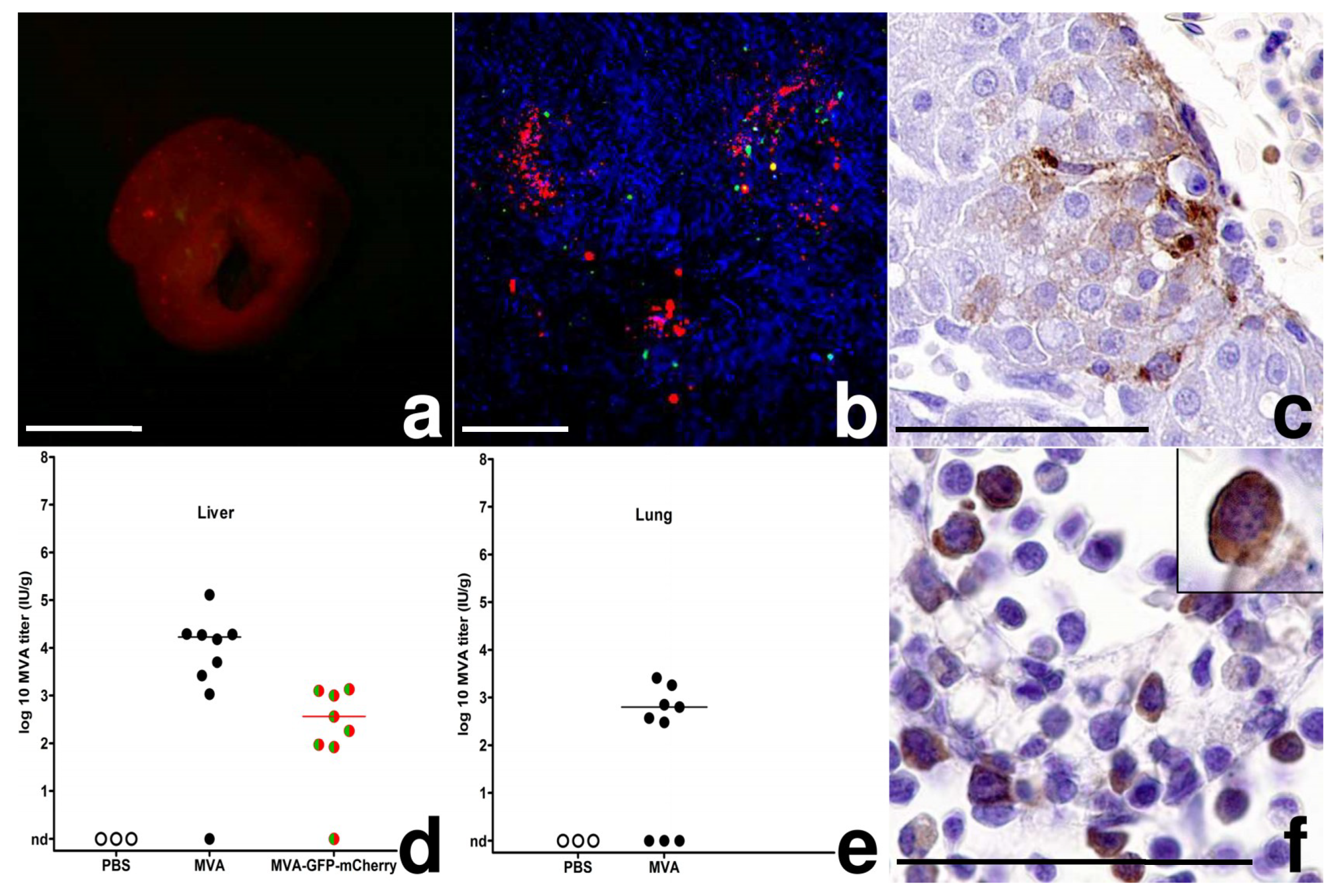
2.4. Histology and Immunohistochemistry
2.5. Virus Titration
2.6. Serum Analysis
2.7. Data Analysis
3. Results
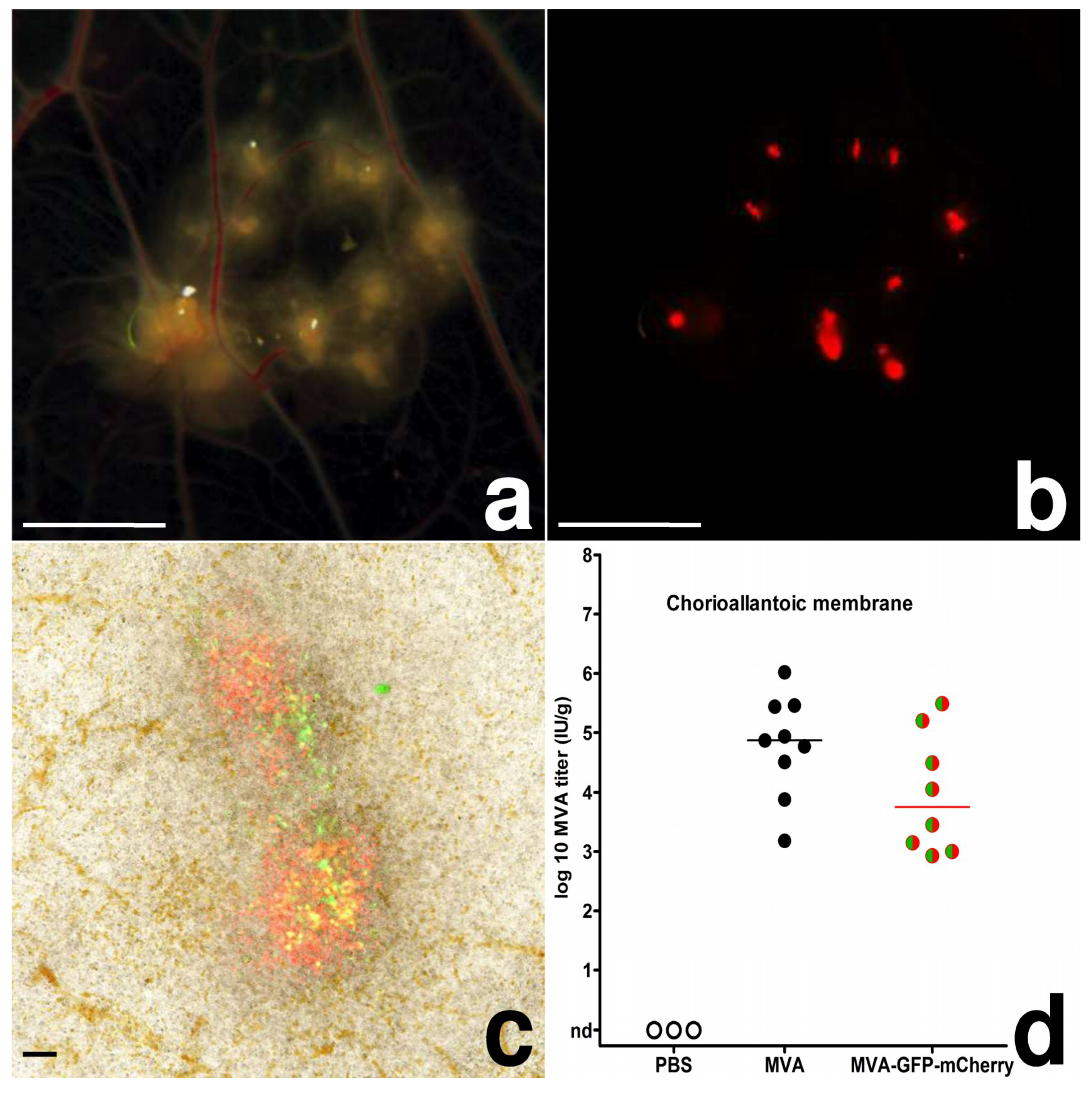
3.1. MVA Infection of the Chorioallantoic Membrane (CAM) in Chicken Eggs
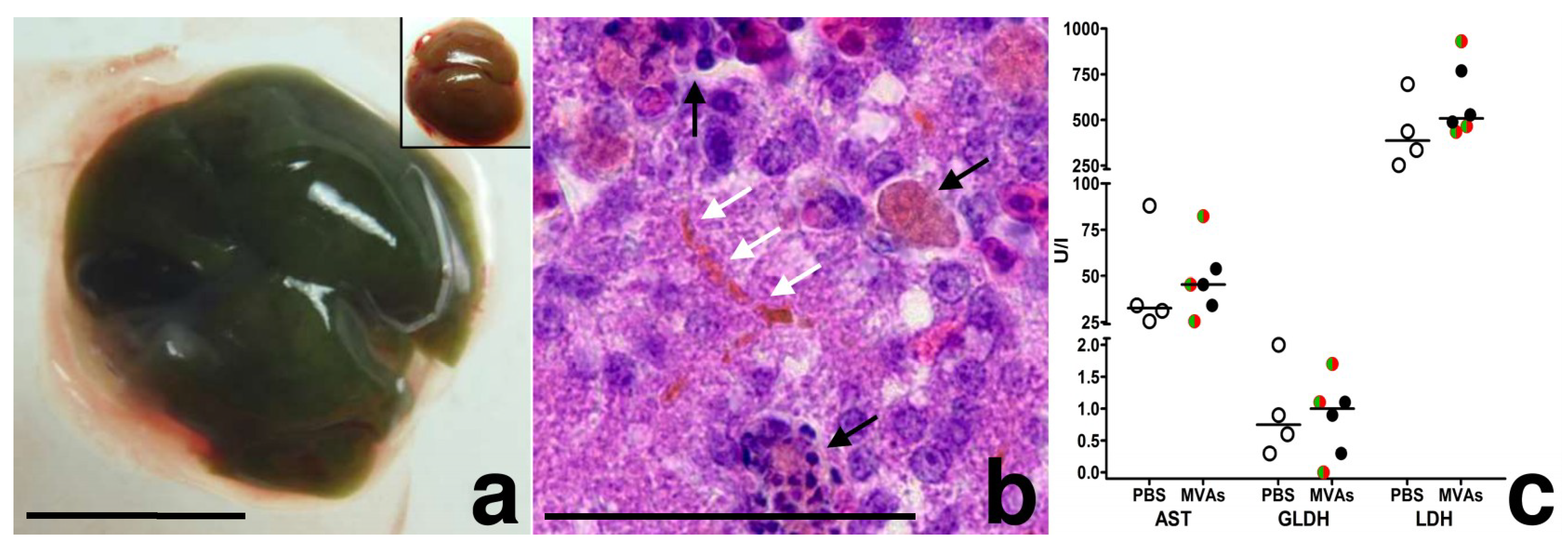
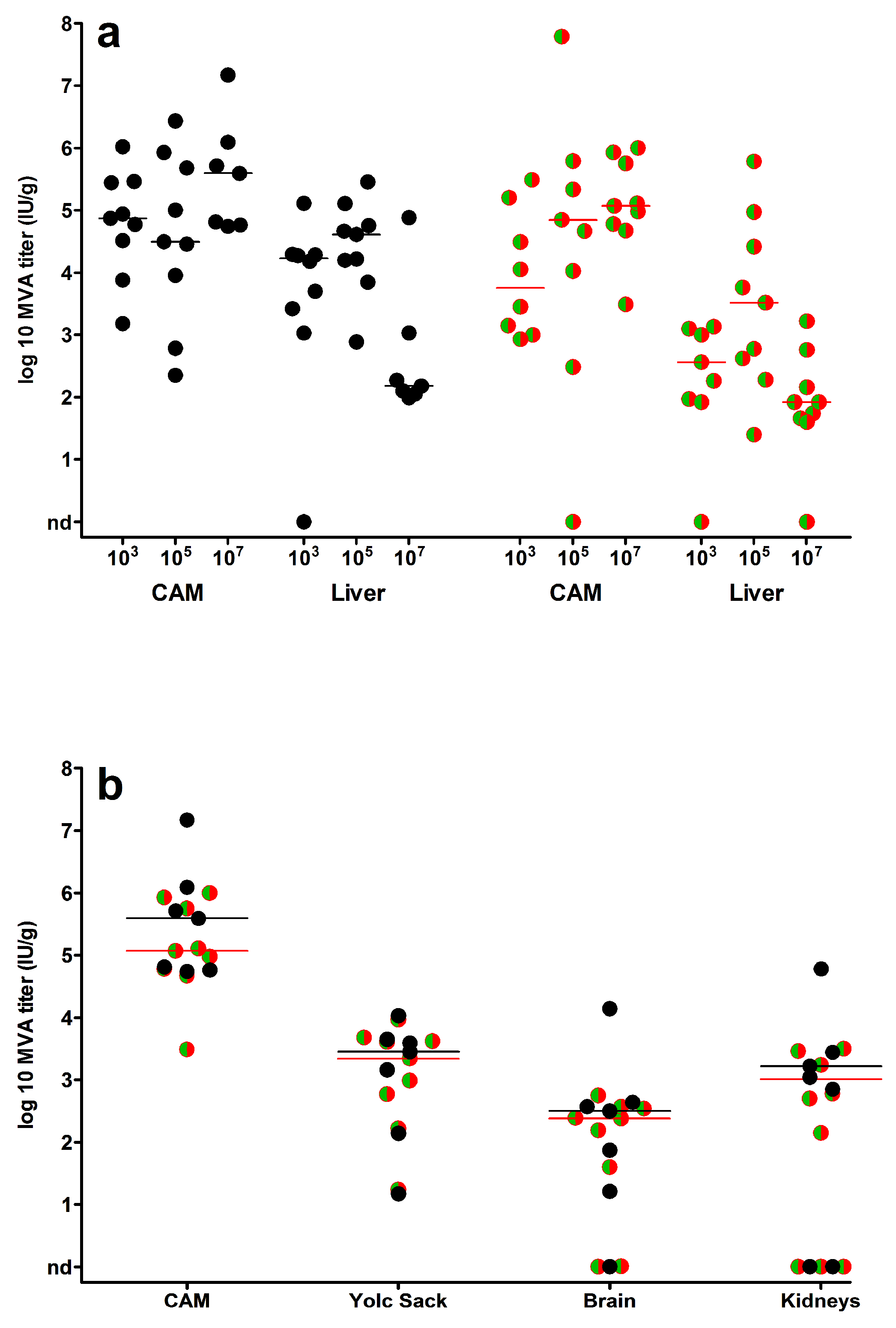
3.2. MVA Spread to the Chicken Embryo and the Extraembryonic Tissues
3.3. Egg Infections Using Escalating MVA Doses
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Mayr, A.; Munz, E. Veränderung von Vaccinevirus durch Dauerpassagen in Hühnerembryofibroblastenkulturen. Zbl. Bakt. I Orig. 1964, 195, 24–35. [Google Scholar]
- Mayr, A.; Hochstein-Mintzel, V.; Stickl, H. Abstammung, Eigenschaften und Verwendung des attenuierten Vaccinia-Stammes MVA. Infection 1975, 3, 6–14. [Google Scholar] [CrossRef]
- Antoine, G.; Scheiflinger, F.; Dorner, F.; Falkner, F.G. The complete genomic sequence of the modified vaccinia Ankara strain: Comparison with other orthopoxviruses. Virology 1998, 244, 365–396. [Google Scholar] [CrossRef] [PubMed]
- Meyer, H.; Sutter, G.; Mayr, A. Mapping of deletions in the genome of the highly attenuated vaccinia virus MVA and their influence on virulence. J. Gen. Virol. 1991, 72 Pt 5, 1031–1038. [Google Scholar] [CrossRef]
- Carroll, M.W.; Moss, B. Host range and cytopathogenicity of the highly attenuated MVA strain of vaccinia virus: Propagation and generation of recombinant viruses in a nonhuman mammalian cell line. Virology 1997, 238, 198–211. [Google Scholar] [CrossRef] [PubMed]
- Drexler, I.; Heller, K.; Wahren, B.; Erfle, V.; Sutter, G. Highly attenuated modified vaccinia virus Ankara replicates in baby hamster kidney cells, a potential host for virus propagation, but not in various human transformed and primary cells. J. Gen. Virol. 1998, 79 Pt 2, 347–352. [Google Scholar] [CrossRef]
- Sutter, G.; Moss, B. Nonreplicating vaccinia vector efficiently expresses recombinant genes. Proc. Natl. Acad. Sci. USA 1992, 89, 10847–10851. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, J.C.; Gherardi, M.M.; Esteban, M. Biology of attenuated modified vaccinia virus Ankara recombinant vector in mice: Virus fate and activation of B- and T-cell immune responses in comparison with the Western Reserve strain and advantages as a vaccine. J. Virol. 2000, 74, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Stittelaar, K.J.; Kuiken, T.; de Swart, R.L.; van Amerongen, G.; Vos, H.W.; Niesters, H.G.; van Schalkwijk, P.; van der Kwast, T.; Wyatt, L.S.; Moss, B.; et al. Safety of modified vaccinia virus Ankara (MVA) in immune-suppressed macaques. Vaccine 2001, 19, 3700–3709. [Google Scholar] [CrossRef]
- Volz, A.; Sutter, G. Modified Vaccinia Virus Ankara: History, Value in Basic Research, and Current Perspectives for Vaccine Development. Adv. Virus Res. 2017, 97, 187–243. [Google Scholar] [CrossRef] [PubMed]
- Veits, J.; Romer-Oberdorfer, A.; Helferich, D.; Durban, M.; Suezer, Y.; Sutter, G.; Mettenleiter, T.C. Protective efficacy of several vaccines against highly pathogenic H5N1 avian influenza virus under experimental conditions. Vaccine 2008, 26, 1688–1696. [Google Scholar] [CrossRef] [PubMed]
- Ducatez, M.F.; Becker, J.; Freudenstein, A.; Delverdier, M.; Delpont, M.; Sutter, G.; Guerin, J.L.; Volz, A. Low pathogenic avian influenza (H9N2) in chicken: Evaluation of an ancestral H9-MVA vaccine. Vet. Microbiol. 2016, 189, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Zanetti, F.A.; Del Medico Zajac, M.P.; Taboga, O.A.; Calamante, G. Evaluation of modified vaccinia virus Ankara expressing VP2 protein of infectious bursal disease virus as an immunogen in chickens. J. Vet. Sci. 2012, 13, 199–201. [Google Scholar] [CrossRef] [PubMed]
- Richetta, M.; Gomez, E.; Lucero, M.S.; Chimeno Zoth, S.; Gravisaco, M.J.; Calamante, G.; Berinstein, A. Comparison of homologous and heterologous prime-boost immunizations combining MVA-vectored and plant-derived VP2 as a strategy against IBDV. Vaccine 2017, 35, 142–148. [Google Scholar] [CrossRef] [PubMed]
- Boyd, A.C.; Ruiz-Hernandez, R.; Peroval, M.Y.; Carson, C.; Balkissoon, D.; Staines, K.; Turner, A.V.; Hill, A.V.; Gilbert, S.C.; Butter, C. Towards a universal vaccine for avian influenza: Protective efficacy of modified Vaccinia virus Ankara and Adenovirus vaccines expressing conserved influenza antigens in chickens challenged with low pathogenic avian influenza virus. Vaccine 2013, 31, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Stickl, H.; Hochstein-Mintzel, V.; Mayr, A.; Huber, H.C.; Schäfer, H.; Holzner, A. MVA-Stufenimpfung gegen Pocken. Klinische Erprobung des attenuierten Pocken-Lebendimpfstoffes, Stamm MVA. Dtsch. Med. Wochenschr. 1974, 99, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Lülf, A.T.; Freudenstein, A.; Marr, L.; Sutter, G.; Volz, A. Non-plaque-forming virions of Modified Vaccinia virus Ankara express viral genes. Virology 2016, 499, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Kremer, M.; Volz, A.; Kreijtz, J.H.; Fux, R.; Lehmann, M.H.; Sutter, G. Easy and efficient protocols for working with recombinant vaccinia virus MVA. Methods Mol. Biol. 2012, 890, 59–92. [Google Scholar] [CrossRef] [PubMed]
- Voss, H. Ein einfaches Hilfsmittel zur exakten histologischen Bearbeitung der normalen und infizierten Chorioallantois des bebrüteten Hühnereies. Zbl. Bakt. I Orig. 1953, 630, 643–646. [Google Scholar]
- Beckstead, J.H. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues: Addendum. J. Histochem. Cytochem. 1995, 43, 345. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Vara, J.A.; Miller, M.A. When tissue antigens and antibodies get along: Revisiting the technical aspects of immunohistochemistry—The red, brown, and blue technique. Vet. Pathol. 2014, 51, 42–87. [Google Scholar] [CrossRef] [PubMed]
- Herrlich, A.; Mayr, A. Die Differenzierung der Tierpockenviren im bebrüteten Hühnerei. Arch. Hyg. 1955, 139, 444–466. [Google Scholar]
- Goossens, M.; Pauwels, K.; Willemarck, N.; Breyer, D. Environmental risk assessment of clinical trials involving modified vaccinia virus Ankara (MVA)-based vectors. Curr. Gene Ther. 2013, 13, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Goodpasture, E.W.; Woodruff, A.M.; Buddingh, G.J. The Cultivation of Vaccine and Other Viruses in the Chorioallantoic Membrane of Chick Embryos. Science 1931, 74, 371–372. [Google Scholar] [CrossRef] [PubMed]
- Morgan, C.; Ellison, S.A.; Rose, H.M.; Moore, D.H. Structure and development of viruses observed in the electron microscope. II. Vaccinia and fowl pox viruses. J. Exp. Med. 1954, 100, 301–310. [Google Scholar] [CrossRef] [PubMed]
- Overman, J.R.; Tamm, I. Quantitative titration of vaccinia virus on the chorioallantoic membrane. J. Immunol. 1956, 76, 228–236. [Google Scholar] [PubMed]
- Beckstead, J.H. A simple technique for preservation of fixation-sensitive antigens in paraffin-embedded tissues. J. Histochem. Cytochem. 1994, 42, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Mori, H.; Soonsawad, P.; Schuetter, L.; Chen, Q.; Hubbard, N.E.; Cardiff, R.D.; Borowsky, A.D. Introduction of zinc-salt fixation for effective detection of immune cell-related markers by immunohistochemistry. Toxicol. Pathol. 2015, 43, 883–889. [Google Scholar] [CrossRef] [PubMed]
- Mast, J.; Goddeeris, B.M.; Peeters, K.; Vandesande, F.; Berghman, L.R. Characterisation of chicken monocytes, macrophages and interdigitating cells by the monoclonal antibody KUL01. Vet. Immunol. Immunopathol. 1998, 61, 343–357. [Google Scholar] [CrossRef]
- Sanchez-Puig, J.M.; Sanchez, L.; Roy, G.; Blasco, R. Susceptibility of different leukocyte cell types to Vaccinia virus infection. Virol. J. 2004, 1, 10. [Google Scholar] [CrossRef] [PubMed]
- Kastenmuller, W.; Drexler, I.; Ludwig, H.; Erfle, V.; Peschel, C.; Bernhard, H.; Sutter, G. Infection of human dendritic cells with recombinant vaccinia virus MVA reveals general persistence of viral early transcription but distinct maturation-dependent cytopathogenicity. Virology 2006, 350, 276–288. [Google Scholar] [CrossRef] [PubMed]
- Altenburg, A.F.; van de Sandt, C.E.; Li, B.W.S.; MacLoughlin, R.J.; Fouchier, R.A.M.; van Amerongen, G.; Volz, A.; Hendriks, R.W.; de Swart, R.L.; Sutter, G.; et al. Modified Vaccinia Virus Ankara preferentially targets antigen presenting cells in vitro, ex vivo and in vivo. Sci. Rep. 2017, 7, 8580. [Google Scholar] [CrossRef] [PubMed]
- Burton, F.G.; Tullett, S.G. Respiration of avian embryos. Comp. Biochem. Physiol. 1985, 82, 735–744. [Google Scholar] [CrossRef]
- Van Golde, J.; Mulder, T.; Straaten, H.; Blanco, C.E. The chorioallantoic artery blood flow of the chick embryo from stage 34 to 43. Pediatr. Res. 1996, 40, 867–871. [Google Scholar] [CrossRef] [PubMed]
- Fellah, J.S.; Jaffredo, T.; Nagy, N.; Dunon, D. Development of the avian immune system. In Avian Immunology; Schat, K.A., Kaspers, B., Kaiser, P., Eds.; Elsevier: San Diego, CA, USA, 2014. [Google Scholar]
- Zon, L.I. Developmental biology of hematopoiesis. Blood 1995, 86, 2876–2891. [Google Scholar] [PubMed]
- Lin, G.L.; Himes, J.A.; Cornelius, C.E. Bilirubin and biliverdin excretion by the chicken. Am. J. Physiol. 1974, 226, 881–885. [Google Scholar] [CrossRef] [PubMed]
- Desmet, V.J. Ductal plates in hepatic ductular reactions. Hypothesis and implications. I. Types of ductular reaction reconsidered. Virchows Arch. 2011, 458, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Bhogal, H.K.; Sanyal, A.J. The molecular pathogenesis of cholestasis in sepsis. Front. Biosci. 2013, 5, 87–96. [Google Scholar] [CrossRef]
- Gilroy, R.K.; Mailliard, M.E.; Gollan, J.L. Gastrointestinal disorders of the critically ill. Cholestasis of sepsis. Best Pract. Res. Clin. Gastroenterol. 2003, 17, 357–367. [Google Scholar] [CrossRef]
- Becker, S.; Quay, J.; Soukup, J. Cytokine (tumor necrosis factor, IL-6, and IL-8) production by respiratory syncytial virus-infected human alveolar macrophages. J. Immunol. 1991, 147, 4307–4312. [Google Scholar] [PubMed]
- Cheung, C.Y.; Poon, L.L.; Lau, A.S.; Luk, W.; Lau, Y.L.; Shortridge, K.F.; Gordon, S.; Guan, Y.; Peiris, J.S. Induction of proinflammatory cytokines in human macrophages by influenza A (H5N1) viruses: A mechanism for the unusual severity of human disease? Lancet 2002, 360, 1831–1837. [Google Scholar] [CrossRef]
- Gomez-Villamandos, J.C.; Bautista, M.J.; Sanchez-Cordon, P.J.; Carrasco, L. Pathology of African swine fever: The role of monocyte-macrophage. Virus Res. 2013, 173, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.H.; Kastenmuller, W.; Kandemir, J.D.; Brandt, F.; Suezer, Y.; Sutter, G. Modified vaccinia virus ankara triggers chemotaxis of monocytes and early respiratory immigration of leukocytes by induction of CCL2 expression. J. Virol. 2009, 83, 2540–2552. [Google Scholar] [CrossRef] [PubMed]
- Delaloye, J.; Roger, T.; Steiner-Tardivel, Q.G.; Le Roy, D.; Knaup Reymond, M.; Akira, S.; Petrilli, V.; Gomez, C.E.; Perdiguero, B.; Tschopp, J.; et al. Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 2009, 5, e1000480. [Google Scholar] [CrossRef] [PubMed]
- Zimmerling, S.; Waibler, Z.; Resch, T.; Sutter, G.; Schwantes, A. Interleukin-1beta receptor expressed by modified vaccinia virus Ankara interferes with interleukin-1β activity produced in various virus-infected antigen-presenting cells. Virol. J. 2013, 10, 34. [Google Scholar] [CrossRef] [PubMed]
- Fredrickson, T.N.; Sechler, J.M.; Palumbo, G.J.; Albert, J.; Khairallah, L.H.; Buller, R.M. Acute inflammatory response to cowpox virus infection of the chorioallantoic membrane of the chick embryo. Virology 1992, 187, 693–704. [Google Scholar] [CrossRef]
- Abdul-Careem, M.F.; Hunter, D.B.; Lambourne, M.D.; Barta, J.; Sharif, S. Ontogeny of cytokine gene expression in the chicken spleen. Poult. Sci. 2007, 86, 1351–1355. [Google Scholar] [CrossRef] [PubMed]
- Lowenthal, J.W.; Connick, T.E.; McWaters, P.G.; York, J.J. Development of T cell immune responsiveness in the chicken. Immunol. Cell Biol. 1994, 72, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.A.; Browning, G.F.; Washington, E.A.; Crabb, B.S.; Kaiser, P. Embryonic age influences the capacity for cytokine induction in chicken thymocytes. Immunology 2003, 110, 358–367. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langenmayer, M.C.; Lülf-Averhoff, A.-T.; Adam-Neumair, S.; Sutter, G.; Volz, A. Tracking Modified Vaccinia Virus Ankara in the Chicken Embryo: In Vivo Tropism and Pathogenesis of Egg Infections. Viruses 2018, 10, 452. https://doi.org/10.3390/v10090452
Langenmayer MC, Lülf-Averhoff A-T, Adam-Neumair S, Sutter G, Volz A. Tracking Modified Vaccinia Virus Ankara in the Chicken Embryo: In Vivo Tropism and Pathogenesis of Egg Infections. Viruses. 2018; 10(9):452. https://doi.org/10.3390/v10090452
Chicago/Turabian StyleLangenmayer, Martin C., Anna-Theresa Lülf-Averhoff, Silvia Adam-Neumair, Gerd Sutter, and Asisa Volz. 2018. "Tracking Modified Vaccinia Virus Ankara in the Chicken Embryo: In Vivo Tropism and Pathogenesis of Egg Infections" Viruses 10, no. 9: 452. https://doi.org/10.3390/v10090452
APA StyleLangenmayer, M. C., Lülf-Averhoff, A.-T., Adam-Neumair, S., Sutter, G., & Volz, A. (2018). Tracking Modified Vaccinia Virus Ankara in the Chicken Embryo: In Vivo Tropism and Pathogenesis of Egg Infections. Viruses, 10(9), 452. https://doi.org/10.3390/v10090452