The Auxiliary Role of the Amidase Domain in Cell Wall Binding and Exolytic Activity of Staphylococcal Phage Endolysins
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Growth Conditions
2.2. In Silico Analysis of Staphylococcal Endolysins
2.3. Cloning, Expression, and Purification of S. aureus Endolysin Derivatives and EGFP Fusion Proteins
2.4. Lytic Activity Assay of Endolysin and Their Derivatives
2.5. EGFP Fusion Protein Binding Assay
3. Results
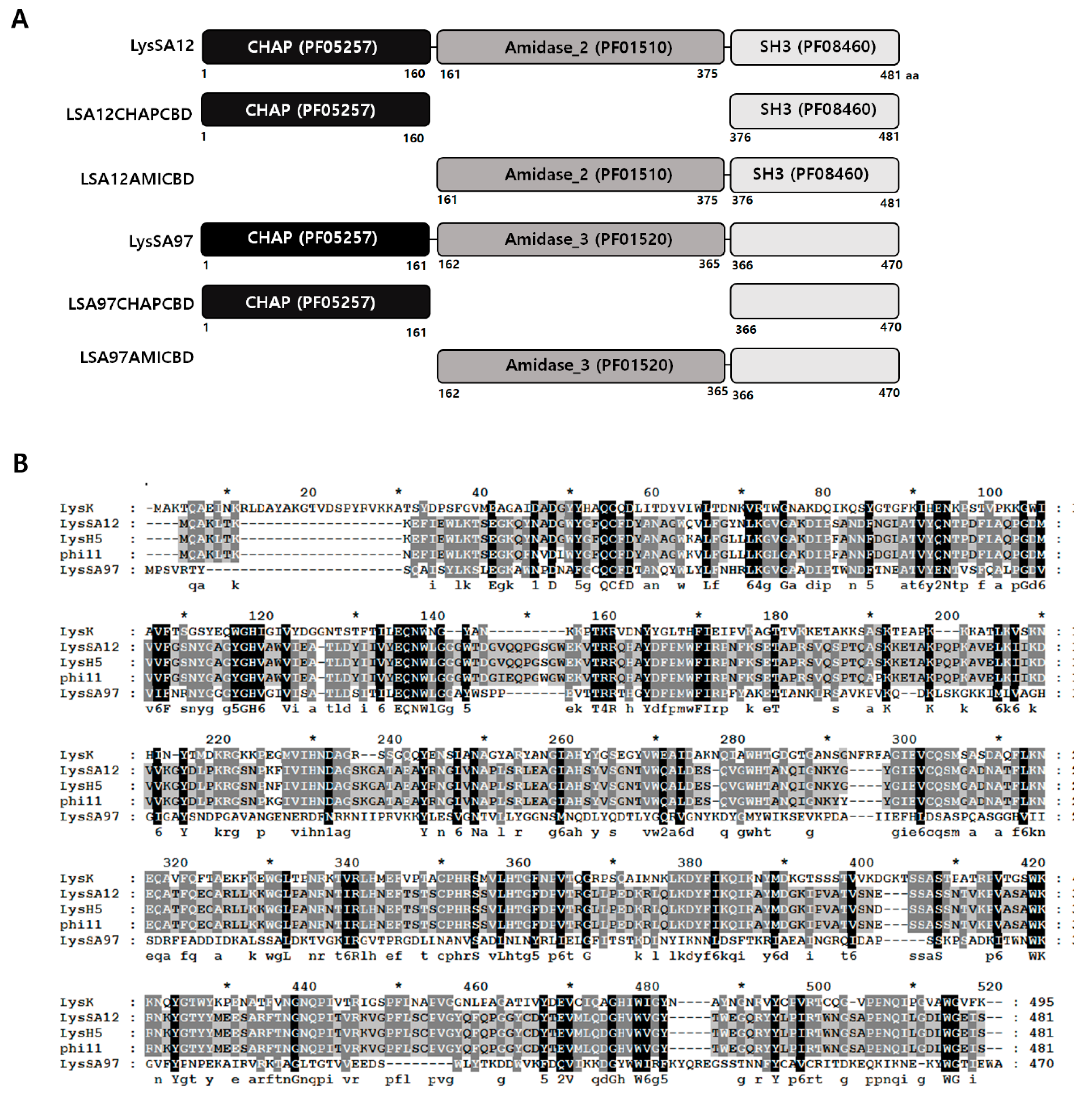
3.1. Modular Structure of LysSA12
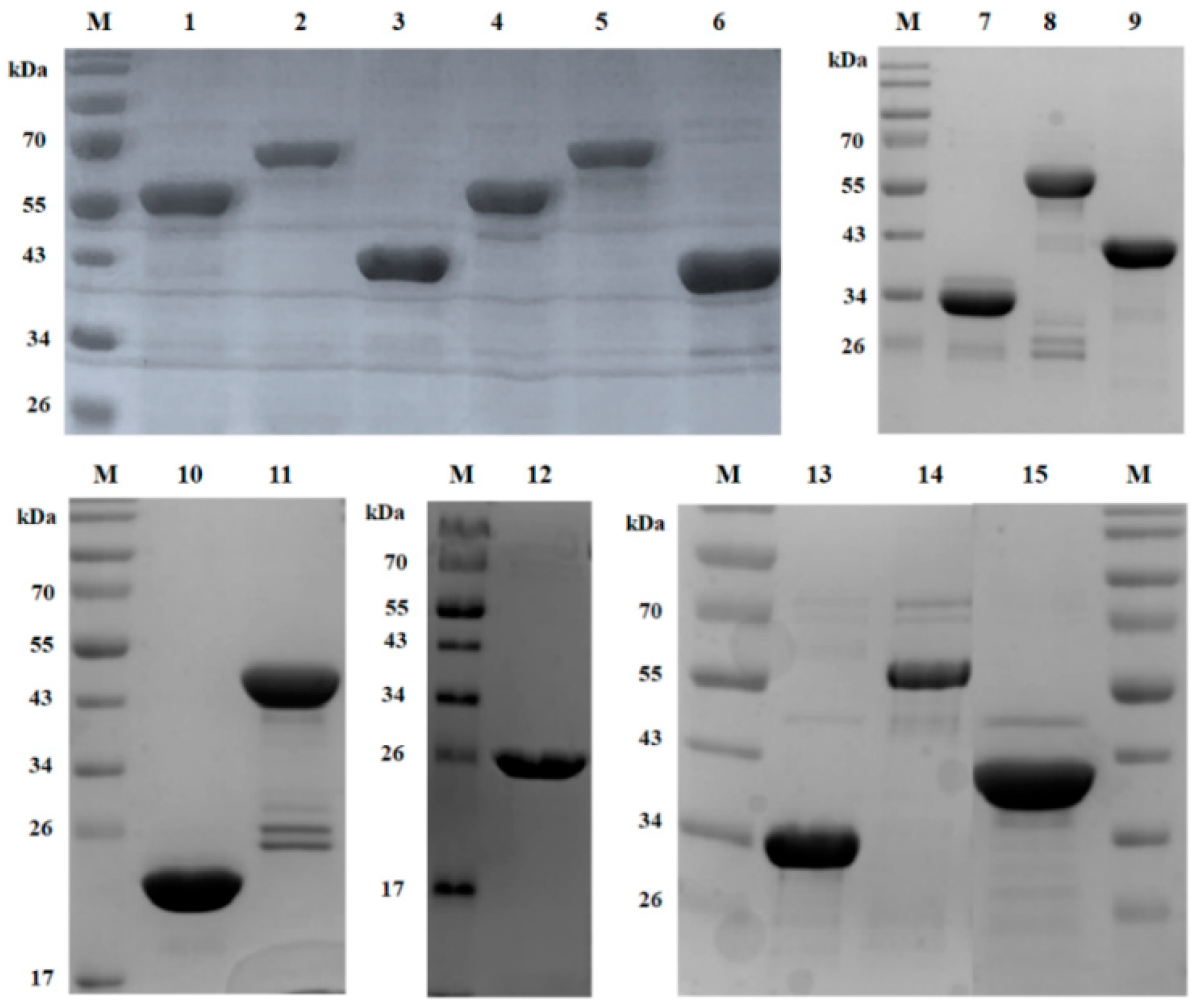
3.2. Expression and Purification of LysSA12 Derivatives
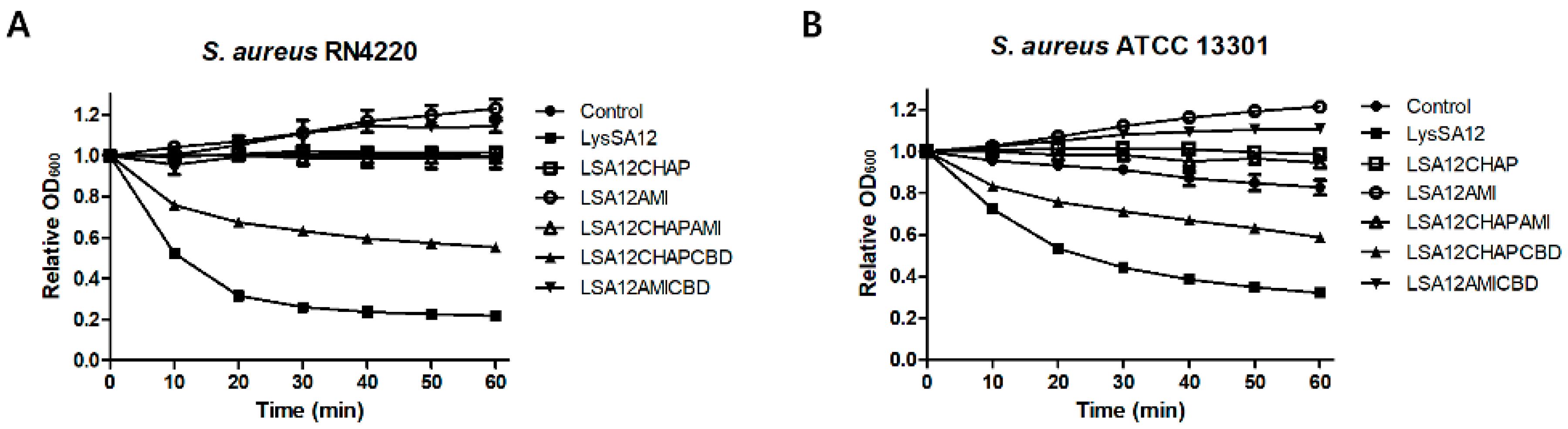
3.3. Lytic Activities of LysSA12 and Its Truncated Proteins
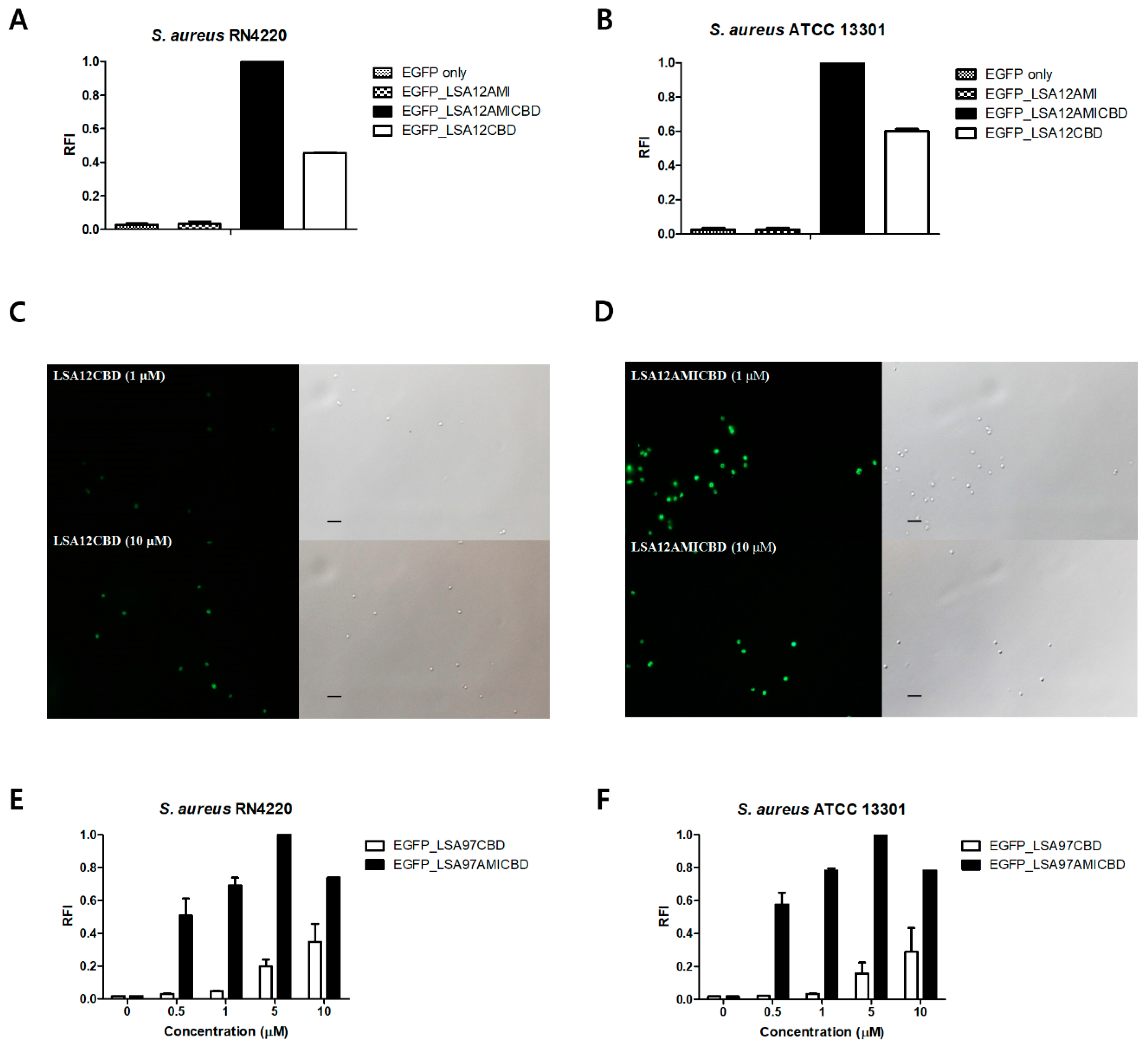
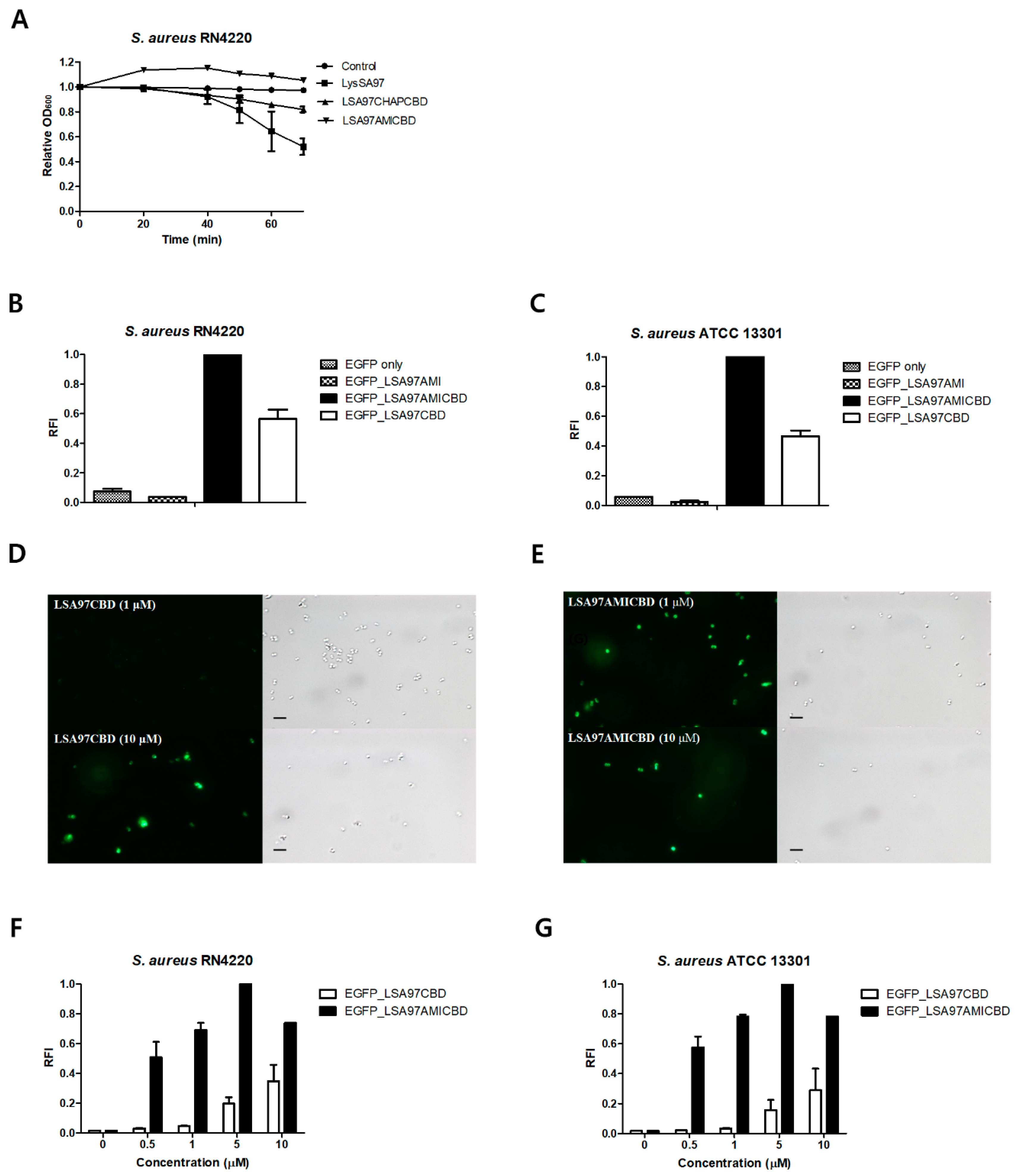
3.4. Amidase Domain Helps CBD Bind to Intact Cells
3.5. Role of the LysSA97 Amidase Domain
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Lindsay, J.A.; Holden, M.T. Staphylococcus aureus: Superbug, super genome? Trends Microbiol. 2004, 12, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Tong, S.Y.; Davis, J.S.; Eichenberger, E.; Holland, T.L.; Fowler, V.G. Staphylococcus aureus infections: Epidemiology, pathophysiology, clinical manifestations, and management. Clin. Microbiol. Rev. 2015, 28, 603–661. [Google Scholar] [CrossRef] [PubMed]
- Furuno, J.P.; Perencevich, E.N.; Johnson, J.A.; Wright, M.-O.; McGregor, J.C.; Morris, J.G., Jr.; Strauss, S.M.; Roghman, M.-C.; Nemoy, L.L.; Standiford, H.C. Methicillin-resistant Staphylococcus aureus and vancomycin-resistant enterococci co-colonization. Emerg. Infect. Dis. 2005, 11, 1539. [Google Scholar] [CrossRef] [PubMed]
- Seybold, U.; Kourbatova, E.V.; Johnson, J.G.; Halvosa, S.J.; Wang, Y.F.; King, M.D.; Ray, S.M.; Blumberg, H.M. Emergence of community-associated methicillin-resistant Staphylococcus aureus USA300 genotype as a major cause of health care—Associated blood stream infections. Clin. Infect. Dis. 2006, 42, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J. Bacteriophage endolysins—Current state of research and applications. Curr. Opin. Microbiol. 2005, 8, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Borysowski, J.; Weber-Dąbrowska, B.; Górski, A. Bacteriophage endolysins as a novel class of antibacterial agents. Exp. Biol. Med. 2006, 231, 366–377. [Google Scholar] [CrossRef]
- Chang, Y.; Ryu, S. Characterization of a novel cell wall binding domain-containing Staphylococcus aureus endolysin LysSA97. Appl. Microbiol. Biotechnol. 2017, 101, 147–158. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Dong, S.; Baker, J.R.; Foster-Frey, J.; Pritchard, D.G.; Donovan, D.M. LysK CHAP endopeptidase domain is required for lysis of live staphylococcal cells. FEMS Microbiol. Lett. 2009, 294, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Navarre, W.W.; Ton-That, H.; Faull, K.F.; Schneewind, O. Multiple Enzymatic Activities of the Murein Hydrolase from Staphylococcal Phage φ11. Identification of a D-alanyl-glycine endopeptidase activity. J. Biol. Chem. 1999, 274, 15847–15856. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Rubio, L.; Martínez, B.; Rodríguez, A.; Donovan, D.M.; Götz, F.; García, P. The phage lytic proteins from the Staphylococcus aureus bacteriophage vB_SauS-phiIPLA88 display multiple active catalytic domains and do not trigger staphylococcal resistance. PLoS ONE 2013, 8, e64671. [Google Scholar]
- Sass, P.; Bierbaum, G. Lytic activity of recombinant bacteriophage φ11 and φ12 endolysins on whole cells and biofilms of Staphylococcus aureus. Appl. Environ. Microbiol. 2007, 73, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Kashani, H.H.; Schmelcher, M.; Sabzalipoor, H.; Hosseini, E.S.; Moniri, R. Recombinant endolysins as potential therapeutics against antibiotic-resistant Staphylococcus aureus: Current status of research and novel delivery strategies. Clin. Microbiol. Rev. 2018, 31, e00071-17. [Google Scholar]
- Abaev, I.; Foster-Frey, J.; Korobova, O.; Shishkova, N.; Kiseleva, N.; Kopylov, P.; Pryamchuk, S.; Schmelcher, M.; Becker, S.C.; Donovan, D.M. Staphylococcal phage 2638A endolysin is lytic for Staphylococcus aureus and harbors an inter-lytic-domain secondary translational start site. Appl. Microbiol. Biotechnol. 2013, 97, 3449–3456. [Google Scholar] [CrossRef] [PubMed]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Altschul, S.F.; Madden, T.L.; Schäffer, A.A.; Zhang, J.; Zhang, Z.; Miller, W.; Lipman, D.J. Gapped BLAST and PSI-BLAST: A new generation of protein database search programs. Nucleic Acids Res. 1997, 25, 3389–3402. [Google Scholar] [CrossRef] [PubMed]
- Finn, R.D.; Bateman, A.; Clements, J.; Coggill, P.; Eberhardt, R.Y.; Eddy, S.R.; Heger, A.; Hetherington, K.; Holm, L.; Mistry, J. Pfam: The protein families database. Nucleic Acids Res. 2013, 42, D222–D230. [Google Scholar] [CrossRef] [PubMed]
- Larkin, M.A.; Blackshields, G.; Brown, N.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [PubMed]
- Gasteiger, E.; Hoogland, C.; Gattiker, A.; Wilkins, M.R.; Appel, R.D.; Bairoch, A. Protein identification and analysis tools on the ExPASy server. In The Proteomics Protocols Handbook; Springer: Berlin, Germany, 2005; pp. 571–607. [Google Scholar]
- Chang, Y.; Yoon, H.; Kang, D.-H.; Chang, P.-S.; Ryu, S. Endolysin LysSA97 is synergistic with carvacrol in controlling Staphylococcus aureus in foods. Int. J. Food Microbiol. 2017, 244, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Shin, H.; Lee, J.-H.; Park, C.J.; Paik, S.-Y.; Ryu, S. Isolation and genome characterization of the virulent Staphylococcus aureus bacteriophage SA97. Viruses 2015, 7, 5225–5242. [Google Scholar] [CrossRef] [PubMed]
- Kong, M.; Sim, J.; Kang, T.; Nguyen, H.H.; Park, H.K.; Chung, B.H.; Ryu, S. A novel and highly specific phage endolysin cell wall binding domain for detection of Bacillus cereus. Eur. Biophys. J. 2015, 44, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Son, B.; Yun, J.; Lim, J.-A.; Shin, H.; Heu, S.; Ryu, S. Characterization of LysB4, an endolysin from the Bacillus cereus-infecting bacteriophage B4. BMC Microbiol. 2012, 12, 33. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, A.; Sekiguchi, J. Cloning, sequencing and genetic mapping of a Bacillus subtilis cell wall hydrolase gene. Microbiology 1990, 136, 2209–2216. [Google Scholar] [CrossRef] [PubMed]
- Loessner, M.J.; Kramer, K.; Ebel, F.; Scherer, S. C-terminal domains of Listeria monocytogenes bacteriophage murein hydrolases determine specific recognition and high-affinity binding to bacterial cell wall carbohydrates. Mol. Microbiol. 2002, 44, 335–349. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.; Lee, J.-H.; Shin, H.; Heu, S.; Ryu, S. Characterization and complete genome sequence analysis of Staphylococcus aureus bacteriophage SA12. Virus Genes 2013, 47, 389–393. [Google Scholar] [CrossRef] [PubMed]
- García, P.; Martínez, B.; Rodríguez, L.; Rodríguez, A. Synergy between the phage endolysin LysH5 and nisin to kill Staphylococcus aureus in pasteurized milk. Int. J. Food Microbiol. 2010, 141, 151–155. [Google Scholar]
- O’flaherty, S.; Coffey, A.; Meaney, W.; Fitzgerald, G.; Ross, R. The recombinant phage lysin LysK has a broad spectrum of lytic activity against clinically relevant staphylococci, including methicillin-resistant Staphylococcus aureus. J. Bacteriol. 2005, 187, 7161–7164. [Google Scholar]
- Takano, M.; Oshida, T.; Yasojima, A.; Yamada, M.; Okagaki, C.; Sugai, M.; Suginaka, H.; Matsushita, T. Modification of autolysis by synthetic peptides derived from the presumptive binding domain of Staphylococcus aureus autolysin. Microbiol. Immunol. 2000, 44, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Foster-Frey, J.; Stodola, A.J.; Anacker, D.; Donovan, D.M. Differentially conserved staphylococcal SH3b_5 cell wall binding domains confer increased staphylolytic and streptolytic activity to a streptococcal prophage endolysin domain. Gene 2009, 443, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M.; Foster-Frey, J. LambdaSa2 prophage endolysin requires Cpl-7-binding domains and amidase-5 domain for antimicrobial lysis of streptococci. FEMS Microbiol. Lett. 2008, 287, 22–33. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Díez-Martínez, R.; de Paz, H.; Bustamante, N.; García, E.; Menéndez, M.; García, P. Improving the lethal effect of Cpl-7, a pneumococcal phage lysozyme with broad bactericidal activity, by inverting the net charge of its cell wall-binding module. Antimicrob. Agents Chemother. 2013, 57, 5355–5365. [Google Scholar] [CrossRef] [PubMed]
- Fischetti, V.A. Bacteriophage endolysins: A novel anti-infective to control Gram-positive pathogens. Int. J. Med. Microbiol. 2010, 300, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Future Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.C.; Foster-Frey, J.; Donovan, D.M. The phage K lytic enzyme LysK and lysostaphin act synergistically to kill MRSA. FEMS Microbiol. Lett. 2008, 287, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Donovan, D.M.; Foster-Frey, J.; Dong, S.; Rousseau, G.M.; Moineau, S.; Pritchard, D.G. The cell lysis activity of the Streptococcus agalactiae bacteriophage B30 endolysin relies on the cysteine, histidine-dependent amidohydrolase/peptidase domain. Appl. Environ. Microbiol. 2006, 72, 5108–5112. [Google Scholar] [CrossRef] [PubMed]
- Pritchard, D.G.; Dong, S.; Kirk, M.C.; Cartee, R.T.; Baker, J.R. LambdaSa1 and LambdaSa2 prophage lysins of Streptococcus agalactiae. Appl. Environ. Microbiol. 2007, 73, 7150–7154. [Google Scholar] [CrossRef] [PubMed]
- Eugster, M.R.; Loessner, M.J. Wall teichoic acids restrict access of bacteriophage endolysin Ply118, Ply511, and PlyP40 cell wall binding domains to the Listeria monocytogenes peptidoglycan. J. Bacteriol. 2012, 194, 6498–6506. [Google Scholar] [CrossRef] [PubMed]
- Schlag, M.; Biswas, R.; Krismer, B.; Kohler, T.; Zoll, S.; Yu, W.; Schwarz, H.; Peschel, A.; Götz, F. Role of staphylococcal wall teichoic acid in targeting the major autolysin Atl. Mol. Microbiol. 2010, 75, 864–873. [Google Scholar] [CrossRef] [PubMed]
- Bhavsar, A.P.; Brown, E.D. Cell wall assembly in Bacillus subtilis: How spirals and spaces challenge paradigms. Mol. Microbiol. 2006, 60, 1077–1090. [Google Scholar] [CrossRef] [PubMed]
| Plasmids | ||
| Description | Reference | |
| pET28a | Kanr, T7 promoter, His-tagged expression vector | Novagen, Wisconsin, SA |
| pET28a-EGFP | pET28a with EGFP | This study |
| pET28a-LysSA12 | pET28a with LysSA12 (56 kDa) | [19] |
| pET28a-LSA12CBD | pET28a with LSA12CBD | This study |
| pET28a-LSA12CHAP | pET28a with LSA12CHAP (20 kDa) | This study |
| pET28a-LSA12CHAPCBD | pET28a-LSA12CBD with LSA12CHAP (32 kDa) | This study |
| pET28a-LSA12AMICBD | pET28a with LSA12AMICBD (44 kDa) | This study |
| pET28a-LysSA97 | pET28a with LysSA97 (56 kDa) | [19] |
| pET28a-LSA97CBD | pET28a with LSA97CBD | This study |
| pET28a-LSA97CHAPCBD | pET28a-LSA97CBD with LSA97CHAP (33 kDa) | This study |
| pET28a-LSA97AMICBD | pET28a with LSA97AMICBD (38 kDa) | This study |
| pET28a-EGFP_LSA12CBD | pET28a-EGFP with LSA12CBD (42 kDa) | This study |
| pET28a-EGFP_LSA12AMICBD | pET28a-EGFP with LSA12AMICBD (66 kDa) | This study |
| pET28a-EGFP_LSA12AMI | pET28a-EGFP with LSA12AMI (54 kDa) | This study |
| pET28a-EGFP_LSA97CBD | pET28a-EGFP with LSA97CBD (42 kDa) | [7] |
| pET28a-EGFP_LSA97AMICBD | pET28a-EGFP with LSA97AMICBD (65 kDa) | This study |
| pET28a-EGFP_LSA97AMI | pET28a-EGFP with LSA97AMI (52 kDa) | This study |
| Primers (5′→3′) a | ||
| Sequence | Purpose | |
| BamH1_LSA12CHAP_F | AAA GGA TCC ATGC AAG CAA AAC TAA CTA AAA A | pET28a-LSA12CHAP and pET28a-LSA12CHAPCBD construction |
| LSA12CHAP_Sal1_R | TTT GTC GAC TGA TCG TGG AGC TGT TTC GCT T | pET28a-LSA12CHAP construction |
| LSA12CHAP_EcoR1_R | TTT GAA TTC TGA TCG TGG AGC TGT TTC GCT T | pET28a-LSA12CHAPCBD construction |
| BamH1_LSA12AMI_F | AAA GGA TCC GTA CAA TCT CCT ACG CAA GCA | pET28a-LSA12AMI, pET28a-LSA12AMICBD, pET28a-EGFP_LSA12AMI and pET28a-EGFP_LSA12AMICBD construction |
| LSA12AMI_Sal1_R | TTT GTC GAC ACT TGA AGC GCT TGA CTC ATT AG | pET28a-LSA12AMI and pET28a-EGFP_LSA12AMI construction |
| EcoR1_LSA12CBD_F | AAA GAA TTC TCA AGT AAT ACA GTT AAA CCA GT | pET28a-LSA12CBD construction |
| LSA12CBD_Sal1_R | TTT GTC GAC ACT GAT TTC TCC CCA TAA GT | pET28a-LSA12CBD, pET28a-LSA12AMICBD and pET28a-EGFP_LSA12AMICBD construction |
| BamH1_LSA97CHAP_F | AAA GGA TCC ATG CCG TCG GTT AGG ACA TAC AG | pET28a-LSA97CHAP and pET28a-LSA97CHAPCBD construction |
| LSA97CHAP_Sal1_R | TTT GTC GAC TTC TTT TGC GTA GAA TGG ACG GAT | pET28a-LSA97CHAPconstruction |
| LSA97CHAP_EcoR1_R | TTT GAA TTC TTC TTT TGC GTA GAA TGG ACG GAT | pET28a-LSA97CHAPCBD construction |
| BamH1_LSA97AMI_F | AAA GGA TCC CAA GAT AAG TTA TCA AAA GGT AAA | pET28a-LSA97AMICBD, pET28a-EGFP_LSA97AMICBD and pET28a-EGFP_LSA97AMI construction |
| LSA97AMI_Sal1_R | TTT GTC GAC ACT ACT TGG CGC ATC AAT TTG TC | pET28a-EGFP_LSA97AMI construction |
| EcoR1_LSA97CBD_F | AAA GAA TTC AGT AGT AAG CCA AGC GCT GAC AA | pET28a-LSA97CBD construction |
| LSA97CBD_Sal1_R | TTT GTC GAC TTA AGC CCA CTC AAT CGT GCC CCA | pET28a-LSA97CBD, pET28a-LSA97AMICBD and pET28a-EGFP_LSA97AMICBD construction |
| Nde1_EGFP_F | AAA CAT ATG ATG GTG AGC AAG GGC GAG GA | pET28a-EGFP construction |
| EGFP_BamH1_R | TTT GGA TCC CTT GTA CAG CTC GTC CAT GCC G | |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Son, B.; Kong, M.; Ryu, S. The Auxiliary Role of the Amidase Domain in Cell Wall Binding and Exolytic Activity of Staphylococcal Phage Endolysins. Viruses 2018, 10, 284. https://doi.org/10.3390/v10060284
Son B, Kong M, Ryu S. The Auxiliary Role of the Amidase Domain in Cell Wall Binding and Exolytic Activity of Staphylococcal Phage Endolysins. Viruses. 2018; 10(6):284. https://doi.org/10.3390/v10060284
Chicago/Turabian StyleSon, Bokyung, Minsuk Kong, and Sangryeol Ryu. 2018. "The Auxiliary Role of the Amidase Domain in Cell Wall Binding and Exolytic Activity of Staphylococcal Phage Endolysins" Viruses 10, no. 6: 284. https://doi.org/10.3390/v10060284
APA StyleSon, B., Kong, M., & Ryu, S. (2018). The Auxiliary Role of the Amidase Domain in Cell Wall Binding and Exolytic Activity of Staphylococcal Phage Endolysins. Viruses, 10(6), 284. https://doi.org/10.3390/v10060284






