The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity
Abstract
1. Introduction
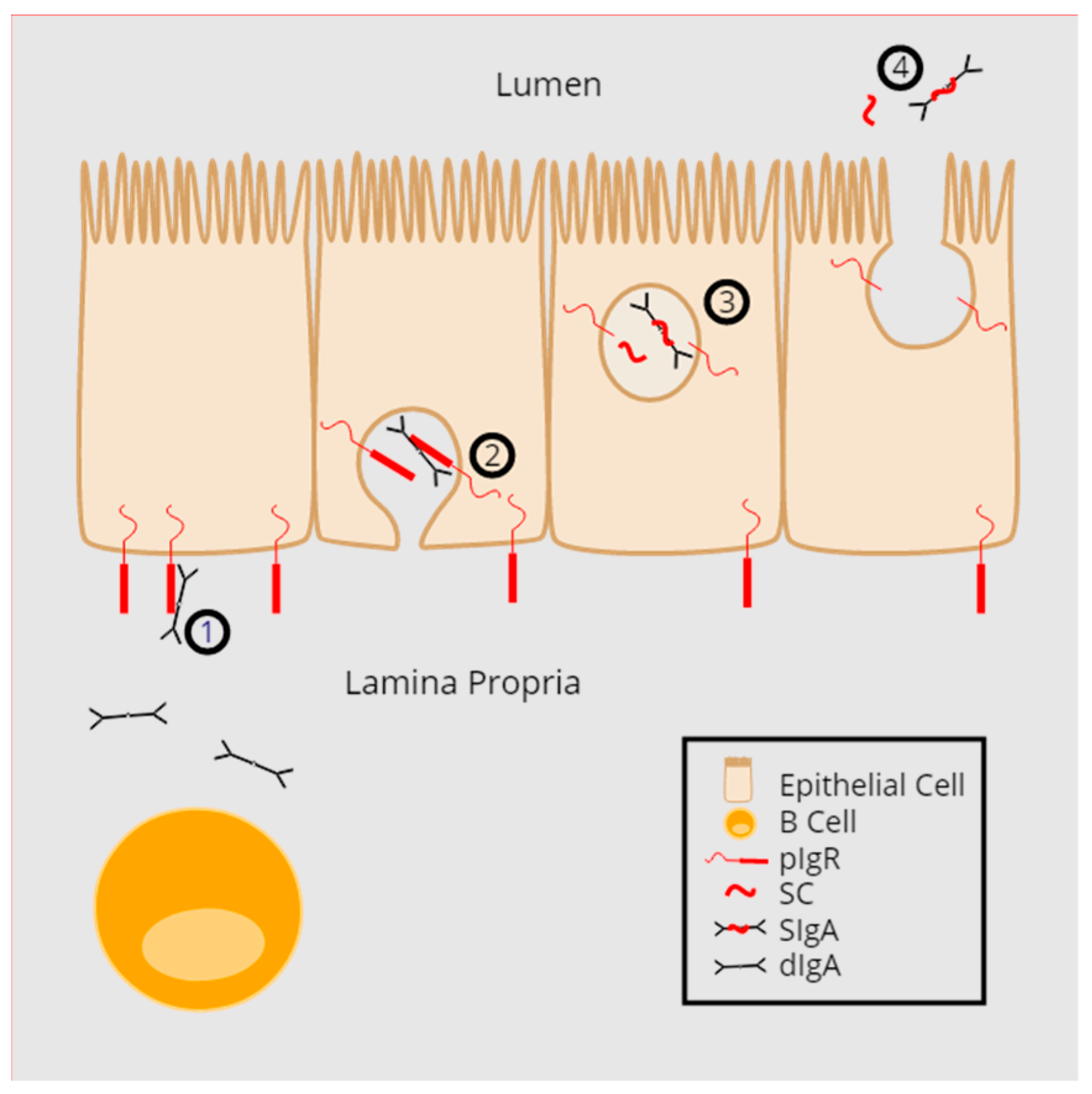
2. pIgR Structure and Function
3. The Multiple Functions of Secretory Component (SC)
4. Regulation of the pIgR/SIg System
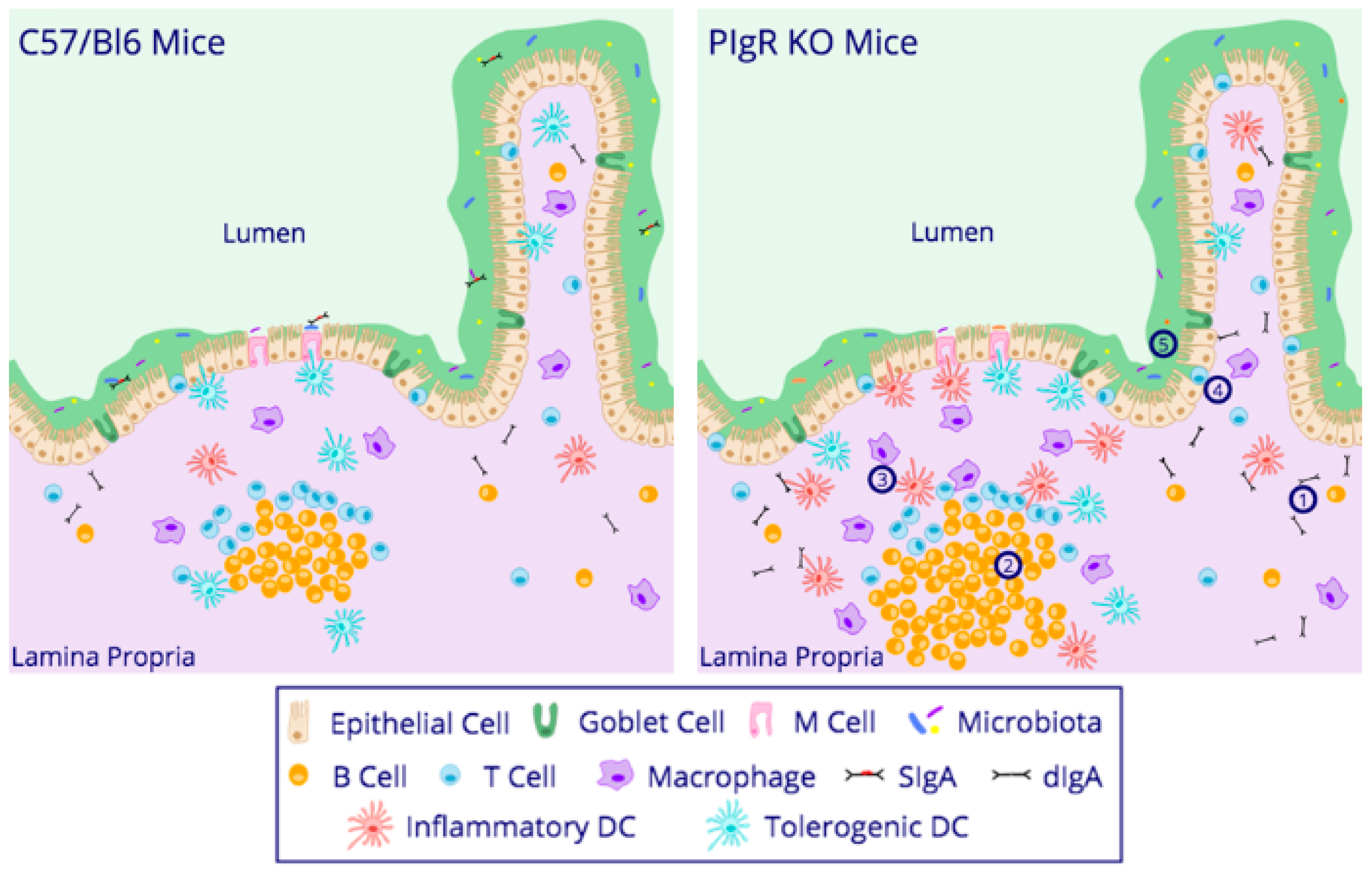
5. Lessons from pIgR-Deficient Mice
6. Agglutination and Exclusion of Pathogens from Mucosal Surfaces (Immune Exclusion)
7. Intracellular Neutralization and Excretion of Pathogens
8. SIg-Mediated Immune Modulation during Infection
9. SIg-Induced Complement Activation and Immune Pathology
10. Subversion of the pIgR/SIg System by Pathogens
11. Conclusions
12. Outstanding Questions
- Are pathogenic infections modulated by natural non-specific SIg or SC and do pathogens modulate that response?
- Can non-bacterial mucosal infections be controlled by SIg-mediated agglutination?
- Does intracellular neutralization of viral infections by SIgA and basolateral to apical excretion of SIgA-immune complexes extend to non-viral infections, occur in vivo, and affect within or between host spread?
- What role does SIg-induced complement activation play during infection with mucosal pathogens and colonization of commensals?
- How common are pIgR/SIg subversion or evasion mechanisms among mucosal pathogens from different kingdoms and are the strategies shared or specific?
- What is the role of SIgM-immune complexes during mucosal homeostasis and pathogenesis?
- What is the identity of the SIg receptor on M cells?
- What breakthroughs will the future hold when organoid technology is applied to the study of the pIgR/SIg cycle?
Acknowledgments
Conflicts of Interest
References
- Bunker, J.J.; Erickson, S.A.; Flynn, T.M.; Henry, C.; Koval, J.C.; Meisel, M.; Jabri, B.; Antonopoulos, D.A.; Wilson, P.C.; Bendelac, A.; et al. Natural polyreactive IgA antibodies coat the intestinal microbiota. Science 2017, 358. [Google Scholar] [CrossRef] [PubMed]
- Macpherson, A.J.; Gatto, D.; Sainsbury, E.; Harriman, G.R.; Hengartner, H.; Zinkernagel, R.M. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science 2000, 288, 2222–2226. [Google Scholar] [CrossRef] [PubMed]
- Quan, C.P.; Berneman, A.; Pires, R.; Avrameas, S.; Bouvet, J.P. Natural polyreactive secretory immunoglobulin A autoantibodies as a possible barrier to infection in humans. Infect. Immun. 1997, 65, 3997–4004. [Google Scholar] [PubMed]
- Pabst, O.; Cerovic, V.; Hornef, M. Secretory IgA in the Coordination of Establishment and Maintenance of the Microbiota. Trends Immunol. 2016, 37, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Mantis, N.J.; Cheung, M.C.; Chintalacharuvu, K.R.; Rey, J.; Corthesy, B.; Neutra, M.R. Selective adherence of IgA to murine Peyer’s patch M cells: Evidence for a novel IgA receptor. J. Immunol. 2002, 169, 1844–1851. [Google Scholar] [CrossRef] [PubMed]
- Stadtmueller, B.M.; Huey-Tubman, K.E.; Lopez, C.J.; Yang, Z.; Hubbell, W.L.; Bjorkman, P.J. The structure and dynamics of secretory component and its interactions with polymeric immunoglobulins. eLife 2016, 5, e10640. [Google Scholar] [CrossRef] [PubMed]
- Asano, M.; Komiyama, K. Polymeric immunoglobulin receptor. J. Oral. Sci. 2011, 53, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Schneeman, T.A.; Bruno, M.E.; Schjerven, H.; Johansen, F.E.; Chady, L.; Kaetzel, C.S. Regulation of the polymeric Ig receptor by signaling through TLRs 3 and 4: Linking innate and adaptive immune responses. J. Immunol. 2005, 175, 376–384. [Google Scholar] [CrossRef] [PubMed]
- Mikami, Y.; Iwase, T.; Komiyama, Y.; Matsumoto, N.; Oki, H.; Komiyama, K. Secretory leukocyte protease inhibitor inhibits expression of polymeric immunoglobulin receptor via the NF-κB signaling pathway. Mol. Immunol. 2015, 67, 568–574. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S.; Robinson, J.K.; Chintalacharuvu, K.R.; Vaerman, J.P.; Lamm, M.E. The polymeric immunoglobulin receptor (secretory component) mediates transport of immune complexes across epithelial cells: A local defense function for IgA. Proc. Natl. Acad. Sci. USA 1991, 88, 8796–8800. [Google Scholar] [CrossRef] [PubMed]
- Johansen, F.E.; Braathen, R.; Brandtzaeg, P. The J chain is essential for polymeric Ig receptor-mediated epithelial transport of IgA. J. Immunol. 2001, 167, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Bakos, M.A.; Kurosky, A.; Goldblum, R.M. Characterization of a critical binding site for human polymeric Ig on secretory component. J. Immunol. 1991, 147, 3419–3426. [Google Scholar] [PubMed]
- Pilette, C.; Ouadrhiri, Y.; Dimanche, F.; Vaerman, J.P.; Sibille, Y. Secretory component is cleaved by neutrophil serine proteinases but its epithelial production is increased by neutrophils through NF-κ B- and p38 mitogen-activated protein kinase-dependent mechanisms. Am. J. Respir. Cell Mol. Biol. 2003, 28, 485–498. [Google Scholar] [CrossRef] [PubMed]
- Rogier, E.W.; Frantz, A.L.; Bruno, M.E.; Kaetzel, C.S. Secretory IgA is Concentrated in the Outer Layer of Colonic Mucus along with Gut Bacteria. Pathogens 2014, 3, 390–403. [Google Scholar] [CrossRef] [PubMed]
- Obara, W.; Iida, A.; Suzuki, Y.; Tanaka, T.; Akiyama, F.; Maeda, S.; Ohnishi, Y.; Yamada, R.; Tsunoda, T.; Takei, T.; et al. Association of single-nucleotide polymorphisms in the polymeric immunoglobulin receptor gene with immunoglobulin A nephropathy (IgAN) in Japanese patients. J. Hum. Genet. 2003, 48, 293–299. [Google Scholar] [PubMed]
- Phalipon, A.; Corthesy, B. Novel functions of the polymeric Ig receptor: Well beyond transport of immunoglobulins. Trends Immunol. 2003, 24, 55–58. [Google Scholar] [CrossRef]
- Kaetzel, C.S. The polymeric immunoglobulin receptor: Bridging innate and adaptive immune responses at mucosal surfaces. Immunol. Rev. 2005, 206, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Lullau, E.; Heyse, S.; Vogel, H.; Marison, I.; von Stockar, U.; Kraehenbuhl, J.P.; Corthesy, B. Antigen binding properties of purified immunoglobulin A and reconstituted secretory immunoglobulin A antibodies. J. Biol. Chem. 1996, 271, 16300–16309. [Google Scholar] [CrossRef] [PubMed]
- Phalipon, A.; Cardona, A.; Kraehenbuhl, J.P.; Edelman, L.; Sansonetti, P.J.; Corthesy, B. Secretory component: A new role in secretory IgA-mediated immune exclusion in vivo. Immunity 2002, 17, 107–115. [Google Scholar] [CrossRef]
- Duc, M.; Johansen, F.E.; Corthesy, B. Antigen binding to secretory immunoglobulin A results in decreased sensitivity to intestinal proteases and increased binding to cellular Fc receptors. J. Biol. Chem. 2010, 285, 953–960. [Google Scholar] [CrossRef] [PubMed]
- Crottet, P.; Corthesy, B. Secretory component delays the conversion of secretory IgA into antigen-binding competent F(ab’)2: A possible implication for mucosal defense. J. Immunol. 1998, 161, 5445–5453. [Google Scholar] [PubMed]
- Fasching, C.E.; Grossman, T.; Corthesy, B.; Plaut, A.G.; Weiser, J.N.; Janoff, E.N. Impact of the molecular form of immunoglobulin a on functional activity in defense against Streptococcus pneumoniae. Infect. Immun. 2007, 75, 1801–1810. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Guerrero, A.; Parker, E.; Strum, J.S.; Smilowitz, J.T.; German, J.B.; Lebrilla, C.B. Site-specific glycosylation of secretory immunoglobulin A from human colostrum. J. Proteom. Res. 2015, 14, 1335–1349. [Google Scholar] [CrossRef] [PubMed]
- Pierce-Cretel, A.; Pamblanco, M.; Strecker, G.; Montreuil, J.; Spik, G.; Dorland, L.; Van Halbeek, H.; Vliegenthart, J.F. Primary structure of the N-glycosidically linked sialoglycans of secretory immunoglobulins A from human milk. Eur. J. Biochem. 1982, 125, 383–388. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, N.; Asano, M.; Ogura, Y.; Takenouchi-Ohkubo, N.; Chihaya, H.; Chung-Hsing, W.; Ishikawa, K.; Zhu, L.; Moro, I. Release of non-glycosylated polymeric immunoglobulin receptor protein. Scand. J. Immunol. 2003, 58, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Corthesy, B. Recognition of gram-positive intestinal bacteria by hybridoma- and colostrum-derived secretory immunoglobulin A is mediated by carbohydrates. J. Biol. Chem. 2011, 286, 17239–17247. [Google Scholar] [CrossRef] [PubMed]
- Dallas, S.D.; Rolfe, R.D. Binding of Clostridium difficile toxin A to human milk secretory component. J. Med. Microbiol. 1998, 47, 879–888. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira, I.R.; de Araujo, A.N.; Bao, S.N.; Giugliano, L.G. Binding of lactoferrin and free secretory component to enterotoxigenic Escherichia coli. FEMS Microbiol. Lett. 2001, 203, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Motegi, Y.; Kita, H. Interaction with secretory component stimulates effector functions of human eosinophils but not of neutrophils. J. Immunol. 1998, 161, 4340–4346. [Google Scholar] [PubMed]
- Marshall, L.J.; Perks, B.; Ferkol, T.; Shute, J.K. IL-8 released constitutively by primary bronchial epithelial cells in culture forms an inactive complex with secretory component. J. Immunol. 2001, 167, 2816–2823. [Google Scholar] [CrossRef] [PubMed]
- Kaetzel, C.S. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol. Lett. 2014, 162, 10–21. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.; Frantz, A.L.; Rogier, E.W.; Johansen, F.E.; Kaetzel, C.S. Regulation of the polymeric immunoglobulin receptor by the classical and alternative NF-κB pathways in intestinal epithelial cells. Mucosal Immunol. 2011, 4, 468–478. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.; Rogier, E.W.; Frantz, A.L.; Stefka, A.T.; Thompson, S.N.; Kaetzel, C.S. Regulation of the polymeric immunoglobulin receptor in intestinal epithelial cells by Enterobacteriaceae: Implications for mucosal homeostasis. Immunol. Investig. 2010, 39, 356–382. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.L.; Rogier, E.W.; Weber, C.R.; Shen, L.; Cohen, D.A.; Fenton, L.A.; Bruno, M.E.; Kaetzel, C.S. Targeted deletion of MyD88 in intestinal epithelial cells results in compromised antibacterial immunity associated with downregulation of polymeric immunoglobulin receptor, mucin-2, and antibacterial peptides. Mucosal Immunol. 2012, 5, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Blanch, V.J.; Piskurich, J.F.; Kaetzel, C.S. Cutting edge: Coordinate regulation of IFN regulatory factor-1 and the polymeric Ig receptor by proinflammatory cytokines. J. Immunol. 1999, 162, 1232–1235. [Google Scholar] [PubMed]
- Moon, C.; VanDussen, K.L.; Miyoshi, H.; Stappenbeck, T.S. Development of a primary mouse intestinal epithelial cell monolayer culture system to evaluate factors that modulate IgA transcytosis. Mucosal Immunol. 2014, 7, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Kvale, D.; Brandtzaeg, P. Butyrate differentially affects constitutive and cytokine-induced expression of HLA molecules, secretory component (SC), and ICAM-1 in a colonic epithelial cell line (HT-29, clone m3). In Advances in Mucosal Immunology; Springer: Boston, MA, USA, 1995; pp. 183–188. [Google Scholar]
- Pal, K.; Kaetzel, C.S.; Brundage, K.; Cunningham, C.A.; Cuff, C.F. Regulation of polymeric immunoglobulin receptor expression by reovirus. J. Gen. Virol. 2005, 86, 2347–23457. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.W.; O’Meara, C.P.; Beagley, K.W. Chlamydial infection enhances expression of the polymeric immunoglobulin receptor (pIgR) and transcytosis of IgA. Am. J. Reprod. Immunol. 2017, 77, e12611. [Google Scholar] [CrossRef] [PubMed]
- Godinez-Victoria, M.; Cruz-Hernandez, T.R.; Reyna-Garfias, H.; Barbosa-Cabrera, R.E.; Drago-Serrano, M.E.; Sanchez-Gomez, M.C.; Campos-Rodriguez, R. Modulation by bovine lactoferrin of parameters associated with the IgA response in the proximal and distal small intestine of BALB/c mice. Immunopharmacol. Immunotoxicol. 2017, 39, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M.; Agace, W.W. Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 2014, 14, 667–685. [Google Scholar] [CrossRef] [PubMed]
- Rincheval-Arnold, A.; Belair, L.; Djiane, J. Developmental expression of pIgR gene in sheep mammary gland and hormonal regulation. J. Dairy Res. 2002, 69, 13–26. [Google Scholar] [PubMed]
- Godinez-Victoria, M.; Campos-Rodriguez, R.; Rivera-Aguilar, V.; Lara-Padilla, E.; Pacheco-Yepez, J.; Jarillo-Luna, R.A.; Drago-Serrano, M.E. Intermittent fasting promotes bacterial clearance and intestinal IgA production in Salmonella typhimurium-infected mice. Scand. J. Immunol. 2014, 79, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Kurimoto, Y.; Saruta, J.; To, M.; Yamamoto, Y.; Kimura, K.; Tsukinoki, K. Voluntary exercise increases IgA concentration and polymeric Ig receptor expression in the rat submandibular gland. Biosci. Biotechnol. Biochem. 2016, 80, 2490–2496. [Google Scholar] [CrossRef] [PubMed]
- Lopez, M.C. Chronic alcohol consumption regulates the expression of poly immunoglobulin receptor (pIgR) and secretory IgA in the gut. Toxicol. Appl. Pharmacol. 2017, 333, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Rusznak, C.; Sapsford, R.J.; Devalia, J.L.; Wang, J.H.; Shah, S.S.; Mills, P.R.; Davies, R.J.; Lozewicz, S. Cigarette smoke decreases the expression of secretory component in human bronchial epithelial cells, in vitro. Acta Microbiol. Immunol. Hung. 2001, 48, 81–94. [Google Scholar] [PubMed]
- Qi, X.; Li, X.; Sun, X. Reduced expression of polymeric immunoglobulin receptor (pIgR) in nasopharyngeal carcinoma and its correlation with prognosis. Tumour. Biol. 2016, 37, 11099–11104. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, P.; Bhattacharya, S.; Chin-Aleong, J.; Capaso, M.; Kocher, H.M. Expression of polymeric immunoglobulin receptor and stromal activity in pancreatic ductal adenocarcinoma. Pancreatology 2017, 17, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Ai, J.; Xu, Y.; Chen, Y.; Huang, M.; Yang, X.; Hu, B.; Zhang, H.; He, C.; Yang, X.; et al. Polymeric immunoglobulin receptor promotes tumor growth in hepatocellular carcinoma. Hepatology 2017, 65, 1948–1962. [Google Scholar] [CrossRef] [PubMed]
- Shimada, S.; Kawaguchi-Miyashita, M.; Kushiro, A.; Sato, T.; Nanno, M.; Sako, T.; Matsuoka, Y.; Sudo, K.; Tagawa, Y.; Iwakura, Y.; et al. Generation of polymeric immunoglobulin receptor-deficient mouse with marked reduction of secretory IgA. J. Immunol. 1999, 163, 5367–5373. [Google Scholar] [PubMed]
- Johansen, F.E.; Pekna, M.; Norderhaug, I.N.; Haneberg, B.; Hietala, M.A.; Krajci, P.; Betsholtz, C.; Brandtzaeg, P. Absence of epithelial immunoglobulin A transport, with increased mucosal leakiness, in polymeric immunoglobulin receptor/secretory component-deficient mice. J. Exp. Med. 1999, 190, 915–922. [Google Scholar] [CrossRef] [PubMed]
- Uren, T.K.; Johansen, F.E.; Wijburg, O.L.; Koentgen, F.; Brandtzaeg, P.; Strugnell, R.A. Role of the polymeric Ig receptor in mucosal B cell homeostasis. J. Immunol. 2003, 170, 2531–2539. [Google Scholar] [CrossRef] [PubMed]
- Turula, H.; Bragazzi-Cunha, J.; Ramakrishnan, S.; Wilke, C.; Gonzalez-Hernandez, M.; Pry, A.; Fava, J.; Svoboda, S.; Shah, Y.; Corthesy, B.; et al. Natural Secretory Immunoglobulins Enhance Norovirus Infection. bioRxiv 2018, 253286. [Google Scholar] [CrossRef]
- Yamazaki, K.; Shimada, S.; Kato-Nagaoka, N.; Soga, H.; Itoh, T.; Nanno, M. Accumulation of intestinal intraepithelial lymphocytes in association with lack of polymeric immunoglobulin receptor. Eur. J. Immunol. 2005, 35, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Richmond, B.W.; Du, R.H.; Han, W.; Benjamin, J.T.; Meer, R.V.; Gleaves, L.; Guo, M.; McKissack, A.; Zhang, Y.; Cheng, D.S.; et al. Bacterial-derived Neutrophilic Inflammation Drives Lung Remodeling in a Mouse Model of COPD. Am. J. Respir. Cell Mol. Biol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Sait, L.; Galic, M.; Strugnell, R.A.; Janssen, P.H. Secretory antibodies do not affect the composition of the bacterial microbiota in the terminal ileum of 10-week-old mice. Appl. Environ. Microbiol. 2003, 69, 2100–2109. [Google Scholar] [CrossRef] [PubMed]
- Van de Perre, P. Transfer of antibody via mother’s milk. Vaccine 2003, 21, 3374–3376. [Google Scholar] [CrossRef]
- Reikvam, D.H.; Derrien, M.; Islam, R.; Erofeev, A.; Grcic, V.; Sandvik, A.; Gaustad, P.; Meza-Zepeda, L.A.; Jahnsen, F.L.; Smidt, H.; et al. Epithelial-microbial crosstalk in polymeric Ig receptor deficient mice. Eur. J. Immunol. 2012, 42, 2959–2970. [Google Scholar] [CrossRef] [PubMed]
- Kato-Nagaoka, N.; Shimada, S.; Yamakawa, Y.; Tsujibe, S.; Naito, T.; Setoyama, H.; Watanabe, Y.; Shida, K.; Matsumoto, S.; Nanno, M. Enhanced differentiation of intraepithelial lymphocytes in the intestine of polymeric immunoglobulin receptor-deficient mice. Immunology 2015, 146, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Polosukhin, V.V.; Cates, J.M.; Lawson, W.E.; Zaynagetdinov, R.; Milstone, A.P.; Massion, P.P.; Ocak, S.; Ware, L.B.; Lee, J.W.; Bowler, R.P.; et al. Bronchial secretory immunoglobulin a deficiency correlates with airway inflammation and progression of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2011, 184, 317–327. [Google Scholar] [CrossRef] [PubMed]
- Gohy, S.T.; Detry, B.R.; Lecocq, M.; Bouzin, C.; Weynand, B.A.; Amatngalim, G.D.; Sibille, Y.M.; Pilette, C. Polymeric immunoglobulin receptor down-regulation in chronic obstructive pulmonary disease. Persistence in the cultured epithelium and role of transforming growth factor-beta. Am. J. Respir. Crit. Care Med. 2014, 190, 509–521. [Google Scholar] [CrossRef] [PubMed]
- Richmond, B.W.; Brucker, R.M.; Han, W.; Du, R.H.; Zhang, Y.; Cheng, D.S.; Gleaves, L.; Abdolrasulnia, R.; Polosukhina, D.; Clark, P.E.; et al. Airway bacteria drive a progressive COPD-like phenotype in mice with polymeric immunoglobulin receptor deficiency. Nat. Commun. 2016, 7, e11240. [Google Scholar] [CrossRef] [PubMed]
- Frantz, A.L.; Bruno, M.E.; Rogier, E.W.; Tuna, H.; Cohen, D.A.; Bondada, S.; Chelvarajan, R.L.; Brandon, J.A.; Jennings, C.D.; Kaetzel, C.S. Multifactorial patterns of gene expression in colonic epithelial cells predict disease phenotypes in experimental colitis. Inflamm. Bowel Dis. 2012, 18, 2138–2148. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Arsenescu, R.; Bruno, M.E.; Rogier, E.W.; Stefka, A.T.; McMahan, A.E.; Wright, T.B.; Nasser, M.S.; de Villiers, W.J.; Kaetzel, C.S. Signature biomarkers in Crohn’s disease: Toward a molecular classification. Mucosal Immunol. 2008, 1, 399–411. [Google Scholar] [CrossRef] [PubMed]
- Bruno, M.E.; Rogier, E.W.; Arsenescu, R.I.; Flomenhoft, D.R.; Kurkjian, C.J.; Ellis, G.I.; Kaetzel, C.S. Correlation of Biomarker Expression in Colonic Mucosa with Disease Phenotype in Crohn's Disease and Ulcerative Colitis. Dig. Dis. Sci. 2015, 60, 2976–2984. [Google Scholar] [CrossRef] [PubMed]
- Wijburg, O.L.; Uren, T.K.; Simpfendorfer, K.; Johansen, F.E.; Brandtzaeg, P.; Strugnell, R.A. Innate secretory antibodies protect against natural Salmonella typhimurium infection. J. Exp. Med. 2006, 203, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Davids, B.J.; Palm, J.E.; Housley, M.P.; Smith, J.R.; Andersen, Y.S.; Martin, M.G.; Hendrickson, B.A.; Johansen, F.E.; Svard, S.G.; Gillin, F.D.; et al. Polymeric immunoglobulin receptor in intestinal immune defense against the lumen-dwelling protozoan parasite Giardia. J. Immunol. 2006, 177, 6281–6290. [Google Scholar] [CrossRef] [PubMed]
- Testerman, T.L.; Morris, J. Beyond the stomach: An updated view of Helicobacter pylori pathogenesis, diagnosis, and treatment. World J. Gastroenterol. 2014, 20, 12781–12808. [Google Scholar] [CrossRef] [PubMed]
- Gorrell, R.J.; Wijburg, O.L.; Pedersen, J.S.; Walduck, A.K.; Kwok, T.; Strugnell, R.A.; Robins-Browne, R.M. Contribution of secretory antibodies to intestinal mucosal immunity against Helicobacter pylori. Infect. Immun. 2013, 81, 3880–3893. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Falk, P.; Roth, K.A.; Boren, T.; Westblom, T.U.; Gordon, J.I.; Normark, S. An in vitro adherence assay reveals that Helicobacter pylori exhibits cell lineage-specific tropism in the human gastric epithelium. Proc. Natl. Acad. Sci. USA 1993, 90, 2035–2039. [Google Scholar] [CrossRef] [PubMed]
- Ahlstedt, I.; Lindholm, C.; Lonroth, H.; Hamlet, A.; Svennerholm, A.M.; Quiding-Jarbrink, M. Role of local cytokines in increased gastric expression of the secretory component in Helicobacter pylori infection. Infect. Immun. 1999, 67, 4921–4925. [Google Scholar] [PubMed]
- Kaneko, T.; Ota, H.; Hayama, M.; Akamatsu, T.; Katsuyama, T. Helicobacter pylori infection produces expression of a secretory component in gastric mucous cells. Virchows Arch. 2000, 437, 514–520. [Google Scholar] [CrossRef] [PubMed]
- Fubara, E.S.; Freter, R. Protection against enteric bacterial infection by secretory IgA antibodies. J. Immunol. 1973, 111, 395–403. [Google Scholar] [PubMed]
- Tokuhara, D.; Yuki, Y.; Nochi, T.; Kodama, T.; Mejima, M.; Kurokawa, S.; Takahashi, Y.; Nanno, M.; Nakanishi, U.; Takaiwa, F.; et al. Secretory IgA-mediated protection against V. cholerae and heat-labile enterotoxin-producing enterotoxigenic Escherichia coli by rice-based vaccine. Proc. Natl. Acad. Sci. USA 2010, 107, 8794–8799. [Google Scholar] [CrossRef] [PubMed]
- Winner, L., 3rd; Mack, J.; Weltzin, R.; Mekalanos, J.J.; Kraehenbuhl, J.P.; Neutra, M.R. New model for analysis of mucosal immunity: Intestinal secretion of specific monoclonal immunoglobulin A from hybridoma tumors protects against Vibrio cholerae infection. Infect. Immun. 1991, 59, 977–982. [Google Scholar] [PubMed]
- Boullier, S.; Tanguy, M.; Kadaoui, K.A.; Caubet, C.; Sansonetti, P.; Corthesy, B.; Phalipon, A. Secretory IgA-mediated neutralization of Shigella flexneri prevents intestinal tissue destruction by down-regulating inflammatory circuits. J. Immunol. 2009, 183, 5879–5885. [Google Scholar] [CrossRef] [PubMed]
- Mathias, A.; Longet, S.; Corthesy, B. Agglutinating secretory IgA preserves intestinal epithelial cell integrity during apical infection by Shigella flexneri. Infect. Immun. 2013, 81, 3027–3034. [Google Scholar] [CrossRef] [PubMed]
- Longet, S.; Vonarburg, C.; Lotscher, M.; Miescher, S.; Zuercher, A.; Corthesy, B. Reconstituted human polyclonal plasma-derived secretory-like IgM and IgA maintain the barrier function of epithelial cells infected with an enteropathogen. J. Biol. Chem. 2014, 289, 21617–21626. [Google Scholar] [CrossRef] [PubMed]
- Armitage, C.W.; O'Meara, C.P.; Harvie, M.C.; Timms, P.; Wijburg, O.L.; Beagley, K.W. Evaluation of intra- and extra-epithelial secretory IgA in chlamydial infections. Immunology 2014, 143, 520–530. [Google Scholar] [CrossRef] [PubMed]
- Perrier, C.; Sprenger, N.; Corthesy, B. Glycans on secretory component participate in innate protection against mucosal pathogens. J. Biol. Chem. 2006, 281, 14280–14287. [Google Scholar] [CrossRef] [PubMed]
- Wold, A.E.; Mestecky, J.; Tomana, M.; Kobata, A.; Ohbayashi, H.; Endo, T.; Eden, C.S. Secretory immunoglobulin A carries oligosaccharide receptors for Escherichia coli type 1 fimbrial lectin. Infect. Immun. 1990, 58, 3073–3077. [Google Scholar] [PubMed]
- Murthy, A.K.; Chaganty, B.K.; Troutman, T.; Guentzel, M.N.; Yu, J.J.; Ali, S.K.; Lauriano, C.M.; Chambers, J.P.; Klose, K.E.; Arulanandam, B.P. Mannose-containing oligosaccharides of non-specific human secretory immunoglobulin A mediate inhibition of Vibrio cholerae biofilm formation. PLoS ONE 2011, 6, e16847. [Google Scholar] [CrossRef] [PubMed]
- Hendrickx, A.P.; Top, J.; Bayjanov, J.R.; Kemperman, H.; Rogers, M.R.; Paganelli, F.L.; Bonten, M.J.; Willems, R.J. Antibiotic-Driven Dysbiosis Mediates Intraluminal Agglutination and Alternative Segregation of Enterococcus faecium from the Intestinal Epithelium. MBio 2015, 6, e01346-15. [Google Scholar] [CrossRef] [PubMed]
- Bioley, G.; Monnerat, J.; Lotscher, M.; Vonarburg, C.; Zuercher, A.; Corthesy, B. Plasma-Derived Polyreactive Secretory-Like IgA and IgM Opsonizing Salmonella enterica Typhimurium Reduces Invasion and Gut Tissue Inflammation through Agglutination. Front. Immunol. 2017, 8, e1043. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Kaetzel, C.S.; Lamm, M.E.; Fletcher, D.; Nedrud, J.G. Intracellular neutralization of virus by immunoglobulin A antibodies. Proc. Natl. Acad. Sci. USA 1992, 89, 6901–6905. [Google Scholar] [CrossRef] [PubMed]
- Mazanec, M.B.; Coudret, C.L.; Fletcher, D.R. Intracellular neutralization of influenza virus by immunoglobulin A anti-hemagglutinin monoclonal antibodies. J. Virol. 1995, 69, 1339–1343. [Google Scholar] [PubMed]
- Yan, H.; Lamm, M.E.; Bjorling, E.; Huang, Y.T. Multiple functions of immunoglobulin A in mucosal defense against viruses: An in vitro measles virus model. J. Virol. 2002, 76, 10972–10979. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wright, A.; Yan, H.; Lamm, M.E.; Huang, Y.T. Immunoglobulin A antibodies against internal HIV-1 proteins neutralize HIV-1 replication inside epithelial cells. Virology 2006, 356, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Corthesy, B.; Benureau, Y.; Perrier, C.; Fourgeux, C.; Parez, N.; Greenberg, H.; Schwartz-Cornil, I. Rotavirus anti-VP6 secretory immunoglobulin A contributes to protection via intracellular neutralization but not via immune exclusion. J. Virol. 2006, 80, 10692–10699. [Google Scholar] [CrossRef] [PubMed]
- Ruggeri, F.M.; Johansen, K.; Basile, G.; Kraehenbuhl, J.P.; Svensson, L. Antirotavirus immunoglobulin A neutralizes virus in vitro after transcytosis through epithelial cells and protects infant mice from diarrhea. J. Virol. 1998, 72, 2708–2714. [Google Scholar] [PubMed]
- Wright, A.; Lamm, M.E.; Huang, Y.T. Excretion of human immunodeficiency virus type 1 through polarized epithelium by immunoglobulin A. J. Virol. 2008, 82, 11526–11535. [Google Scholar] [CrossRef] [PubMed]
- Burns, J.W.; Siadat-Pajouh, M.; Krishnaney, A.A.; Greenberg, H.B. Protective effect of rotavirus VP6-specific IgA monoclonal antibodies that lack neutralizing activity. Science 1996, 272, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Schwartz-Cornil, I.; Benureau, Y.; Greenberg, H.; Hendrickson, B.A.; Cohen, J. Heterologous protection induced by the inner capsid proteins of rotavirus requires transcytosis of mucosal immunoglobulins. J. Virol. 2002, 76, 8110–8117. [Google Scholar] [CrossRef] [PubMed]
- Rey, J.; Garin, N.; Spertini, F.; Corthesy, B. Targeting of secretory IgA to Peyer’s patch dendritic and T cells after transport by intestinal M cells. J. Immunol. 2004, 172, 3026–3033. [Google Scholar] [CrossRef] [PubMed]
- Kadaoui, K.A.; Corthesy, B. Secretory IgA mediates bacterial translocation to dendritic cells in mouse Peyer’s patches with restriction to mucosal compartment. J. Immunol. 2007, 179, 7751–7757. [Google Scholar] [CrossRef] [PubMed]
- Diana, J.; Moura, I.C.; Vaugier, C.; Gestin, A.; Tissandie, E.; Beaudoin, L.; Corthesy, B.; Hocini, H.; Lehuen, A.; Monteiro, R.C. Secretory IgA induces tolerogenic dendritic cells through SIGNR1 dampening autoimmunity in mice. J. Immunol. 2013, 191, 2335–2343. [Google Scholar] [CrossRef] [PubMed]
- Baumann, J.; Park, C.G.; Mantis, N.J. Recognition of secretory IgA by DC-SIGN: Implications for immune surveillance in the intestine. Immunol. Lett. 2010, 131, 59–66. [Google Scholar] [CrossRef] [PubMed]
- Mikulic, J.; Bioley, G.; Corthesy, B. SIgA-Shigella Immune Complexes Interact with Dectin-1 and SIGNR3 to Differentially Regulate Mouse Peyer’s Patch and Mesenteric Lymph Node Dendritic Cell’s Responsiveness. J. Mol. Biol. 2017, 429, 2387–2400. [Google Scholar] [CrossRef] [PubMed]
- Hansen, I.S.; Hoepel, W.; Zaat, S.A.J.; Baeten, D.L.P.; den Dunnen, J. Serum IgA Immune Complexes Promote Proinflammatory Cytokine Production by Human Macrophages, Monocytes, and Kupffer Cells through FcalphaRI-TLR Cross-Talk. J. Immunol. 2017, 199, 4124–4131. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.L.; Suscovich, T.J.; Fortune, S.M.; Alter, G. Beyond binding: Antibody effector functions in infectious diseases. Nat. Rev. Immunol. 2018, 18, 46–61. [Google Scholar] [CrossRef] [PubMed]
- Michaelsen, T.E.; Emilsen, S.; Sandin, R.H.; Granerud, B.K.; Bratlie, D.; Ihle, O.; Sandlie, I. Human Secretory IgM Antibodies Activate Human Complement and Offer Protection at Mucosal Surface. Scand. J. Immunol. 2017, 85, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Hendrickson, B.A.; Guo, J.; Brown, I.; Dennis, K.; Marcellino, D.; Hetzel, J.; Herold, B.C. Decreased vaginal disease in J-chain-deficient mice following herpes simplex type 2 genital infection. Virology 2000, 271, 155–162. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Salerno-Goncalves, R.; Safavie, F.; Fasano, A.; Sztein, M.B. Free and complexed-secretory immunoglobulin A triggers distinct intestinal epithelial cell responses. Clin. Exp. Immunol. 2016, 185, 338–347. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Church, D.; Vanderkooi, O.G.; Low, D.E.; Pillai, D.R. Streptococcus pneumoniae infection: A Canadian perspective. Expert Rev. Anti-Infect. Ther. 2013, 11, 781–791. [Google Scholar] [CrossRef] [PubMed]
- Hammerschmidt, S.; Talay, S.R.; Brandtzaeg, P.; Chhatwal, G.S. SpsA, a novel pneumococcal surface protein with specific binding to secretory immunoglobulin A and secretory component. Mol. Microbiol. 1997, 25, 1113–1124. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.R.; Mostov, K.E.; Lamm, M.E.; Nanno, M.; Shimida, S.; Ohwaki, M.; Tuomanen, E. The polymeric immunoglobulin receptor translocates pneumococci across human nasopharyngeal epithelial cells. Cell 2000, 102, 827–837. [Google Scholar] [CrossRef]
- Asmat, T.M.; Agarwal, V.; Rath, S.; Hildebrandt, J.P.; Hammerschmidt, S. Streptococcus pneumoniae infection of host epithelial cells via polymeric immunoglobulin receptor transiently induces calcium release from intracellular stores. J. Biol. Chem. 2011, 286, 17861–17869. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Johansen, F.E.; Eckmann, L.; Metzger, D.W. An important role for polymeric Ig receptor-mediated transport of IgA in protection against Streptococcus pneumoniae nasopharyngeal carriage. J. Immunol. 2004, 173, 4576–4581. [Google Scholar] [CrossRef] [PubMed]
- Boehme, J.D.; Stegemann-Koniszewski, S.; Autengruber, A.; Peters, N.; Wissing, J.; Jansch, L.; Jeron, A.; Bruder, D. Chronic lung inflammation primes humoral immunity and augments antipneumococcal resistance. Sci. Rep. 2017, 7, 4972. [Google Scholar] [CrossRef] [PubMed]
- ovino, F.; Engelen-Lee, J.Y.; Brouwer, M.; van de Beek, D.; van der Ende, A.; Valls Seron, M.; Mellroth, P.; Muschiol, S.; Bergstrand, J.; Widengren, J.; et al. pIgR and PECAM-1 bind to pneumococcal adhesins RrgA and PspC mediating bacterial brain invasion. J. Exp. Med. 2017, 214, 1619–1630. [Google Scholar]
- Poulain, D. Candida albicans, plasticity and pathogenesis. Crit. Rev. Microbiol. 2015, 41, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Holmes, A.R.; Bandara, B.M.; Cannon, R.D. Saliva promotes Candida albicans adherence to human epithelial cells. J. Dent. Res. 2002, 81, 28–32. [Google Scholar] [CrossRef] [PubMed]
- Van der Wielen, P.A.; Holmes, A.R.; Cannon, R.D. Secretory component mediates Candida albicans binding to epithelial cells. Oral. Dis. 2016, 22, 69–74. [Google Scholar] [CrossRef] [PubMed]
- Vetsika, E.K.; Callan, M. Infectious mononucleosis and Epstein-Barr virus. Expert Rev. Mol. Med. 2004, 6, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Sixbey, J.W.; Yao, Q.Y. Immunoglobulin A-induced shift of Epstein-Barr virus tissue tropism. Science 1992, 255, 1578–1580. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.T.; Lin, C.R.; Tan, G.K.; Chen, W.; Dee, A.N.; Chan, W.Y. The mechanism of Epstein-Barr virus infection in nasopharyngeal carcinoma cells. Am. J. Pathol. 1997, 150, 1745–1756. [Google Scholar] [PubMed]
- Gan, Y.J.; Chodosh, J.; Morgan, A.; Sixbey, J.W. Epithelial cell polarization is a determinant in the infectious outcome of immunoglobulin A-mediated entry by Epstein-Barr virus. J. Virol. 1997, 71, 519–526. [Google Scholar] [PubMed]
- Liu, G.; Ren, W.; Fang, J.; Hu, C.A.; Guan, G.; Al-Dhabi, N.A.; Yin, J.; Duraipandiyan, V.; Chen, S.; Peng, Y.; et al. l-Glutamine and l-arginine protect against enterotoxigenic Escherichia coli infection via intestinal innate immunity in mice. Amino Acids 2017, 49, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wang, F.J.; Yu, L.; Yao, W.R.; Cui, Y.F.; Yang, G.B. Expression of pIgR in the tracheal mucosa of SHIV/SIV-infected rhesus macaques. Zool Res. 2017, 38, 44–48. [Google Scholar] [PubMed]
- Wang, Y.; Yang, G.B. Alteration of Polymeric Immunoglobulin Receptor and Neonatal Fc Receptor Expression in the Gut Mucosa of Immunodeficiency Virus-Infected Rhesus Macaques. Scand. J. Immunol. 2016, 83, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Aurora, M.; Spence, J.R. hPSC-derived lung and intestinal organoids as models of human fetal tissue. Dev. Biol. 2016, 420, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [PubMed]
- Noel, G.; Baetz, N.W.; Staab, J.F.; Donowitz, M.; Kovbasnjuk, O.; Pasetti, M.F.; Zachos, N.C. A primary human macrophage-enteroid co-culture model to investigate mucosal gut physiology and host-pathogen interactions. Sci. Rep. 2017, 7, 45270. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Murphy, S.V.; Devarasetty, M.; Mead, I.; Kang, H.W.; Seol, Y.J.; Shrike Zhang, Y.; Shin, S.R.; Zhao, L.; Aleman, J.; et al. Multi-tissue interactions in an integrated three-tissue organ-on-a-chip platform. Sci. Rep. 2017, 7, 8837. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Turula, H.; Wobus, C.E. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses 2018, 10, 237. https://doi.org/10.3390/v10050237
Turula H, Wobus CE. The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses. 2018; 10(5):237. https://doi.org/10.3390/v10050237
Chicago/Turabian StyleTurula, Holly, and Christiane E. Wobus. 2018. "The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity" Viruses 10, no. 5: 237. https://doi.org/10.3390/v10050237
APA StyleTurula, H., & Wobus, C. E. (2018). The Role of the Polymeric Immunoglobulin Receptor and Secretory Immunoglobulins during Mucosal Infection and Immunity. Viruses, 10(5), 237. https://doi.org/10.3390/v10050237




