A Novel Squirrel Respirovirus with Putative Zoonotic Potential
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection
2.2. Bacteriological Examination
2.3. Virus Isolation
2.4. Hemadsorption and Hemagglutination
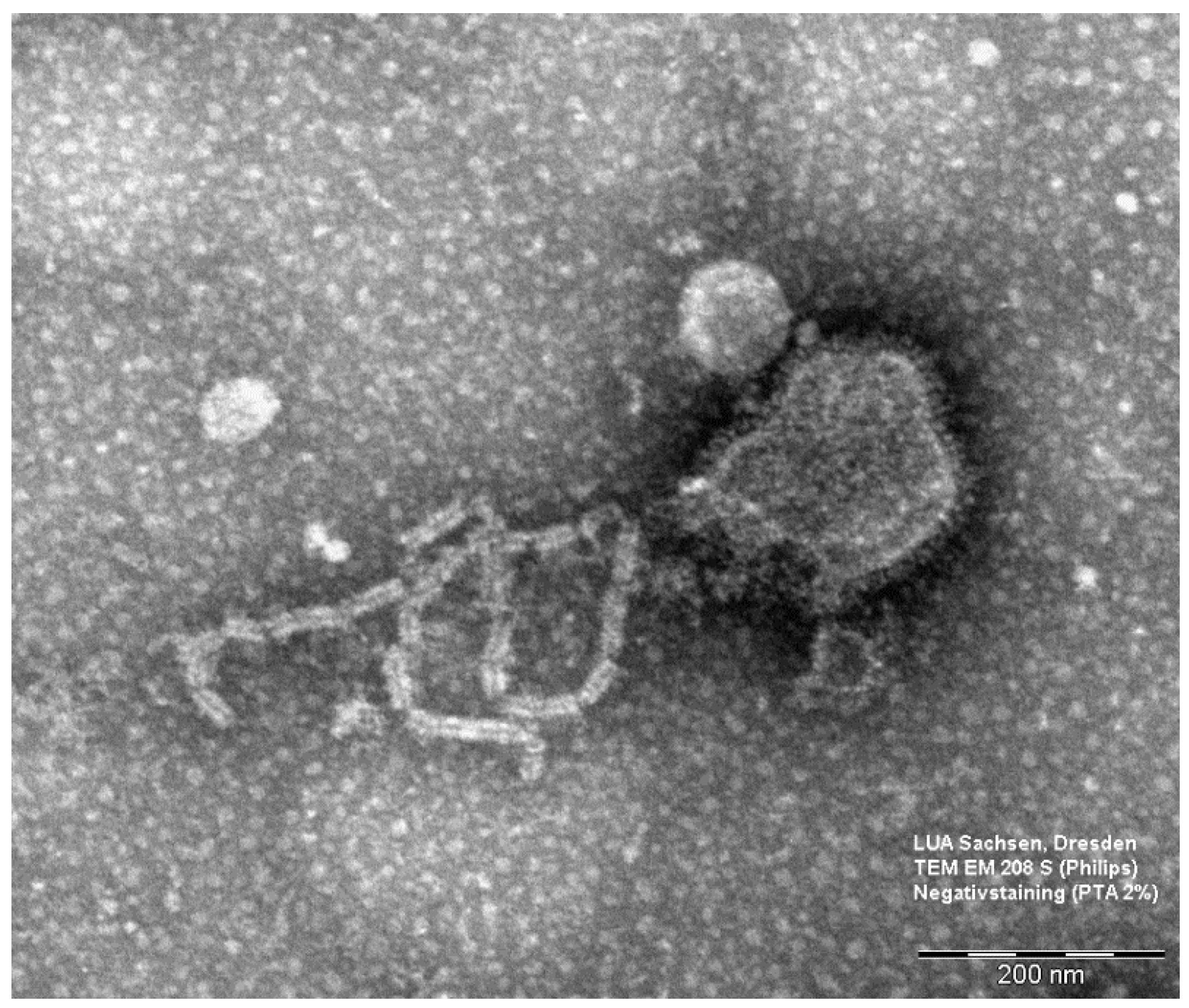
2.5. Electron Microscopy
2.6. Extraction of Nucleic Acids
2.7. Full-Genome Sequencing and Data Analysis
2.8. Real-Time Reverse Transcription-Polymerase Chain Reaction (RT-qPCR)
3. Results and Discussion
3.1. Pathological Findings and Initial Pathogen Screening
3.2. Viral Genome Loads in Different Organs of the Sri Lankan Giant Squirrel
3.3. Genome Analysis and Features of GSqRV
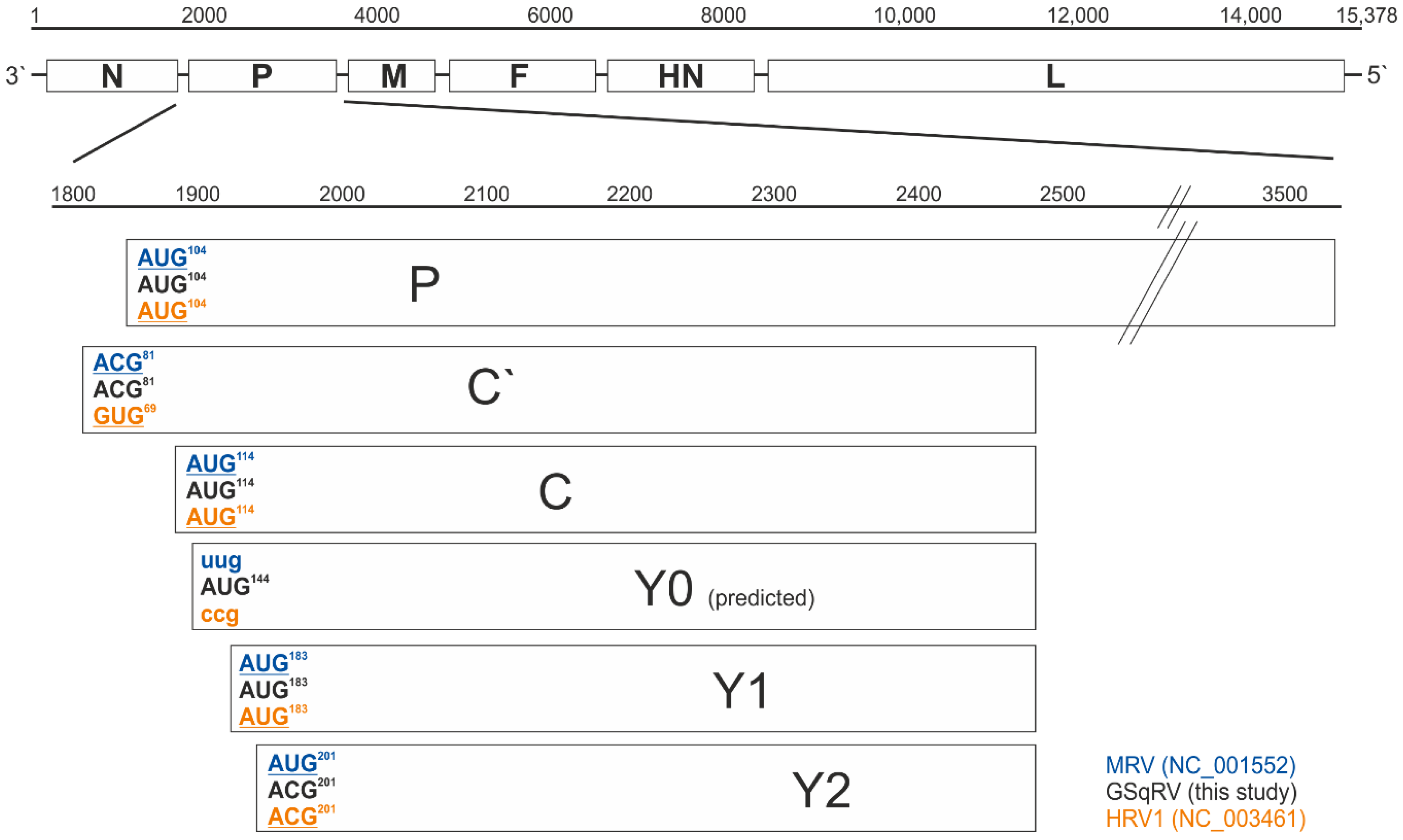
3.3.1. Genome Organization
3.3.2. Putative Additional Accessory Protein
3.3.3. RNA Editing
3.3.4. Single Nucleotide Variants (SNVs)
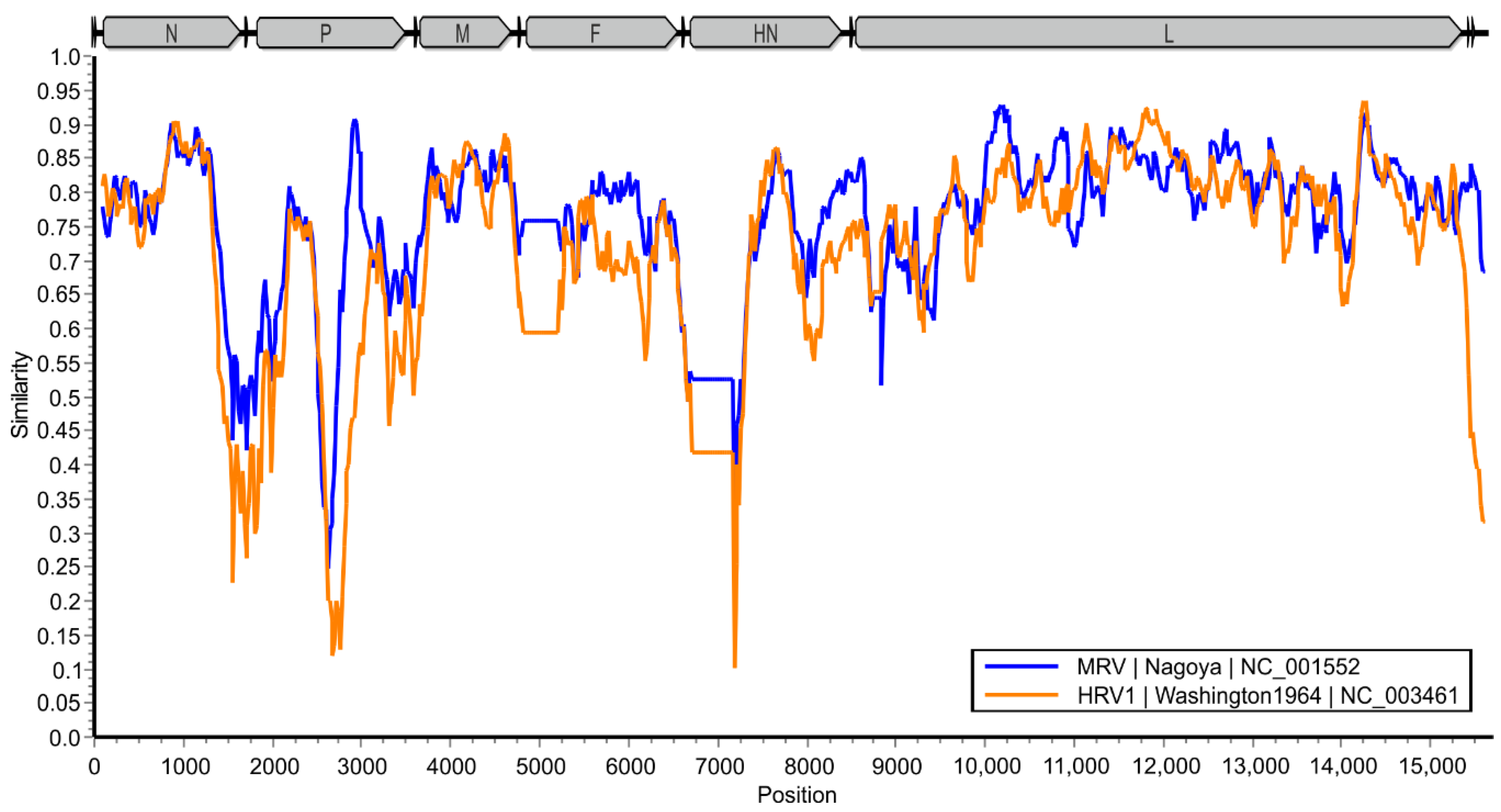
3.4. Sequence Identities to Other Paramyxoviruses
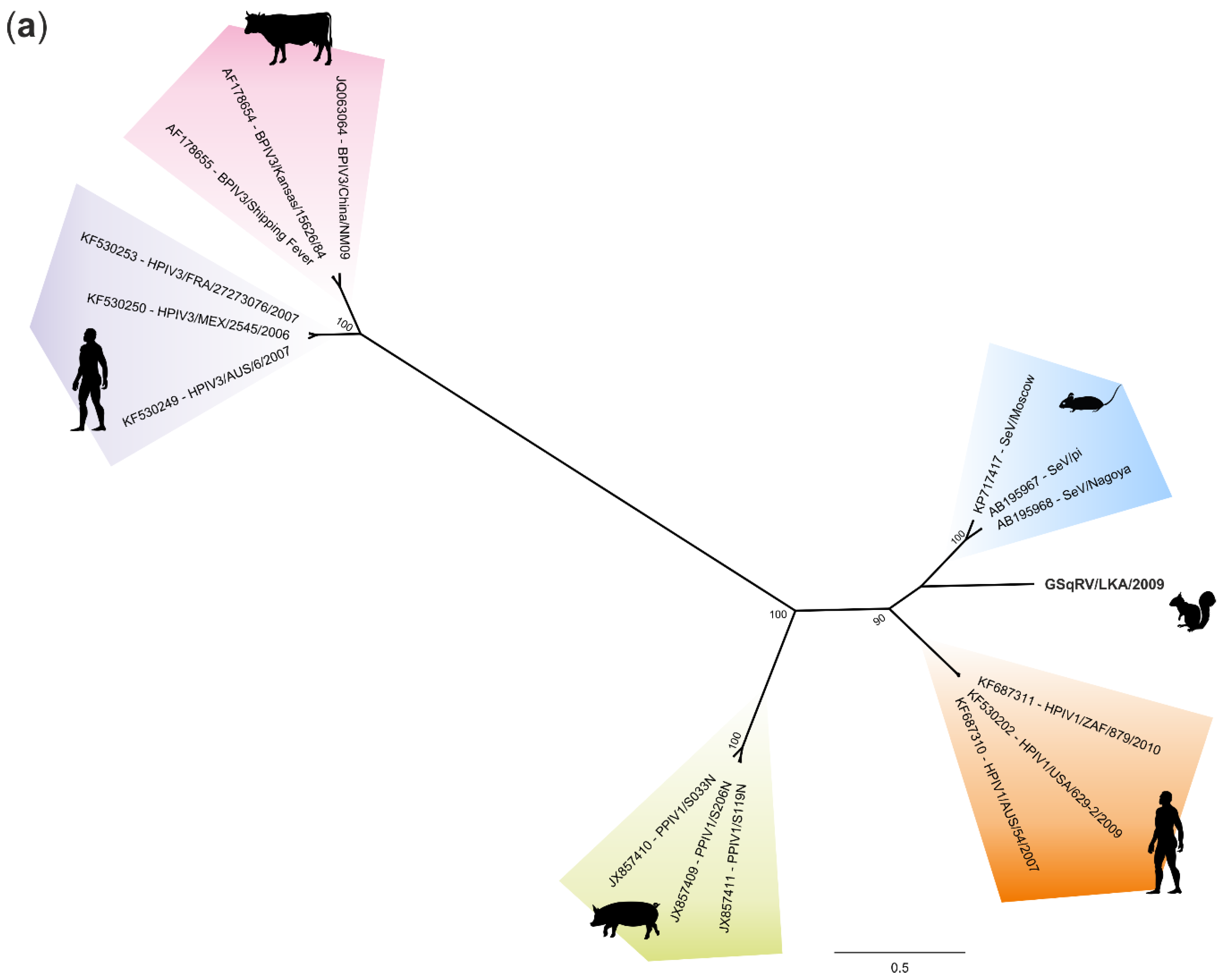
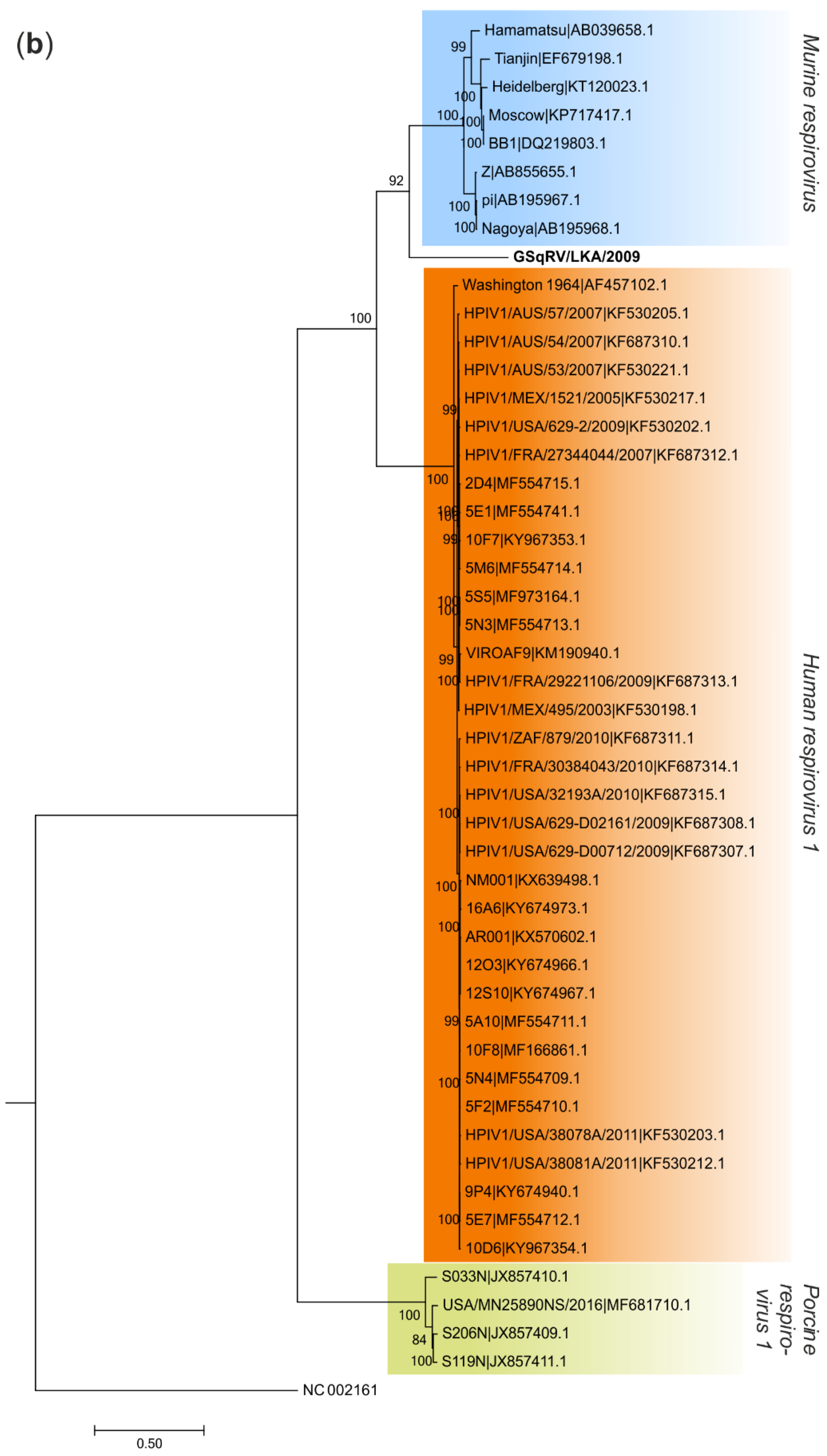
3.5. Phylogenetic Analysis
3.6. Pilot Screening for MRV and GSqRV in Squirrels
3.7. Evaluation of GSqRV as a Potential Zoonotic Agent
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, L.-F.; Chua, K.; Yu, M.; Eaton, B.T. Genome Diversity of Emerging Paramyxoviruses. Curr. Genom. 2003, 4, 263–273. [Google Scholar] [CrossRef]
- Drexler, J.F.; Corman, V.M.; Müller, M.A.; Maganga, G.D.; Vallo, P.; Binger, T.; Gloza-Rausch, F.; Cottontail, V.M.; Rasche, A.; Yordanov, S.; et al. Bats host major mammalian paramyxoviruses. Nat. Commun. 2012, 3, 796. [Google Scholar] [CrossRef] [PubMed]
- Lambeth, L.S.; Yu, M.; Anderson, D.E.; Crameri, G.; Eaton, B.T.; Wang, L.F. Complete genome sequence of Nariva virus, a rodent paramyxovirus. Arch. Virol. 2009, 154, 199–207. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.P.; Woo, P.C.Y.; Wong, B.H.L.; Wong, A.Y.P.; Tsoi, H.-W.; Wang, M.; Lee, P.; Xu, H.; Poon, R.W.S.; Guo, R.; et al. Identification and complete genome analysis of three novel paramyxoviruses, Tuhoko virus 1, 2 and 3, in fruit bats from China. Virology 2010, 404, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Yu, M.; Zhang, H.; Magoffin, D.E.; Jack, P.J.; Hyatt, A.; Wang, H.Y.; Wang, L.F. Beilong virus, a novel paramyxovirus with the largest genome of non-segmented negative-stranded RNA viruses. Virology 2006, 346, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tidona, C.A.; Kurz, H.W.; Gelderblom, H.R.; Darai, G. Isolation and molecular characterization of a novel cytopathogenic paramyxovirus from tree shrews. Virology 1999, 258, 425–434. [Google Scholar] [CrossRef] [PubMed]
- Drewes, S.; Straková, P.; Drexler, J.F.; Jacob, J.; Ulrich, R.G. Assessing the Diversity of Rodent-Borne Viruses: Exploring of High-Throughput Sequencing and Classical Amplification/Sequencing Approaches. In Advances in Virus Research; Beer, M., Höper, D., Eds.; Academic Press: Cambridge, MA, USA, 2017; Volume 99, pp. 61–108. [Google Scholar]
- Adams, M.J.; Lefkowitz, E.J.; King, A.M.Q.; Harrach, B.; Harrison, R.L.; Knowles, N.J.; Kropinski, A.M.; Krupovic, M.; Kuhn, J.H.; Mushegian, A.R.; et al. Changes to taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2017). Arch. Virol. 2017, 162, 2505–2538. [Google Scholar] [CrossRef] [PubMed]
- Kuroya, M.; Ishida, N.; Shiratori, T. Newborn Virus Pneumonitis (Type Sendai), II. The Isolation of A New Virus. Tohoku J. Exp. Med. 1953, 58, 62. [Google Scholar] [CrossRef] [PubMed]
- Parker, J.C.; Tennant, R.W.; Ward, T.G.; Rowe, W.P. Enzootic Sendai virus infections in mouse breeder colonies within the United States. Science 1964, 146, 936–938. [Google Scholar] [CrossRef] [PubMed]
- Abolins, S.; Lazarou, L.; Weldon, L.; Hughes, L.; King, E.C.; Drescher, P.; Pocock, M.J.O.; Hafalla, J.C.R.; Riley, E.M.; Viney, M. The ecology of immune state in a wild mammal, Mus musculus domesticus. PLoS Biol. 2018, 16, e2003538. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, C.; Healing, T.D.; Evans, N.; Healing, L.; Prior, A. Evidence of Infection by Viruses in Small British Field Rodents. J. Hyg. 1980, 84, 285–294. [Google Scholar] [CrossRef] [PubMed]
- Van der Veen, J.; Poort, Y.; Birchfield, D.J. Experimental transmission of Sendai virus infection in mice. Arch. Gesamte Virusforsch. 1970, 31, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Harris, R.J.; Ellis, J.; Donati, M.; Pebody, R.G. Epidemiology of parainfluenza infection in England and Wales, 1998–2013: Any evidence of change? Epidemiol. Infect. 2017, 145, 1210–1220. [Google Scholar] [CrossRef] [PubMed]
- Branche, A.R.; Falsey, A.R. Parainfluenza Virus Infection. Semin. Respir. Crit. Care Med. 2016, 37, 538–554. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Schaap-Nutt, A.; Bartlett, E.J.; Schomacker, H.; Boonyaratanakornkit, J.; Karron, R.A.; Collins, P.L. Progress in the development of human parainfluenza virus vaccines. Expert Rev. Respir. Med. 2011, 5, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Joshua, J.; de A. Goonatilake, W.L.D.P.T.S.; Molur, S. Ratufa Macroura (Amended Version of 2008 Assessment). The IUCN Red List of Threatened Species 2017; Available online: http://dx.doi.org/10.2305/IUCN.UK.2017-2.RLTS.T19381A117059627.en (accessed on 29 May 2018).
- Mulisch, M.; Welsch, U. Romeis—Mikroskopische Technik, 18th ed.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2010. [Google Scholar]
- Schlottau, K.; Hoffmann, B.; Homeier-Bachmann, T.; Fast, C.; Ulrich, R.G.; Beer, M.; Hoffmann, D. Multiple detection of zoonotic variegated squirrel bornavirus 1 RNA in different squirrel species suggests a possible unknown origin for the virus. Arch. Virol. 2017, 162, 2747–2754. [Google Scholar] [CrossRef] [PubMed]
- Schlottau, K.; Jenckel, M.; van den Brand, J.; Fast, C.; Herden, C.; Höper, D.; Homeier-Bachmann, T.; Thielebein, J.; Mensing, N.; Diender, B.; et al. Variegated Squirrel Bornavirus 1 in Squirrels, Germany and the Netherlands. Emerg. Infect. Dis. 2017, 23, 477–481. [Google Scholar] [CrossRef] [PubMed]
- Bisping, W.; Amtsberg, G. Colour Atlas for the Diagnosis of Bacterial Pathogens in Animals; Paul Parey Scientific Publishers: Berlin/Hamburg, Germany, 1988. [Google Scholar]
- Hanke, D.; Pohlmann, A.; Sauter-Louis, C.; Höper, D.; Stadler, J.; Ritzmann, M.; Steinrigl, A.; Schwarz, B.A.; Akimkin, V.; Fux, R.; et al. Porcine Epidemic Diarrhea in Europe: In-Detail Analyses of Disease Dynamics and Molecular Epidemiology. Viruses 2017, 9, E177. [Google Scholar] [CrossRef] [PubMed]
- Scheuch, M.; Höper, D.; Beer, M. RIEMS: A software pipeline for sensitive and comprehensive taxonomic classification of reads from metagenomics datasets. BMC Bioinform. 2015, 16, 69. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT Multiple Sequence Alignment Software Version 7: Improvements in Performance and Usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [PubMed]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Lole, K.S.; Bollinger, R.C.; Paranjape, R.S.; Gadkari, D.; Kulkarni, S.S.; Novak, N.G.; Ingersoll, R.; Sheppard, H.W.; Ray, S.C. Full-length human immunodeficiency virus type 1 genomes from subtype C-infected seroconverters in India, with evidence of intersubtype recombination. J. Virol. 1999, 73, 152–160. [Google Scholar] [PubMed]
- Toussaint, J.F.; Sailleau, C.; Breard, E.; Zientara, S.; de Clercq, K. Bluetongue virus detection by two real-time RT-qPCRs targeting two different genomic segments. J. Virol. Methods 2007, 140, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Sugai, K.; Tsukagoshi, H.; Nojima, I.; Fujiwara, K.; Kodera, A.; Kimura, N.; Tsuchimoto, K.; Sekimoto, K.; Kitada, K.; Takahashi, N.; et al. Severe acute encephalopathy related to human parainfluenza virus type 2 infection in an infant: A case report. JMM Case Rep. 2015, 2. [Google Scholar] [CrossRef]
- Voudris, K.A.; Vagiakou, E.A.; Skardoutsou, A. Acute disseminated encephalomyelitis associated with parainfluenza virus infection of childhood. Brain Dev. 2002, 24, 112–114. [Google Scholar] [CrossRef]
- Calain, P.; Roux, L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 1993, 67, 4822–4830. [Google Scholar] [PubMed]
- Kozak, M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell 1986, 44, 283–292. [Google Scholar] [CrossRef]
- Patwardhan, S.; Gupta, K.C. Translation initiation potential of the 5′ proximal AUGs of the polycistronic P/C mRNA of Sendai virus. A multipurpose vector for site-specific mutagenesis. J. Biol. Chem. 1988, 263, 4907–4913. [Google Scholar] [PubMed]
- Kozak, M. An analysis of 5′-noncoding sequences from 699 vertebrate messenger RNAs. Nucleic Acids Res. 1987, 15, 8125–8148. [Google Scholar] [CrossRef] [PubMed]
- De Breyne, S.; Simonet, V.; Pelet, T.; Curran, J. Identification of a cis-acting element required for shunt-mediated translational initiation of the Sendai virus Y proteins. Nucleic Acids Res. 2003, 31, 608–618. [Google Scholar] [CrossRef] [PubMed]
- Latorre, P.; Kolakofsky, D.; Curran, J. Sendai virus Y proteins are initiated by a ribosomal shunt. Mol. Cell. Biol. 1998, 18, 5021–5031. [Google Scholar] [CrossRef] [PubMed]
- Irie, T.; Nagata, N.; Yoshida, T.; Sakaguchi, T. Paramyxovirus Sendai virus C proteins are essential for maintenance of negative-sense RNA genome in virus particles. Virology 2008, 374, 495–505. [Google Scholar] [CrossRef] [PubMed]
- Curran, J.; Marq, J.B.; Kolakofsky, D. The Sendai virus nonstructural C proteins specifically inhibit viral mRNA synthesis. Virology 1992, 189, 647–656. [Google Scholar] [CrossRef]
- Tapparel, C.; Hausmann, S.; Pelet, T.; Curran, J.; Kolakofsky, D.; Roux, L. Inhibition of Sendai virus genome replication due to promoter-increased selectivity: A possible role for the accessory C proteins. J. Virol. 1997, 71, 9588–9599. [Google Scholar] [PubMed]
- Bartlett, E.J.; Cruz, A.M.; Esker, J.; Castano, A.; Schomacker, H.; Surman, S.R.; Hennessey, M.; Boonyaratanakornkit, J.; Pickles, R.J.; Collins, P.L.; et al. Human parainfluenza virus type 1 C proteins are nonessential proteins that inhibit the host interferon and apoptotic responses and are required for efficient replication in nonhuman primates. J. Virol. 2008, 82, 8965–8977. [Google Scholar] [CrossRef] [PubMed]
- Schomacker, H.; Hebner, R.M.; Boonyaratanakornkit, J.; Surman, S.; Amaro-Carambot, E.; Collins, P.L.; Schmidt, A.C. The C proteins of human parainfluenza virus type 1 block IFN signaling by binding and retaining STAT1 in perinuclear aggregates at the late endosome. PLoS ONE 2012, 7, e28382. [Google Scholar] [CrossRef] [PubMed]
- Boeck, R.; Curran, J.; Matsuoka, Y.; Compans, R.; Kolakofsky, D. The parainfluenza virus type 1 P/C gene uses a very efficient GUG codon to start its C′ protein. J. Virol. 1992, 66, 1765–1768. [Google Scholar] [PubMed]
- Dillon, P.J.; Gupta, K.C. Expression of five proteins from the Sendai virus P/C mRNA in infected cells. J. Virol. 1989, 63, 974–977. [Google Scholar] [PubMed]
- Vidal, S.; Curran, J.; Kolakofsky, D. Editing of the Sendai virus P/C mRNA by G insertion occurs during mRNA synthesis via a virus-encoded activity. J. Virol. 1990, 64, 239–246. [Google Scholar] [PubMed]
- Horikami, S.M.; Smallwood, S.; Moyer, S.A. The Sendai virus V protein interacts with the NP protein to regulate viral genome RNA replication. Virology 1996, 222, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Fukuhara, N.; Huang, C.; Kiyotani, K.; Yoshida, T.; Sakaguchi, T. Mutational analysis of the Sendai virus V protein: Importance of the conserved residues for Zn binding, virus pathogenesis, and efficient RNA editing. Virology 2002, 299, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Kiyotani, K.; Sakaguchi, T.; Kato, A.; Nagai, Y.; Yoshida, T. Paramyxovirus Sendai virus V protein counteracts innate virus clearance through IRF-3 activation, but not via interferon, in mice. Virology 2007, 359, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Rochat, S.; Komada, H.; Kolakofsky, D. Loss of V protein expression in human parainfluenza virus type 1 is not a recent event. Virus Res. 1992, 24, 137–144. [Google Scholar] [CrossRef]
- Henrickson, K.J.; Savatski, L.L. Antigenic structure, function, and evolution of the hemagglutinin-neuraminidase protein of human parainfluenza virus type 1. J. Infect. Dis. 1997, 176, 867–875. [Google Scholar] [CrossRef] [PubMed]
- King, A.; Adams, M.; Carstens, E.; Lefkowitz, E. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses; Waltham Academic Press: London, UK, 2012. [Google Scholar]
- Greenwood, A.G.; Sanchez, S. Serological evidence of murine pathogens in wild grey squirrels (Sciurus carolinensis) in North Wales. Vet. Rec. 2002, 150, 543–546. [Google Scholar] [CrossRef] [PubMed]
- Brooks, F.; Wood, A.R.; Thomson, J.; Deane, D.; Everest, D.J.; McInnes, C.J. Preliminary characterisation of Pentlands paramyxovirus-1, -2 and -3, three new paramyxoviruses of rodents. Vet. Microbiol. 2014, 170, 391–397. [Google Scholar] [CrossRef] [PubMed]
- International Committee on Taxonomy of Viruses (ICTV). ICTV Master Species List #32, 2018 ed.; ICTV: Wayne, PA, USA, 12 March 2018. [Google Scholar]
- Skiadopoulos, M.H.; Surman, S.R.; Riggs, J.M.; Elkins, W.R.; St Claire, M.; Nishio, M.; Garcin, D.; Kolakofsky, D.; Collins, P.L.; Murphy, B.R. Sendai virus, a murine parainfluenza virus type 1, replicates to a level similar to human PIV1 in the upper and lower respiratory tract of African green monkeys and chimpanzees. Virology 2002, 297, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Lyn, D.; Gill, D.S.; Scroggs, R.A.; Portner, A. The nucleoproteins of human parainfluenza virus type 1 and Sendai virus share amino acid sequences and antigenic and structural determinants. J. Gen. Virol. 1991, 72, 983–987. [Google Scholar] [CrossRef] [PubMed]
- Adderson, E.; Branum, K.; Sealy, R.E.; Jones, B.G.; Surman, S.L.; Penkert, R.; Freiden, P.; Slobod, K.S.; Gaur, A.H.; Hayden, R.T.; et al. Safety and immunogenicity of an intranasal Sendai virus-based human parainfluenza virus type 1 vaccine in 3- to 6-year-old children. Clin. Vaccine Immunol. 2015, 22, 298–303. [Google Scholar] [CrossRef] [PubMed]
- Slobod, K.S.; Shenep, J.L.; Lujan-Zilbermann, J.; Allison, K.; Brown, B.; Scroggs, R.A.; Portner, A.; Coleclough, C.; Hurwitz, J.L. Safety and immunogenicity of intranasal murine parainfluenza virus type 1 (Sendai virus) in healthy human adults. Vaccine 2004, 22, 3182–3186. [Google Scholar] [CrossRef] [PubMed]

| Function | MRV (Nagoya) | GSqRV (Sri Lanka) | HRV1 (Washington) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Start | Stop | Length | Start | Stop | Length | Start | Stop | Length | ||
| N | RNA-binding protein | 120 | 1694 | 1575 | 120 | 1694 | 1575 | 120 | 1694 | 1575 |
| C’ | Accessory protein | 1821 | 2468 | 648 | 1821 | 2468 | 648 | 1809 | 2468 | 660 |
| P | Phosphoprotein | 1844 | 3550 | 1707 | 1844 | 3550 | 1707 | 1844 | 3550 | 1707 |
| C | Accessory protein | 1854 | 2468 | 615 | 1854 | 2468 | 615 | 1854 | 2468 | 615 |
| Y0 | Putative accessory protein | - | - | - | 1884 | 2468 | 585 | - | - | - |
| Y1 | Accessory protein | 1923 | 2468 | 546 | 1923 | 2468 | 546 | 1923 | 2468 | 546 |
| Y2 | Accessory protein | 1941 | 2468 | 528 | 1941 | 2468 | 528 | 1941 | 2468 | 528 |
| M | Matrix protein | 3669 | 4715 | 1047 | 3669 | 4715 | 1047 | 3669 | 4715 | 1047 |
| F | Fusion protein | 4866 | 6563 | 1698 | 4860 | 6581 | 1722 | 5088 | 6755 | 1668 |
| HN | Attachment protein | 6693 | 8420 | 1728 | 6687 | 8411 | 1725 | 6903 | 8630 | 1728 |
| L | Large polymerase protein | 8556 | 15,242 | 6687 | 8550 | 15,236 | 6687 | 8772 | 15,443 | 6672 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Forth, L.F.; Konrath, A.; Klose, K.; Schlottau, K.; Hoffmann, K.; Ulrich, R.G.; Höper, D.; Pohlmann, A.; Beer, M. A Novel Squirrel Respirovirus with Putative Zoonotic Potential. Viruses 2018, 10, 373. https://doi.org/10.3390/v10070373
Forth LF, Konrath A, Klose K, Schlottau K, Hoffmann K, Ulrich RG, Höper D, Pohlmann A, Beer M. A Novel Squirrel Respirovirus with Putative Zoonotic Potential. Viruses. 2018; 10(7):373. https://doi.org/10.3390/v10070373
Chicago/Turabian StyleForth, Leonie F., Andrea Konrath, Kristin Klose, Kore Schlottau, Kathrin Hoffmann, Rainer G. Ulrich, Dirk Höper, Anne Pohlmann, and Martin Beer. 2018. "A Novel Squirrel Respirovirus with Putative Zoonotic Potential" Viruses 10, no. 7: 373. https://doi.org/10.3390/v10070373
APA StyleForth, L. F., Konrath, A., Klose, K., Schlottau, K., Hoffmann, K., Ulrich, R. G., Höper, D., Pohlmann, A., & Beer, M. (2018). A Novel Squirrel Respirovirus with Putative Zoonotic Potential. Viruses, 10(7), 373. https://doi.org/10.3390/v10070373





