Honey Bee and Bumble Bee Antiviral Defense
Abstract
1. Introduction
1.1. Bees—Hymenopteran Insects That Play an Important Ecological Role as Plant Pollinators
1.2. Bee Viruses
1.3. Bee Virology
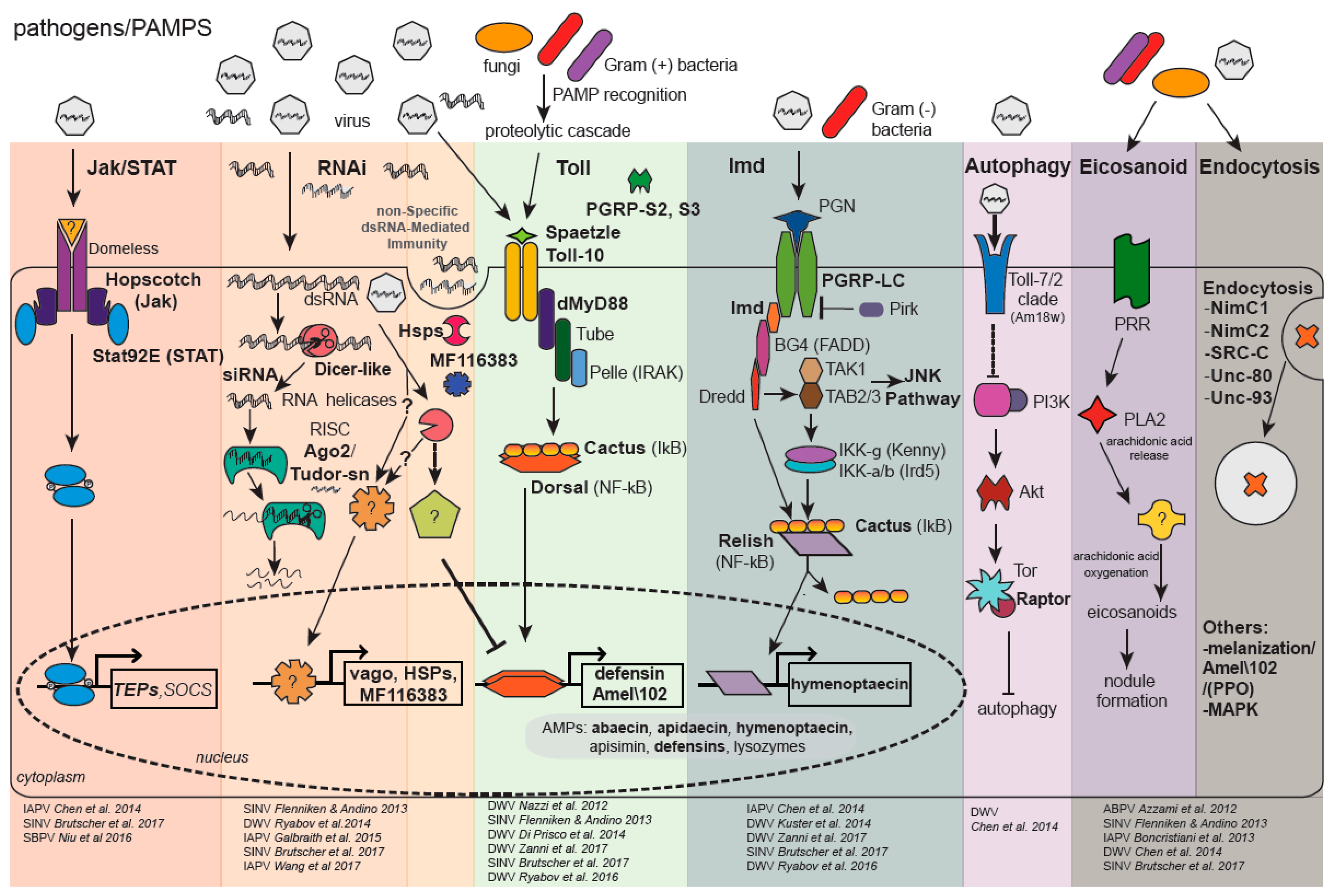
2. Bee Antiviral Defense
2.1. Viral dsRNA-Triggered Antiviral Responses in Honey Bees and Bumble Bees, Including Sequence-Specific RNAi and Non-Sequence-Specific Pathways
2.2. Honey Bee Antiviral RNA Interference
2.3. Honey Bee Non-Sequence-Specific dsRNA-Mediated Antiviral Response
2.4. Bumble Bee RNA Interference
2.5. Bumble Bee Non-Sequence-Specific dsRNA-Mediated Antiviral Response
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Michener, C.D. The Bees of the World, 2nd ed.; John Hopkins University Press: Baltimore, MD, USA; London, UK, 2007. [Google Scholar]
- Calderone, N.W. Insect pollinated crops, insect pollinators and us agriculture: Trend analysis of aggregate data for the period 1992–2009. PLoS ONE 2012, 7, e37235. [Google Scholar] [CrossRef] [PubMed]
- Gallai, N.; Salles, J.-M.; Settele, J.; Vaissière, B.E. Economic valuation of the vulnerability of world agriculture confronted with pollinator decline. Ecol. Econ. 2009, 68, 810–821. [Google Scholar] [CrossRef]
- Cridland, J.M.; Tsutsui, N.D.; Ramirez, S.R. The complex demographic history and evolutionary origin of the western honey bee, Apis mellifera. Genome Biol. Evol. 2017, 9, 457–472. [Google Scholar] [CrossRef] [PubMed]
- Sheppard, W.S. A history of the introduction of honey bee races into the United-States 2. Am. Bee J. 1989, 129, 664–667. [Google Scholar]
- Han, F.; Wallberg, A.; Webster, M.T. From where did the western honeybee (Apis mellifera) originate? Ecol. Evol. 2012, 2, 1949–1957. [Google Scholar] [CrossRef] [PubMed]
- Diao, Q.; Sun, L.; Zheng, H.; Zeng, Z.; Wang, S.; Xu, S.; Zheng, H.; Chen, Y.; Shi, Y.; Wang, Y.; et al. Genomic and transcriptomic analysis of the asian honeybee Apis cerana provides novel insights into honeybee biology. Sci. Rep. 2018, 8, 822. [Google Scholar] [CrossRef] [PubMed]
- Government, T.A. Status of the Asian Honey Bee in Mainland Australia. Available online: http://www.agriculture.gov.au/pests-diseases-weeds/bees/the-asian-honey-bee-in-australia (accessed on 2 July 2018).
- Winston, M.L. The Biology of the Honey Bee; Harvard University Press: Cambridge, MA, USA, 1991. [Google Scholar]
- Van Engelsdorp, D.; Meixner, M.D. A historical review of managed honey bee populations in Europe and the United States and the factors that may affect them. J. Invertebr. Pathol. 2010, 103, S80–S95. [Google Scholar] [CrossRef] [PubMed]
- Flenniken, M.L. Almond Almanac; Almond Board of California: Modesto, CA, USA, 2014. [Google Scholar]
- Traynor, K.S.; Rennich, K.; Forsgren, E.; Rose, R.; Pettis, J.; Kunkel, G.; Madella, S.; Evans, J.; Lopez, D.; van Engelsdorp, D. Multiyear survey targeting disease incidence in US honey bees. Apidologie 2016, 47, 325–347. [Google Scholar] [CrossRef]
- Spleen, A.M.; Lengerich, E.J.; Rennich, K.; Caron, D.; Rose, R.; Pettis, J.S.; Henson, M.; Wilkes, J.T.; Wilson, M.; Stitzinger, J.; et al. A national survey of managed honey bee 2011–2012 winter colony losses in the United States: Results from the bee informed partnership. J. Apicult. Res. 2015, 52, 44–53. [Google Scholar] [CrossRef]
- Steinhauer, N.A.; Rennich, K.; Wilson, M.E.; Caron, D.M.; Lengerich, E.J.; Pettis, J.S.; Rose, R.; Skinner, J.A.; Tarpy, D.R.; Wilkes, J.T.; et al. A national survey of managed honey bee 2012–2013 annual colony losses in the USA: Results from the bee informed partnership. J. Apicult. Res. 2015, 53, 1–18. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Caron, D.; Hayes, J.; Underwood, R.; Henson, M.; Rennich, K.; Spleen, A.; Andree, M.; Snyder, R.; Lee, K.; et al. A national survey of managed honey bee 2010–2011 winter colony losses in the USA: Results from the bee informed partnership. J. Apicult. Res. 2015, 51, 115–124. [Google Scholar] [CrossRef]
- Van Engelsdorp, D.; Evans, J.D.; Saegerman, C.; Mullin, C.; Haubruge, E.; Nguyen, B.K.; Frazier, M.; Frazier, J.; Cox-Foster, D.; Chen, Y.; et al. Colony collapse disorder: A descriptive study. PLoS ONE 2009, 4, e6481. [Google Scholar]
- Van Engelsdorp, D.; Hayes, J.; Underwood, R.M.; Pettis, J. A survey of honey bee colony losses in the U.S., fall 2007 to spring 2008. PLoS ONE 2008, 3, e4071. [Google Scholar]
- McMenamin, A.J.; Brutscher, L.M.; Glenny, W.; Flenniken, M.L. Abiotic and biotic factors affecting the replication and pathogenicity of bee viruses. Curr. Opin. Insect Sci. 2016, 16, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Vaudo, A.D.; Tooker, J.F.; Grozinger, C.M.; Patch, H.M. Bee nutrition and floral resource restoration. Curr. Opin. Insect Sci. 2015, 10, 133–141. [Google Scholar] [CrossRef] [PubMed]
- DeGrandi-Hoffman, G.; Chen, Y. Nutrition, immunity and viral infections in honey bees. Curr. Opin. Insect Sci. 2015, 10, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Barron, A.B. Death of the bee hive: Understanding the failure of an insect society. Curr. Opin. Insect Sci. 2015, 10, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M. Honey bee toxicology. Annu. Rev. Entomol. 2015, 60, 415–434. [Google Scholar] [CrossRef] [PubMed]
- Johnson, R.M.; Ellis, M.D.; Mullin, C.A.; Frazier, M. Pesticides and honey bee toxicity—USA. Apidologie 2010, 41, 312–331. [Google Scholar] [CrossRef]
- Simone-Finstrom, M.; Li-Byarlay, H.; Huang, M.H.; Strand, M.K.; Rueppell, O.; Tarpy, D.R. Migratory management and environmental conditions affect lifespan and oxidative stress in honey bees. Sci. Rep. 2016, 6, 810. [Google Scholar] [CrossRef] [PubMed]
- Velthuis, H.H.W.; van Doorn, A. A century of advances in bumblebee domestication and the economic and environmental aspects of its commercialization for pollination. Apidologie 2006, 37, 421–451. [Google Scholar] [CrossRef]
- Goulson, D. Bumblebees: Their Behaviour and Ecology; Oxford University Press: Oxford, UK; New York, NY, USA, 2003. [Google Scholar]
- Goulson, D. Effects of introduced bees on native ecosystems. Annu. Rev. Ecol. Evol. Syst. 2003, 34, 1–26. [Google Scholar] [CrossRef]
- Amsalem, E.; Galbraith, D.A.; Cnaani, J.; Teal, P.E.A.; Grozinger, C.M. Conservation and modification of genetic and physiological toolkits underpinning diapause in bumble bee queens. Mol. Ecol. 2015, 24, 5596–5615. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Grozinger, C.M.; Padilla, M.; Hefetz, A. The physiological and genomic bases of bumble bee social behaviour. In Genomics, Physiology and Behaviour of Social Insects; Elsevier: London, UK, 2015; Volume 48, pp. 37–93. [Google Scholar]
- Owen, R.E.; Otterstatter, M.C.; Carter, R.V.; Farmer, A.; Colla, S.R.; O’Toole, N. Significant expansion of the distribution of the bumble bee Bombus moderatus (hymenoptera: Apidae) in alberta over 20 years. Can. J. Zool.-Revue Can. Zool. 2012, 90, 133–138. [Google Scholar] [CrossRef]
- Burkle, L.A.; Marlin, J.C.; Knight, T.M. Plant-pollinator interactions over 120 years: Loss of species, co-occurrence, and function. Science 2013, 339, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Cameron, S.A.; Lozier, J.D.; Strange, J.P.; Koch, J.B.; Cordes, N.; Solter, L.F.; Griswold, T.L. Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. USA 2011, 108, 662–667. [Google Scholar] [CrossRef] [PubMed]
- Goulson, D.; Nicholls, E.; Botias, C.; Rotheray, E.L. Bee declines driven by combined stress from parasites, pesticides, and lack of flowers. Science 2015, 347, 1255957. [Google Scholar] [PubMed]
- Hatten, T.D.; Looney, C.; Strange, J.P.; Bosque-Perez, N.A. Bumble bee fauna of palouse prairie: Survey of native bee pollinators in a fragmented ecosystem. J. Insect Sci. 2013, 13, 26. [Google Scholar] [CrossRef] [PubMed]
- Woodard, S.H. Bumble bee ecophysiology: Integrating the changing environment and the organism. Curr. Opin. Insect Sci. 2017, 22, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Memmott, J.; Craze, P.G.; Waser, N.M.; Price, M.V. Global warming and the disruption of plant-pollinator interactions. Ecol. Lett. 2007, 10, 710–717. [Google Scholar] [CrossRef] [PubMed]
- Meeus, I.; Pisman, M.; Smagghe, G.; Piot, N. Interaction effects of different drivers of wild bee decline and their influence on host-pathogen dynamics. Curr. Opin. Insect Sci. 2018, 26, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Koch, H.; Schmid-Hempel, P. Socially transmitted gut microbiota protect bumble bees against an intestinal parasite. Proc. Natl. Acad. Sci. USA 2011, 108, 19288–19292. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Schmid-Hempel, P. Qualitatively different immune response of the bumblebee host, Bombus terrestris, to infection by different genotypes of the trypanosome gut parasite, Crithidia bombi. Infect. Genet. Evol. 2013, 20, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Cordes, N.; Huang, W.-F.; Strange, J.P.; Cameron, S.A.; Griswold, T.L.; Lozier, J.D.; Solter, L.F. Interspecific geographic distribution and variation of the pathogens Nosema bombi and crithidia species in United States bumble bee populations. J. Invertebr. Pathol. 2012, 109, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Barribeau, S.M.; Sadd, B.M.; du Plessis, L.; Schmid-Hempel, P. Gene expression differences underlying genotype-by-genotype specificity in a host–parasite system. Proc. Natl. Acad. Sci. USA 2014, 111, 3496–3501. [Google Scholar] [CrossRef] [PubMed]
- Imhoof, B.; Schmid-Hempel, P. Colony success of the bumble bee, Bombus terrestris, in relation to infections by two protozoan parasites, Crithidia bombi and Nosema bombi. Insectes Soc. 2014, 46, 233–238. [Google Scholar] [CrossRef]
- Parmentier, L.; Smagghe, G.; de Graaf, D.C.; Meeus, I. Varroa destructor macula-like virus, lake sinai virus and other new RNA viruses in wild bumblebee hosts (Bombus pascuorum, Bombus lapidarius, and Bombus pratorum). J. Invertebr. Pathol. 2016, 134, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Smagghe, G.; De Coninck, D.I.M.; Van Nieuwerburgh, F.; Deforce, D.; Meeus, I. In vivo study of dicer-2-mediated immune response of the small interfering rna pathway upon systemic infections of virulent and avirulent viruses in Bombus terrestris. Insect Biochem. Mol. Biol. 2016, 70, 127–137. [Google Scholar] [CrossRef] [PubMed]
- Meeus, I.; Brown, M.J.F.; de Graaf, D.C.; Smagghe, G. Effects of invasive parasites on bumble bee declines. Conserv. Biol. 2011, 25, 662–671. [Google Scholar] [CrossRef] [PubMed]
- Meeus, I.; de Miranda, J.R.; de Graaf, D.C.; Wäckers, F.; Smagghe, G. Effect of oral infection with kashmir bee virus and israeli acute paralysis virus on bumblebee (Bombus terrestris) reproductive success. J. Invertebr. Pathol. 2014, 121, 64–69. [Google Scholar] [CrossRef] [PubMed]
- Piot, N.; Snoeck, S.; Vanlede, M.; Smagghe, G.; Meeus, I. The effect of oral administration of dsrna on viral replication and mortality in Bombus terrestris. Viruses 2015, 7, 3172–3185. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Cappelle, K.; de Miranda, J.R.; Smagghe, G.; Meeus, I. Analysis of reference gene stability after israeli acute paralysis virus infection in bumblebees Bombus terrestris. J. Invertebr. Pathol. 2014, 115, 76–79. [Google Scholar] [CrossRef] [PubMed]
- Schoonvaere, K.; Smagghe, G.; Francis, F.; de Graaf, D.C. Study of the metatranscriptome of eight social and solitary wild bee species reveals novel viruses and bee parasites. Front. Microbiol. 2018, 9, 754. [Google Scholar] [CrossRef] [PubMed]
- Sagili, R.R.; Burgett, D.M. Evaluating Honey Bee Colonies for Pollination: A Guide for Commercial Growers and Beekeepers; Oregon State University Extension Service: Corvallis, OR, USA, 2011; pp. 1–8. [Google Scholar]
- Amiri, E.; Meixner, M.; Nielsen, S.L.; Kryger, P. Four categories of viral infection describe the health status of honey bee colonies. PLoS ONE 2015, 10, e0140272. [Google Scholar] [CrossRef] [PubMed]
- Pettis, J.S.; Rice, N.; Joselow, K.; vanEngelsdorp, D.; Chaimanee, V. Colony failure linked to low sperm viability in honey bee (Apis mellifera) queens and an exploration of potential causative factors. PLoS ONE 2016, 11, e0147220. [Google Scholar]
- Nguyen, B.K.; Ribière, M.; vanEngelsdorp, D.; Snoeck, C.; Saegerman, C.; Kalkstein, A.L.; Schurr, F.; Brostaux, Y.; Faucon, J.-P.; Haubruge, E. Effects of honey bee virus prevalence, Varroa destructor load and queen condition on honey bee colony survival over the winter in Belgium. J. Apicult. Res. 2015, 50, 195–202. [Google Scholar] [CrossRef]
- Glenny, W.; Cavigli, I.; Daughenbaugh, K.F.; Radford, R.; Kegley, S.E.; Flenniken, M.L. Honey bee (Apis mellifera) colony health and pathogen composition in migratory beekeeping operations involved in California almond pollination. PLoS ONE 2017, 12, e0182814. [Google Scholar] [CrossRef] [PubMed]
- Cavigli, I.; Daughenbaugh, K.F.; Martin, M.; Lerch, M.; Banner, K.; Garcia, E.; Brutscher, L.M.; Flenniken, M.L. Pathogen prevalence and abundance in honey bee colonies involved in almond pollination. Apidologie 2016, 47, 251–266. [Google Scholar] [CrossRef] [PubMed]
- Brosi, B.J. Pollinator specialization: From the individual to the community. New Phytol. 2016, 210, 1190–1194. [Google Scholar] [CrossRef] [PubMed]
- Garibaldi, L.A.; Steffan-Dewenter, I.; Winfree, R.; Aizen, M.A.; Bommarco, R.; Cunningham, S.A.; Kremen, C.; Carvalheiro, L.G.; Harder, L.D.; Afik, O.; et al. Wild pollinators enhance fruit set of crops regardless of honey bee abundance. Science 2013, 339, 1608–1611. [Google Scholar] [CrossRef] [PubMed]
- Greenleaf, S.S.; Kremen, C. Wild bees enhance honey bees’ pollination of hybrid sunflower. Proc. Natl. Acad. Sci. USA 2006, 103, 13890–13895. [Google Scholar] [CrossRef] [PubMed]
- Brittain, C.; Williams, N.; Kremen, C.; Klein, A.M. Synergistic effects of non-apis bees and honey bees for pollination services. Proc. R. Soc. B-Biol. Sci. 2013, 280, 20122767. [Google Scholar] [CrossRef] [PubMed]
- Winfree, R.; Williams, N.M.; Dushoff, J.; Kremen, C. Native bees provide insurance against ongoing honey bee losses. Ecol. Lett. 2007, 10, 1105–1113. [Google Scholar] [CrossRef] [PubMed]
- Dolezal, A.G.; Hendrix, S.D.; Scavo, N.A.; Carrillo-Tripp, J.; Harris, M.A.; Wheelock, M.J.; O’Neal, M.E.; Toth, A.L. Honey bee viruses in wild bees: Viral prevalence, loads, and experimental inoculation. PLoS ONE 2016, 11, e0166190. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Levitt, A.L.; Rajotte, E.G.; Holmes, E.C.; Ostiguy, N.; vanEngelsdorp, D.; Lipkin, W.I.; Depamphilis, C.W.; Toth, A.L.; Cox-Foster, D.L. RNA viruses in hymenopteran pollinators: Evidence of inter-taxa virus transmission via pollen and potential impact on non-apis hymenopteran species. PLoS ONE 2010, 5, e14357. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Fürst, M.A.; Caspar, J.; Theodorou, P.; Brown, M.J.F.; Paxton, R.J. A sting in the spit: Widespread cross-infection of multiple rna viruses across wild and managed bees. J. Anim. Ecol. 2015, 84, 615–624. [Google Scholar] [CrossRef] [PubMed]
- Fürst, M.A.; McMahon, D.P.; Osborne, J.L.; Paxton, R.J.; Brown, M.J.F. Disease associations between honeybees and bumblebees as a threat to wild pollinators. Nature 2014, 506, 364–366. [Google Scholar] [CrossRef] [PubMed]
- Levitt, A.L.; Singh, R.; Cox-Foster, D.L.; Rajotte, E.; Hoover, K.; Ostiguy, N.; Holmes, E.C. Cross-species transmission of honey bee viruses in associated arthropods. Virus Res. 2013, 176, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Tehel, A.; Brown, M.J.; Paxton, R.J. Impact of managed honey bee viruses on wild bees. Curr. Opin. Virol. 2016, 19, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Bigot, D.; Dalmon, A.; Roy, B.; Hou, C.; Germain, M.; Romary, M.; Deng, S.; Diao, Q.; Weinert, L.A.; Cook, J.M.; et al. The discovery of halictivirus resolves the sinaivirus phylogeny. J. Gen. Virol. 2017, 98, 2864–2875. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; McMenamin, A.J.; Flenniken, M.L. The buzz about honey bee viruses. PLoS Pathog. 2016, 12, e1005757. [Google Scholar] [CrossRef] [PubMed]
- Boncristiani, H.F.; Di Prisco, G.; Pettis, J.S.; Hamilton, M.; Chen, Y. Molecular approaches to the analysis of deformed wing virus replication and pathogenesis in the honey bee, Apis mellifera. Virol. J. 2009, 6, 221. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Siede, R. Honey bee viruses. In Advances in Virus Research; Elsevier: Amsterdam, The Netherlands, 2007; Volume 70, pp. 33–80. [Google Scholar]
- Genersch, E.; Aubert, M. Emerging and re-emerging viruses of the honey bee (Apis mellifera L.). Vet. Res. 2010, 41, 54. [Google Scholar] [CrossRef] [PubMed]
- McMenamin, A.J.; Flenniken, M.L. Recently identified bee viruses and their impact on bee pollinators. Curr. Opin. Insect Sci. 2018, 26, 120–129. [Google Scholar] [CrossRef] [PubMed]
- Remnant, E.J.; Shi, M.; Buchmann, G.; Blacquiere, T.; Holmes, E.C.; Beekman, M.; Ashe, A. A diverse range of novel rna viruses in geographically distinct honey bee populations. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Cornman, S.; Hartmann, U.; Cousserans, F.; Evans, J.; de Miranda, J.; Neumann, P. The Apis mellifera filamentous virus genome. Viruses 2015, 7, 3798–3815. [Google Scholar] [CrossRef] [PubMed]
- Grozinger, C.M.; Flenniken, M.L. Bee viruses: Ecology, pathogenicity, and impacts. Annu. Rev. Entomol. 2018, in press. [Google Scholar]
- Mazzei, M.; Carrozza, M.L.; Luisi, E.; Forzan, M.; Giusti, M.; Sagona, S.; Tolari, F.; Felicioli, A. Infectivity of dwv associated to flower pollen: Experimental evidence of a horizontal transmission route. PLoS ONE 2014, 9, e113448. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Evans, J.; Feldlaufer, M. Horizontal and vertical transmission of viruses in the honey bee, Apis mellifera. J. Invertebr. Pathol. 2006, 92, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Shen, M. Intricate transmission routes and interactions between picorna-like viruses (kashmir bee virus and sacbrood virus) with the honeybee host and the parasitic varroa mite. J. Gen. Virol. 2005, 86, 2281–2289. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Yang, X.; Cox-Foster, D.; Cui, L. The role of varroa mites in infections of kashmir bee virus (KBV) and deformed wing virus (DWV) in honey bees. Virology 2005, 342, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Yue, C.; Schroder, M.; Gisder, S.; Genersch, E. Vertical-transmission routes for deformed wing virus of honeybees (Apis mellifera). J. Gen. Virol. 2007, 88, 2329–2336. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Meixner, M.; Büchler, R.; Kryger, P. Chronic bee paralysis virus in honeybee queens: Evaluating susceptibility and infection routes. Viruses 2014, 6, 1188–1201. [Google Scholar] [CrossRef] [PubMed]
- Amiri, E.; Kryger, P.; Meixner, M.D.; Strand, M.K.; Tarpy, D.R.; Rueppell, O. Quantitative patterns of vertical transmission of deformed wing virus in honey bees. PLoS ONE 2018, 13, e0195283. [Google Scholar] [CrossRef] [PubMed]
- Gisder, S.; Aumeier, P.; Genersch, E. Deformed wing virus: Replication and viral load in mites (Varroa destructor). J. Gen. Virol. 2009, 90, 463–467. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Le Conte, Y. Ecology of Varroa destructor, the major ectoparasite of the western honey bee, Apis mellifera. Annu. Rev. Entomol. 2016, 61, 417–432. [Google Scholar] [CrossRef] [PubMed]
- Di Prisco, G.; Annoscia, D.; Margiotta, M.; Ferrara, R.; Varricchio, P.; Zanni, V.; Caprio, E.; Nazzi, F.; Pennacchio, F. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 2016, 113, 3203–3208. [Google Scholar] [CrossRef] [PubMed]
- Martin, S.J.; Highfield, A.C.; Brettell, L.; Villalobos, E.M.; Budge, G.E.; Powell, M.; Nikaido, S.; Schroeder, D.C. Global honey bee viral landscape altered by a parasitic mite. Science 2012, 336, 1304–1306. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Wood, G.R.; Fannon, J.M.; Moore, J.D.; Bull, J.C.; Chandler, D.; Mead, A.; Burroughs, N.; Evans, D.J. A virulent strain of deformed wing virus (DWV) of honeybees (Apis mellifera) prevails after Varroa destructor-mediated, or in vitro, transmission. PLoS Pathog. 2014, 10, e1004230. [Google Scholar] [CrossRef] [PubMed]
- McMahon, D.P.; Natsopoulou, M.E.; Doublet, V.; Fürst, M.; Weging, S.; Brown, M.J.F.; Gogol-Döring, A.; Paxton, R.J. Elevated virulence of an emerging viral genotype as a driver of honeybee loss. Proc. R. Soc. B-Biol. Sci. 2016, 283, 20160811. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Genersch, E. Deformed wing virus. J. Invertebr. Pathol. 2010, 103, S48–S61. [Google Scholar] [CrossRef] [PubMed]
- Genersch, E.; von der Ohe, W.; Kaatz, H.; Schroeder, A.; Otten, C.; Büchler, R.; Berg, S.; Ritter, W.; Mühlen, W.; Gisder, S.; et al. The german bee monitoring project: A long term study to understand periodically high winter losses of honey bee colonies. Apidologie 2010, 41, 332–352. [Google Scholar] [CrossRef]
- Annoscia, D.; Brown, S.; Di Prisco, G.; De Paoli, E.; Del Fabbro, S.; Zanni, V.; Galbraith, D.; Caprio, E.; Grozinger, C.M.; Pennacchio, F.; et al. Haemolymph removal by the parasite Varroa destructor can trigger the proliferation of the deformed wing virus in mite infested bees (Apis mellifera), contributing to enhanced pathogen virulence. bioRxiv 2018, 257667. [Google Scholar] [CrossRef]
- Nazzi, F.; Pennacchio, F. Disentangling multiple interactions in the hive ecosystem. Trends Parasitol. 2014, 30, 556–561. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Brown, S.P.; Annoscia, D.; Del Piccolo, F.; Di Prisco, G.; Varricchio, P.; Della Vedova, G.; Cattonaro, F.; Caprio, E.; Pennacchio, F. Synergistic parasite-pathogen interactions mediated by host immunity can drive the collapse of honeybee colonies. PLoS Pathog. 2012, 8, e1002735. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Steinhauer, N.; Travis, D.A.; Meixner, M.D.; Deen, J.; vanEngelsdorp, D. Honey bee surveillance: A tool for understanding and improving honey bee health. Curr. Opin. Insect Sci. 2015, 10, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Asensio, I.; Vicente-Rubiano, M.; Munoz, M.J.; Fernandez-Carrion, E.; Sanchez-Vizcaino, J.M.; Carballo, M. Importance of ecological factors and colony handling for optimizing health status of apiaries in mediterranean ecosystems. PLoS ONE 2016, 11, e0164205. [Google Scholar] [CrossRef] [PubMed]
- Olivier, V.; Blanchard, P.; Chaouch, S.; Lallemand, P.; Schurr, F.; Celle, O.; Dubois, E.; Tordo, N.; Thiéry, R.; Houlgatte, R.; et al. Molecular characterisation and phylogenetic analysis of chronic bee paralysis virus, a honey bee virus. Virus Res. 2008, 132, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Pettis, J.S.; Corona, M.; Chen, W.P.; Li, C.J.; Spivak, M.; Visscher, P.K.; DeGrandi-Hoffman, G.; Boncristiani, H.; Zhao, Y.; et al. Israeli acute paralysis virus: Epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014, 10, e1004261. [Google Scholar] [CrossRef] [PubMed]
- Bailey, L.; Fernando, E.F. Effects of sacbrood virus on adult honey-bees. Ann. Appl. Biol. 1972, 72, 27–35. [Google Scholar] [CrossRef]
- Bailey, L. Acute bee-paralysis virus in adult honey bees injected with sacbrood virus. Virology 1967, 33, 368. [Google Scholar] [CrossRef]
- Bailey, L. Honey bee pathology. Annu. Rev. Entomol. 1968, 13, 191–212. [Google Scholar] [CrossRef]
- Di Prisco, G.; Cavaliere, V.; Annoscia, D.; Varricchio, P.; Caprio, E.; Nazzi, F.; Gargiulo, G.; Pennacchio, F. Neonicotinoid clothianidin adversely affects insect immunity and promotes replication of a viral pathogen in honey bees. Proc. Natl. Acad. Sci. USA 2013, 110, 18466–18471. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Antiviral defense mechanisms in honey bees. Curr. Opin. Insect Sci. 2015, 10, 71–82. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Aronstein, K.; Chen, Y.P.; Hetru, C.; Imler, J.L.; Jiang, H.; Kanost, M.; Thompson, G.J.; Zou, Z.; Hultmark, D. Immune pathways and defence mechanisms in honey bees Apis mellifera. Insect Mol. Biol. 2006, 15, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Spain Ministry of Agriculture—Ministerio de Agricultura y Pesca. Informe de Resultados del Programa de Vigilancia Sobre las Pérdidas de Colonias de Abejas 2012–2015; Spain Ministry of Agriculture: Madrid, Spain, 2016. [Google Scholar]
- Runckel, C.; Flenniken, M.L.; Engel, J.C.; Ruby, J.G.; Ganem, D.; Andino, R.; DeRisi, J.L. Temporal analysis of the honey bee microbiome reveals four novel viruses and seasonal prevalence of known viruses, nosema, and crithidia. PLoS ONE 2011, 6, e20656. [Google Scholar] [CrossRef] [PubMed]
- Tentcheva, D.; Gauthier, L.; Zappulla, N.; Dainat, B.; Cousserans, F.; Colin, M.E.; Bergoin, M. Prevalence and seasonal variations of six bee viruses in Apis mellifera L. and Varroa destructor mite populations in France. Appl. Environ. Microbiol. 2004, 70, 7185–7191. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, B.K.; Mignon, J.; Laget, D.; de Graaf, D.C.; Jacobs, F.J.; vanEngelsdorp, D.; Brostaux, Y.; Saegerman, C.; Haubruge, E. Honey bee colony losses in Belgium during the 2008–2009 winter. J. Apicult. Res. 2015, 49, 337–339. [Google Scholar] [CrossRef]
- Lee, K.V.; Steinhauer, N.; Rennich, K.; Wilson, M.E.; Tarpy, D.R.; Caron, D.M.; Rose, R.; Delaplane, K.S.; Baylis, K.; Lengerich, E.J.; et al. A national survey of managed honey bee 2013–2014 annual colony losses in the USA. Apidologie 2015, 46, 292–305. [Google Scholar] [CrossRef]
- Cornman, R.S.; Tarpy, D.R.; Chen, Y.; Jeffreys, L.; Lopez, D.; Pettis, J.S.; vanEngelsdorp, D.; Evans, J.D. Pathogen webs in collapsing honey bee colonies. PLoS ONE 2012, 7, e43562. [Google Scholar] [CrossRef] [PubMed]
- Cox-Foster, D.L.; Conlan, S.; Holmes, E.C.; Palacios, G.; Evans, J.D.; Moran, N.A.; Quan, P.L.; Briese, T.; Hornig, M.; Geiser, D.M.; et al. A metagenomic survey of microbes in honey bee colony collapse disorder. Science 2007, 318, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Canadian National Honey Bee Health Survey, GPRC National Bee Diagnostic Centre. Available online: https://www.gprc.ab.ca/doc.php?d=2016NHBHS (accessed on 23 May 2018).
- Daughenbaugh, K.F.; Martin, M.; Brutscher, L.M.; Cavigli, I.; Garcia, E.; Lavin, M.; Flenniken, M.L. Honey bee infecting lake sinai viruses. Viruses 2015, 7, 3285–3309. [Google Scholar] [CrossRef] [PubMed]
- Van der Zee, R.; Pisa, L.; Andonov, S.; Brodschneider, R.; Charriere, J.-D.; Chlebo, R.; Coffey, M.F.; Crailsheim, K.; Dahle, B.; Gajda, A.; et al. Managed honey bee colony losses in Canada, China, Europe, Israel and Turkey, for the winters of 2008–2209 and 2009–2010. J. Apicult. Res. 2015, 51, 100–114. [Google Scholar] [CrossRef]
- Van der Zee, R.; Gray, A.; Pisa, L.; de Rijk, T. An observational study of honey bee colony winter losses and their association with Varroa destructor, neonicotinoids and other risk factors. PLoS ONE 2015, 10, e0131611. [Google Scholar] [CrossRef] [PubMed]
- De Miranda, J.R.; Bailey, L.; Ball, B.V.; Blanchard, P.; Budge, G.E.; Chejanovsky, N.; Chen, Y.-P.; Gauthier, L.; Genersch, E.; de Graaf, D.C.; et al. Standard methods for virus research in Apis mellifera. J. Apicult. Res. 2015, 52, 1–56. [Google Scholar] [CrossRef]
- De Miranda, J.R.; Cordoni, G.; Budge, G. The acute bee paralysis virus–kashmir bee virus–israeli acute paralysis virus complex. J. Invertebr. Pathol. 2010, 103, S30–S47. [Google Scholar] [CrossRef] [PubMed]
- Benaets, K.; Van Geystelen, A.; Cardoen, D.; De Smet, L.; de Graaf, D.C.; Schoofs, L.; Larmuseau, M.H.D.; Brettell, L.E.; Martin, S.J.; Wenseleers, T. Covert deformed wing virus infections have long-term deleterious effects on honeybee foraging and survival. Proc. R. Soc. B-Biol. Sci. 2017, 284, 20162149. [Google Scholar] [CrossRef] [PubMed]
- Benjeddou, M.; Leat, N.; Allsopp, M.; Davison, S. Development of infectious transcripts and genome manipulation of black queen-cell virus of honey bees. J. Gen. Virol. 2002, 83, 3139–3146. [Google Scholar] [CrossRef] [PubMed]
- Lamp, B.; Url, A.; Seitz, K.; Eichhorn, J.; Riedel, C.; Sinn, L.J.; Indik, S.; Köglberger, H.; Rümenapf, T. Construction and rescue of a molecular clone of deformed wing virus (dwv). PLoS ONE 2016, 11, e0164639. [Google Scholar] [CrossRef] [PubMed]
- Goblirsch, M.J.; Spivak, M.S.; Kurtti, T.J. A cell line resource derived from honey bee (Apis mellifera) embryonic tissues. PLoS ONE 2013, 8, e69831. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Tripp, J.; Dolezal, A.G.; Goblirsch, M.J.; Miller, W.A.; Toth, A.L.; Bonning, B.C. In vivo and in vitro infection dynamics of honey bee viruses. Sci. Rep. 2016, 6, S50. [Google Scholar] [CrossRef] [PubMed]
- Boncristiani, H.F.; Evans, J.D.; Chen, Y.; Pettis, J.; Murphy, C.; Lopez, D.L.; Simone-Finstrom, M.; Strand, M.; Tarpy, D.R.; Rueppell, O. In vitro infection of pupae with israeli acute paralysis virus suggests disturbance of transcriptional homeostasis in honey bees (Apis mellifera). PLoS ONE 2013, 8, e73429. [Google Scholar] [CrossRef] [PubMed]
- Skubnik, K.; Novacek, J.; Fuzik, T.; Pridal, A.; Paxton, R.J.; Plevka, P. Structure of deformed wing virus, a major honey bee pathogen. Proc. Natl. Acad. Sci. USA 2017, 114, 3210–3215. [Google Scholar] [CrossRef] [PubMed]
- Organtini, L.J.; Shingler, K.L.; Ashley, R.E.; Capaldi, E.A.; Durrani, K.; Dryden, K.A.; Makhov, A.M.; Conway, J.F.; Pizzorno, M.C.; Hafenstein, S. Honey bee deformed wing virus structures reveal that conformational changes accompany genome release. J. Virol. 2017, 91, e01795–16. [Google Scholar] [CrossRef] [PubMed]
- Galbraith, D.A.; Yang, X.; Nino, E.L.; Yi, S.; Grozinger, C. Parallel epigenomic and transcriptomic responses to viral infection in honey bees (Apis mellifera). PLoS Pathog. 2015, 11, e1004713. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Daughenbaugh, K.F.; Flenniken, M.L. Virus and dsrna-triggered transcriptional responses reveal key components of honey bee antiviral defense. Sci. Rep. 2017, 7, 6448. [Google Scholar] [CrossRef] [PubMed]
- Flenniken, M.L.; Andino, R. Non-specific dsrna-mediated antiviral response in the honey bee. PLoS ONE 2013, 8, e77263. [Google Scholar] [CrossRef] [PubMed]
- O'Neal, S.T.; Swale, D.R.; Anderson, T.D. Atp-sensitive inwardly rectifying potassium channel regulation of viral infections in honey bees. Sci. Rep. 2017, 7, 614. [Google Scholar] [CrossRef] [PubMed]
- O'Neal, S.T.; Brewster, C.C.; Bloomquist, J.R.; Anderson, T.D. Amitraz and its metabolite modulate honey bee cardiac function and tolerance to viral infection. J. Invertebr. Pathol. 2017, 149, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.D.; Maleszka, R.; Hartfelder, K.; Worley, K.C.; Amdam, G.V.; Bitondi, M.M.G.; Collins, A.M.; Cristino, A.S.; Michael, H.; Lattorff, G.; et al. Insights into social insects from the genome of the honeybee Apis mellifera. Nature 2006, 443, 931–949. [Google Scholar]
- Sadd, B.M.; Barribeau, S.M.; Bloch, G.; de Graaf, D.C.; Dearden, P.; Elsik, C.G.; Gadau, J.; Grimmelikhuijzen, C.J.P.; Hasselmann, M.; Lozier, J.D.; et al. The genomes of two key bumblebee species with primitive eusocial organization. Genome Biol. 2015, 16, 227. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Torres, M.C.; Reese, J.T.; Childers, C.P.; Bennett, A.K.; Sundaram, J.P.; Childs, K.L.; Anzola, J.M.; Milshina, N.; Elsik, C.G. Hymenoptera genome database: Integrated community resources for insect species of the order hymenoptera. Nucleic Acids Res. 2010, 39, D658–D662. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; van Rij, R.P. Analysis of resistance and tolerance to virus infection in drosophila. Nat. Protocols 2015, 10, 1084–1097. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; van Rij, R.P. Beyond rnai: Antiviral defense strategies in drosophila and mosquito. J. Insect Physiol. 2013, 59, 159–170. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, M.B.; Hardy, R.W. Making connections in insect innate immunity. Proc. Natl. Acad. Sci. USA 2012, 109, 18639–18640. [Google Scholar] [CrossRef] [PubMed]
- Lemaitre, B.; Hoffmann, J. The host defense of Drosophila melanogaster. Annu. Rev. Immunol. 2007, 25, 697–743. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A. The immune response of drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.L.; Hoffmann, J.A. Toll receptors in innate immunity. Trends Cell Biol. 2001, 11, 304–311. [Google Scholar] [CrossRef]
- Lamiable, O.; Imler, J.-L. Induced antiviral innate immunity in drosophila. Curr. Opin. Microbiol. 2014, 20, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Antiviral immunity and virus-mediated antagonism in disease vector mosquitoes. Trends Microbiol. 2018, 26, 447–461. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.; Olson, K. The role of rna interference (rnai) in arbovirus-vector interactions. Viruses 2015, 7, 820–843. [Google Scholar] [CrossRef] [PubMed]
- Imler, J.-L.; Bulet, P. Antimicrobial peptides in drosophila: Structures, activities and gene regulation. In Mechanisms of Epithelial Defense; KARGER: Basel, Switzerland, 2005; Volume 86, pp. 1–21. [Google Scholar]
- Lourenco, A.P.; Florecki, M.M.; Simões, Z.L.P.; Evans, J.D. Silencing of Apis mellifera dorsal genes reveals their role in expression of the antimicrobial peptide defensin-1. Insect Mol. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Danihlík, J.; Aronstein, K.; Petřivalský, M. Antimicrobial peptides: A key component of honey bee innate immunity. J. Apicult. Res. 2016, 54, 123–136. [Google Scholar] [CrossRef]
- Schlüns, H.; Crozier, R.H. Relish regulates expression of antimicrobial peptide genes in the honeybee, Apis mellifera, shown by rna interference. Insect Mol. Biol. 2007, 16, 753–759. [Google Scholar] [CrossRef] [PubMed]
- Riddell, C.; Adams, S.; Schmid-Hempel, P.; Mallon, E.B. Differential expression of immune defences is associated with specific host-parasite interactions in insects. PLoS ONE 2009, 4, e7621. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Shi, M.; Chen, X.-X. Antimicrobial peptide evolution in the asiatic honey bee apis cerana. PLoS ONE 2009, 4, e4239. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.S.; Choo, Y.M.; Lee, K.S.; Yoon, H.J.; Kim, I.; Je, Y.H.; Sohn, H.D.; Jin, B.R. Cloning and expression profiling of four antibacterial peptide genes from the bumblebee Bombus ignitus. Comp. Biochem. Physiol. B-Biochem. Mol. Biol. 2008, 150, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Doublet, V.; Poeschl, Y.; Gogol-Döring, A.; Alaux, C.; Annoscia, D.; Aurori, C.; Barribeau, S.M.; Bedoya-Reina, O.C.; Brown, M.J.F.; Bull, J.C.; et al. Unity in defence: Honeybee workers exhibit conserved molecular responses to diverse pathogens. BMC Genom. 2017, 18, 207. [Google Scholar] [CrossRef] [PubMed]
- Zanni, V.; Galbraith, D.A.; Annoscia, D.; Grozinger, C.M.; Nazzi, F. Transcriptional signatures of parasitization and markers of colony decline in varroa-infested honey bees (Apis mellifera). Insect Biochem. Mol. Biol. 2017, 87, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Fannon, J.M.; Moore, J.D.; Wood, G.R.; Evans, D.J. The iflaviruses sacbrood virus and deformed wing virus evoke different transcriptional responses in the honeybee which may facilitate their horizontal or vertical transmission. PeerJ 2016, 4, e1591. [Google Scholar] [CrossRef] [PubMed]
- Steinmann, N.; Corona, M.; Neumann, P.; Dainat, B. Overwintering is associated with reduced expression of immune genes and higher susceptibility to virus infection in honey bees. PLoS ONE 2015, 10, e0129956. [Google Scholar] [CrossRef] [PubMed]
- Merkling, S.H.; Overheul, G.J.; van Mierlo, J.T.; Arends, D.; Gilissen, C.; van Rij, R.P. The heat shock response restricts virus infection in drosophila. Sci. Rep. 2015, 5, 33. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Takeuchi, A.; Siomi, H.; Siomi, M.C. A direct role for hsp90 in pre-risc formation in drosophila. Nat. Struct. Mol. Biol. 2010, 17, 1024–1026. [Google Scholar] [CrossRef] [PubMed]
- Sim, C.; Hong, Y.S.; Tsetsarkin, K.A.; Vanlandingham, D.L.; Higgs, S.; Collins, F.H. Anopheles gambiae heat shock protein cognate 70b impedes o’nyong-nyong virus replication. BMC Genom. 2007, 8, 231. [Google Scholar] [CrossRef] [PubMed]
- Mockel, N.; Gisder, S.; Genersch, E. Horizontal transmission of deformed wing virus: Pathological consequences in adult bees (Apis mellifera) depend on the transmission route. J. Gen. Virol. 2011, 92, 370–377. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Walker, P.L.; Martin, S.J.; Gunn, A. The transmission of deformed wing virus between honeybees (Apis mellifera L.) by the ectoparasitic mite Varroa jacobsoni oud. J. Invertebr. Pathol. 1999, 73, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Nazzi, F.; Pennacchio, F. Honey bee antiviral immune barriers as affected by multiple stress factors: A novel paradigm to interpret colony health decline and collapse. Viruses 2018, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Kuster, R.D.; Boncristiani, H.F.; Rueppell, O. Immunogene and viral transcript dynamics during parasitic Varroa destructor mite infection of developing honey bee (Apis mellifera) pupae. J. Exp. Biol. 2014, 217, 1710–1718. [Google Scholar] [CrossRef] [PubMed]
- Chejanovsky, N.; Ophir, R.; Schwager, M.S.; Slabezki, Y.; Grossman, S.; Cox-Foster, D. Characterization of viral sirna populations in honey bee colony collapse disorder. Virology 2014, 454, 176–183. [Google Scholar] [CrossRef] [PubMed]
- Desai, S.D.; Eu, Y.J.; Whyard, S.; Currie, R.W. Reduction in deformed wing virus infection in larval and adult honey bees (Apis mellifera L.) by double-stranded rna ingestion. Insect Mol. Biol. 2012, 21, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Eleftherianos, I.; Won, S.; Chtarbanova, S.; Squiban, B.; Ocorr, K.; Bodmer, R.; Beutler, B.; Hoffmann, J.A.; Imler, J.L. Atp-sensitive potassium channel (katp)-dependent regulation of cardiotropic viral infections. Proc. Natl. Acad. Sci. USA 2011, 108, 12024–12029. [Google Scholar] [CrossRef] [PubMed]
- Palmer, W.H.; Varghese, F.S.; van Rij, R.P. Natural variation in resistance to virus infection in dipteran insects. Viruses 2018, 10, 118. [Google Scholar] [CrossRef] [PubMed]
- Rückert, C.; Bell-Sakyi, L.; Fazakerley, J.K.; Fragkoudis, R. Antiviral responses of arthropod vectors: An update on recent advances. Virusdisease 2014, 25, 249–260. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vargas, I.; Travanty, E.A.; Keene, K.M.; Franz, A.W.E.; Beaty, B.J.; Blair, C.D.; Olson, K.E. Rna interference, arthropod-borne viruses, and mosquitoes. Virus Res. 2004, 102, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.-W. RNA-based antiviral immunity. Nat. Rev. Immunol. 2010, 10, 632–644. [Google Scholar] [CrossRef] [PubMed]
- Fire, A. RNA-triggered gene silencing. Trends Genet. 1999, 15, 358–363. [Google Scholar] [CrossRef]
- Flenniken, M.L.; Kunitomi, M.; Tassetto, M.; Andino, R. The antiviral role of rna interference. In Insect Virology; Asgari, S., Johnson, K.N., Eds.; Caister Academic Press: Norfolk, UK, 2010; pp. 367–388. [Google Scholar]
- Hammond, S.M. Argonaute2, a link between genetic and biochemical analyses of rnai. Science 2001, 293, 1146–1150. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D. Mosquito rnai is the major innate immune pathway controlling arbovirus infection and transmission. Future Microbiol. 2011, 6, 265–277. [Google Scholar] [CrossRef] [PubMed]
- Bronkhorst, A.W.; van Rij, R.P. The long and short of antiviral defense: Small RNA-based immunity in insects. Curr. Opin. Virol. 2014, 7, 19–28. [Google Scholar] [CrossRef] [PubMed]
- Brutscher, L.M.; Flenniken, M.L. RNAi and antiviral defense in the honey bee. J. Immunol. Res. 2015, 2015, 941897. [Google Scholar] [CrossRef] [PubMed]
- Wilkins, C.; Dishongh, R.; Moore, S.C.; Whitt, M.A.; Chow, M.; Machaca, K. Rna interference is an antiviral defence mechanism in Caenorhabditis elegans. Nature 2005, 436, 1044–1047. [Google Scholar] [CrossRef] [PubMed]
- Zamore, P.D. Ancient pathways programmed by small rnas. Science 2002, 296, 1265–1269. [Google Scholar] [CrossRef] [PubMed]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded rna in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef] [PubMed]
- Baulcombe, D. Rna silencing in plants. Nature 2004, 431, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ye, X.; Jiang, F.; Liang, C.; Chen, D.; Peng, J.; Kinch, L.N.; Grishin, N.V.; Liu, Q. C3po, an endoribonuclease that promotes rnai by facilitating risc activation. Science 2009, 325, 750–753. [Google Scholar] [CrossRef] [PubMed]
- Li, H. Induction and suppression of rna silencing by an animal virus. Science 2002, 296, 1319–1321. [Google Scholar] [CrossRef] [PubMed]
- Van Rij, R.P.; Saleh, M.C.; Berry, B.; Foo, C.; Houk, A.; Antoniewski, C.; Andino, R. The rna silencing endonuclease argonaute 2 mediates specific antiviral immunity in Drosophila melanogaster. Genes Dev. 2006, 20, 2985–2995. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.-C.; van Rij, R.P.; Hekele, A.; Gillis, A.; Foley, E.; O’Farrell, P.H.; Andino, R. The endocytic pathway mediates cell entry of dsrna to induce rnai silencing. Nat. Cell Biol. 2006, 8, 793–802. [Google Scholar] [CrossRef] [PubMed]
- Saleh, M.-C.; Tassetto, M.; van Rij, R.P.; Goic, B.; Gausson, V.; Berry, B.; Jacquier, C.; Antoniewski, C.; Andino, R. Antiviral immunity in drosophila requires systemic RNA interference spread. Nature 2009, 458, 346–350. [Google Scholar] [CrossRef] [PubMed]
- Goic, B.; Vodovar, N.; Mondotte, J.A.; Monot, C.; Frangeul, L.; Blanc, H.; Gausson, V.; Vera-Otarola, J.; Cristofari, G.; Saleh, M.-C. Rna-mediated interference and reverse transcription control the persistence of rna viruses in the insect model drosophila. Nat. Immunol. 2013, 14, 396–403. [Google Scholar] [CrossRef] [PubMed]
- Tassetto, M.; Kunitomi, M.; Andino, R. Circulating immune cells mediate a systemic rnai-based adaptive antiviral response in drosophila. Cell 2017, 169, 314–325. [Google Scholar] [CrossRef] [PubMed]
- Poirier, E.Z.; Goic, B.; Tome-Poderti, L.; Frangeul, L.; Boussier, J.; Gausson, V.; Blanc, H.; Vallet, T.; Loyd, H.; Levi, L.I.; et al. Dicer-2-dependent generation of viral DNA from defective genomes of rna viruses modulates antiviral immunity in insects. Cell Host Microbe 2018, 23, 353–365. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, Y.; Yan, X.; Han, R. Prevention of chinese sacbrood virus infection in Apis cerana using RNA interference. Curr. Microbiol. 2010, 61, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Paldi, N.; Shafir, S.; Kalev, H.; Tsur, E.; Glick, E.; Sela, I. Iapv, a bee-affecting virus associated with colony collapse disorder can be silenced by dsrna ingestion. Insect Mol. Biol. 2009, 18, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Nunes, F.; Aleixo, A.; Barchuk, A.; Bomtorin, A.; Grozinger, C.; Simões, Z. Non-target effects of green fluorescent protein (gfp)-derived double-stranded rna (dsrna-gfp) used in honey bee rna interference (RNAi) assays. Insects 2013, 4, 90–103. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, J.G.; Duan, J.J. Rnai-based insecticidal crops: Potential effects on nontarget species. Bioscience 2013, 63, 657–665. [Google Scholar] [CrossRef]
- Cappelle, K.; Smagghe, G.; Dhaenens, M.; Meeus, I. Israeli acute paralysis virus infection leads to an enhanced rna interference response and not its suppression in the bumblebee Bombus terrestris. Viruses 2016, 8, 334. [Google Scholar] [CrossRef] [PubMed]
- Jarosch, A.; Moritz, R.F.A. Rna interference in honeybees: Off-target effects caused by dsrna. Apidologie 2012, 43, 128–138. [Google Scholar] [CrossRef]
- Hunter, W.; Ellis, J.; vanEngelsdorp, D.; Hayes, J.; Westervelt, D.; Glick, E.; Williams, M.; Sela, I.; Maori, E.; Pettis, J.; et al. Large-scale field application of rnai technology reducing israeli acute paralysis virus disease in honey bees (Apis mellifera, hymenoptera: Apidae). PLoS Pathog. 2010, 6, e1001160. [Google Scholar] [CrossRef] [PubMed]
- Beye, M.; Hartel, S.; Hagen, A.; Hasselmann, M.; Omholt, S.W. Specific developmental gene silencing in the honey bee using a homeobox motif. Insect Mol. Biol. 2002, 11, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Aronstein, K.; Saldivar, E. Characterization of a honey bee toll related receptor gene am18w and its potential involvement in antimicrobial immune defense. Apidologie 2005, 36, 3–14. [Google Scholar] [CrossRef]
- Patel, A.; Fondrk, M.K.; Kaftanoglu, O.; Emore, C.; Hunt, G.; Frederick, K.; Amdam, G.V. The making of a queen: Tor pathway is a key player in diphenic caste development. PLoS ONE 2007, 2, e509. [Google Scholar] [CrossRef] [PubMed]
- Barchuk, A.R.; Figueiredo, V.L.C.; Simões, Z.L.P. Downregulation of ultraspiracle gene expression delays pupal development in honeybees. J. Insect Physiol. 2008, 54, 1035–1040. [Google Scholar] [CrossRef] [PubMed]
- Nilsen, K.A.; Ihle, K.E.; Frederick, K.; Fondrk, M.K.; Smedal, B.; Hartfelder, K.; Amdam, G.V. Insulin-like peptide genes in honey bee fat body respond differently to manipulation of social behavioral physiology. J. Exp. Biol. 2011, 214, 1488–1497. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Baker, N.; Amdam, G.V. Rnai-mediated double gene knockdown and gustatory perception measurement in honey bees (Apis mellifera). J. Vis. Exp. 2013. [Google Scholar] [CrossRef] [PubMed]
- Ihle, K.E.; Fondrk, M.K.; Page, R.E.; Amdam, G.V. Genotype effect on lifespan following vitellogenin knockdown. Exp. Gerontol. 2015, 61, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; Ihle, K.E.; Fondrk, M.K.; Page, R.E.; Amdam, G.V. The gene vitellogenin has multiple coordinating effects on social organization. PLoS Biol. 2007, 5, e62. [Google Scholar] [CrossRef] [PubMed]
- Deddouche, S.; Matt, N.; Budd, A.; Mueller, S.; Kemp, C.; Galiana-Arnoux, D.; Dostert, C.; Antoniewski, C.; Hoffmann, J.A.; Imler, J.-L. The dexd/h-box helicase dicer-2 mediates the induction of antiviral activity in drosophila. Nat. Immunol. 2008, 9, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Trinidad, L.; Voysey, R.; Duchemin, J.B.; Walker, P.J. Secreted vago restricts west nile virus infection in culex mosquito cells by activating the Jak-stat pathway. Proc. Natl. Acad. Sci. USA 2012, 109, 18915–18920. [Google Scholar] [CrossRef] [PubMed]
- Paradkar, P.N.; Duchemin, J.B.; Voysey, R.; Walker, P.J. Dicer-2-dependent activation of culex vago occurs via the traf-rel2 signaling pathway. PLoS Negl. Trop. Dis. 2014, 8, e2823. [Google Scholar] [CrossRef] [PubMed]
- Pichlmair, A.; Schulz, O.; Tan, C.P.; Rehwinkel, J.; Kato, H.; Takeuchi, O.; Akira, S.; Way, M.; Schiavo, G.; Reis e Sousa, C. Activation of mda5 requires higher-order rna structures generated during virus infection. J. Virol. 2009, 83, 10761–10769. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, O.; Akira, S. Rig-i-like antiviral protein in flies. Nat. Immunol. 2008, 9, 1327–1328. [Google Scholar] [CrossRef] [PubMed]
- Yoneyama, M.; Kikuchi, M.; Natsukawa, T.; Shinobu, N.; Imaizumi, T.; Miyagishi, M.; Taira, K.; Akira, S.; Fujita, T. The rna helicase rig-i has an essential function in double-stranded rna-induced innate antiviral responses. Nat. Immunol. 2004, 5, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Kingsolver, M.B.; Huang, Z.; Hardy, R.W. Insect antiviral innate immunity: Pathways, effectors, and connections. J. Mol. Biol. 2013, 425, 4921–4936. [Google Scholar] [CrossRef] [PubMed]
- Cardinal, S.; Straka, J.; Danforth, B.N. Comprehensive phylogeny of apid bees reveals the evolutionary origins and antiquity of cleptoparasitism. Proc. Natl. Acad. Sci. USA 2010, 107, 16207–16211. [Google Scholar] [CrossRef] [PubMed]
- Terenius, O.; Papanicolaou, A.; Garbutt, J.S.; Eleftherianos, I.; Huvenne, H.; Kanginakudru, S.; Albrechtsen, M.; An, C.; Aymeric, J.-L.; Barthel, A.; et al. Rna interference in lepidoptera: An overview of successful and unsuccessful studies and implications for experimental design. J. Insect Physiol. 2011, 57, 231–245. [Google Scholar] [CrossRef] [PubMed]
- Boisson, B.; Jacques, J.C.; Choumet, V.; Martin, E.; Xu, J.; Vernick, K.; Bourgouin, C. Gene silencing in mosquito salivary glands by rnai. FEBS Lett. 2006, 580, 1988–1992. [Google Scholar] [CrossRef] [PubMed]
- Nayak, A.; Berry, B.; Tassetto, M.; Kunitomi, M.; Acevedo, A.; Deng, C.; Krutchinsky, A.; Gross, J.; Antoniewski, C.; Andino, R. Cricket paralysis virus antagonizes argonaute 2 to modulate antiviral defense in drosophila. Nat. Struct. Mol. Biol. 2010, 17, 547–554. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Smagghe, G.; Meeus, I. The role of a single gene encoding the single von willebrand factor c-domain protein (svc) in bumblebee immunity extends beyond antiviral defense. Insect Biochem. Mol. Biol. 2017, 91, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Ronald, P.C.; Beutler, B. Plant and animal sensors of conserved microbial signatures. Science 2010, 330, 1061–1064. [Google Scholar] [CrossRef] [PubMed]
- Coffman, S.R.; Lu, J.; Guo, X.; Zhong, J.; Jiang, H.; Broitman-Maduro, G.; Li, W.-X.; Lu, R.; Maduro, M.; Ding, S.-W. Caenorhabditis elegans rig-i homolog mediates antiviral RNA interference downstream of dicer-dependent biogenesis of viral small interfering rnas. mBio 2017, 8, e00264. [Google Scholar] [CrossRef] [PubMed]
- Ashe, A.; Sarkies, P.; Le Pen, J.; Tanguy, M.; Miska, E.A. Antiviral rna interference against orsay virus is neither systemic nor transgenerational in Caenorhabditis elegans. J. Virol. 2015, 89, 12035–12046. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Maduro, M.; Li, F.; Li, H.W.; Broitman-Maduro, G.; Li, W.X.; Ding, S.W. Animal virus replication and RNAi-mediated antiviral silencing in Caenorhabditis elegans. Nature 2005, 436, 1040–1043. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Tykalová, H.; Watson, M.; Sharma, M.; Sterken, M.G.; Obbard, D.J.; Lewis, S.H.; McFarlane, M.; Bell-Sakyi, L.; Barry, G.; et al. Induction and suppression of tick cell antiviral rnai responses by tick-borne flaviviruses. Nucleic Acids Res. 2014, 42, 9436–9446. [Google Scholar] [CrossRef] [PubMed]
- Green, T.J.; Speck, P. Antiviral defense and innate immune memory in the oyster. Viruses 2018, 10, 133. [Google Scholar] [CrossRef] [PubMed]
- Robalino, J.; Bartlett, T.C.; Chapman, R.W.; Gross, P.S.; Browdy, C.L.; Warr, G.W. Double-stranded RNA and antiviral immunity in marine shrimp: Inducible host mechanisms and evidence for the evolution of viral counter-responses. Dev. Comp. Immunol. 2007, 31, 539–547. [Google Scholar] [CrossRef] [PubMed]
- Pitaluga, A.N.; Mason, P.W.; Traub-Cseko, Y.M. Non-specific antiviral response detected in RNA-treated cultured cells of the sandfly, Lutzomyia longipalpis. Dev. Comp. Immunol. 2008, 32, 191–197. [Google Scholar] [CrossRef] [PubMed]
- Martins-da-Silva, A.; Telleria, E.; Batista, M.; Marchini, F.; Traub-Csekö, Y.; Tempone, A. Identification of secreted proteins involved in nonspecific dsrna-mediated Lutzomyia longipalpis ll5 cell antiviral response. Viruses 2018, 10, 43. [Google Scholar] [CrossRef] [PubMed]
- Van Mierlo, J.T.; van Cleef, K.W.R.; van Rij, R.P. Defense and counterdefense in the RNAi-based antiviral immune system in insects. In Antiviral RNAi. Methods in Molecular Biology (Methods and Protocols); van Rij, R., Ed.; Humana Press: New York, NY, USA, 2011; Volume 721, pp. 3–22. [Google Scholar]
- De Maio, F.A.; Risso, G.; Iglesias, N.G.; Shah, P.; Pozzi, B.; Gebhard, L.G.; Mammi, P.; Mancini, E.; Yanovsky, M.J.; Andino, R.; et al. The dengue virus ns5 protein intrudes in the cellular spliceosome and modulates splicing. PLoS Pathog. 2016, 12, e1005841. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jorda, M.; Jones, P.L.; Maleszka, R.; Ling, X.; Robertson, H.M.; Mizzen, C.A.; Peinado, M.A.; Robinson, G.E. Functional cpg methylation system in a social insect. Science 2006, 314, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Tidbury, H.J.; Pedersen, A.B.; Boots, M. Within and transgenerational immune priming in an insect to a DNA virus. Proc. Biol. Sci. 2011, 278, 871–876. [Google Scholar] [CrossRef] [PubMed]
- Gourbal, B.; Pinaud, S.; Beckers, G.J.M.; Van Der Meer, J.W.M.; Conrath, U.; Netea, M.G. Innate immune memory: An evolutionary perspective. Immunol. Rev. 2018, 283, 21–40. [Google Scholar] [CrossRef] [PubMed]
- Maori, E.; Tanne, E.; Sela, I. Reciprocal sequence exchange between non-retro viruses and hosts leading to the appearance of new host phenotypes. Virology 2007, 362, 342–349. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
McMenamin, A.J.; Daughenbaugh, K.F.; Parekh, F.; Pizzorno, M.C.; Flenniken, M.L. Honey Bee and Bumble Bee Antiviral Defense. Viruses 2018, 10, 395. https://doi.org/10.3390/v10080395
McMenamin AJ, Daughenbaugh KF, Parekh F, Pizzorno MC, Flenniken ML. Honey Bee and Bumble Bee Antiviral Defense. Viruses. 2018; 10(8):395. https://doi.org/10.3390/v10080395
Chicago/Turabian StyleMcMenamin, Alexander J., Katie F. Daughenbaugh, Fenali Parekh, Marie C. Pizzorno, and Michelle L. Flenniken. 2018. "Honey Bee and Bumble Bee Antiviral Defense" Viruses 10, no. 8: 395. https://doi.org/10.3390/v10080395
APA StyleMcMenamin, A. J., Daughenbaugh, K. F., Parekh, F., Pizzorno, M. C., & Flenniken, M. L. (2018). Honey Bee and Bumble Bee Antiviral Defense. Viruses, 10(8), 395. https://doi.org/10.3390/v10080395





