Attenuated Phenotype and Immunogenic Characteristics of a Mutated Herpes Simplex Virus 1 Strain in the Rhesus Macaque
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of the M3 Strain
2.2. Rhesus Administration and Ethics Statement
2.3. Rhesus Macaque Experimental Design and Sample Collection
2.4. q-PCR Detection of the Viral Load in Various Tissues
2.5. In Situ Hybridization of the LAT mRNA in the TG
2.6. Histopathological Detection
2.7. Co-Culture of TG and Vero Cells
2.8. Serum Antibody Detection by ELISA
2.9. Serum Neutralizing Antibody Assay
2.10. ELISpot Detection of Cell-Mediating Immune Responses in Macaques
2.11. Statistical Analysis
3. Results
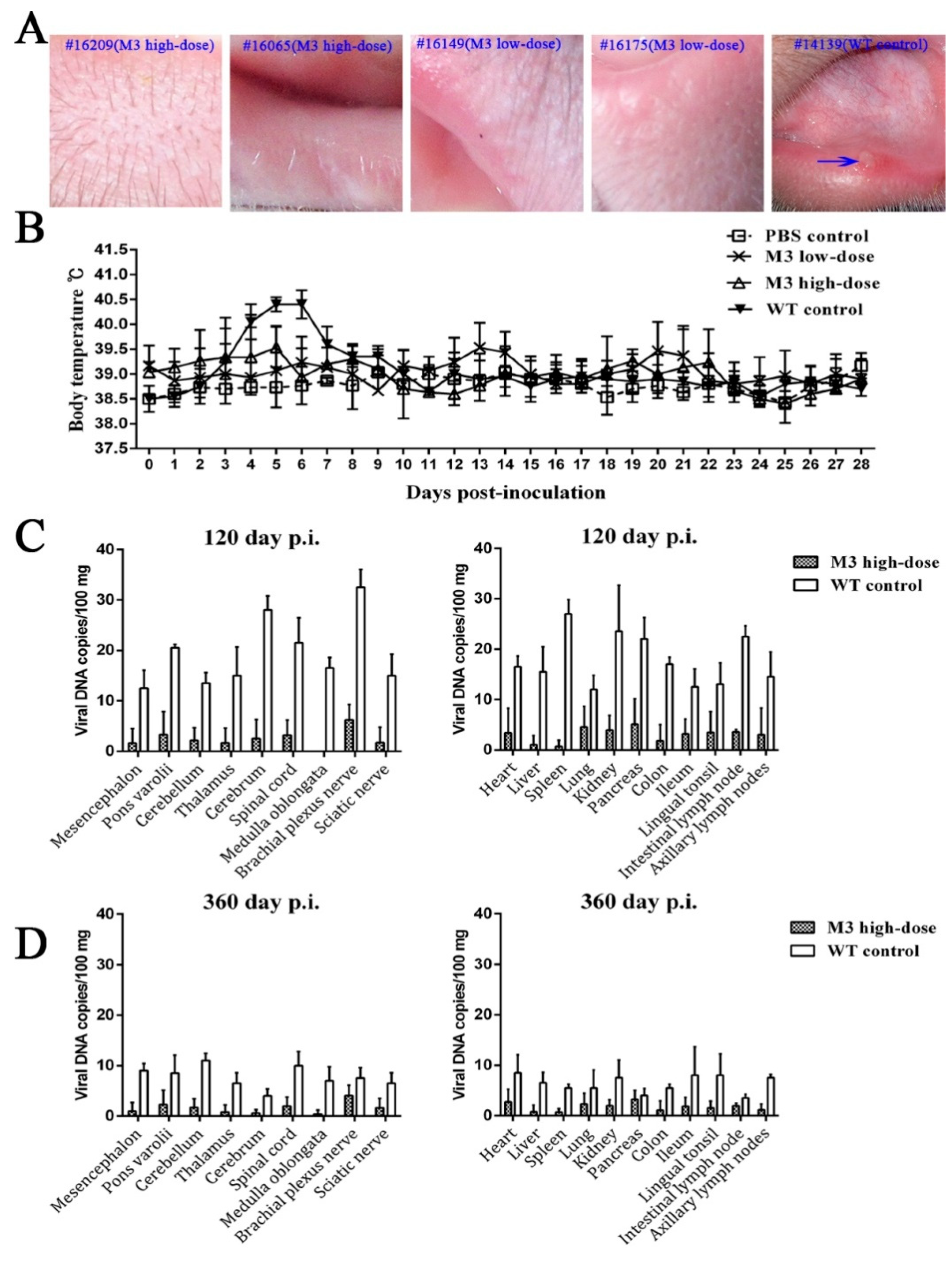
3.1. Clinical Manifestations of the M3 Strain at Different Doses
3.2. M3 Proliferation and Pathogenicity of Inoculated Animals
3.3. Observations of Latent Infection of the M3 Strain in Macaques
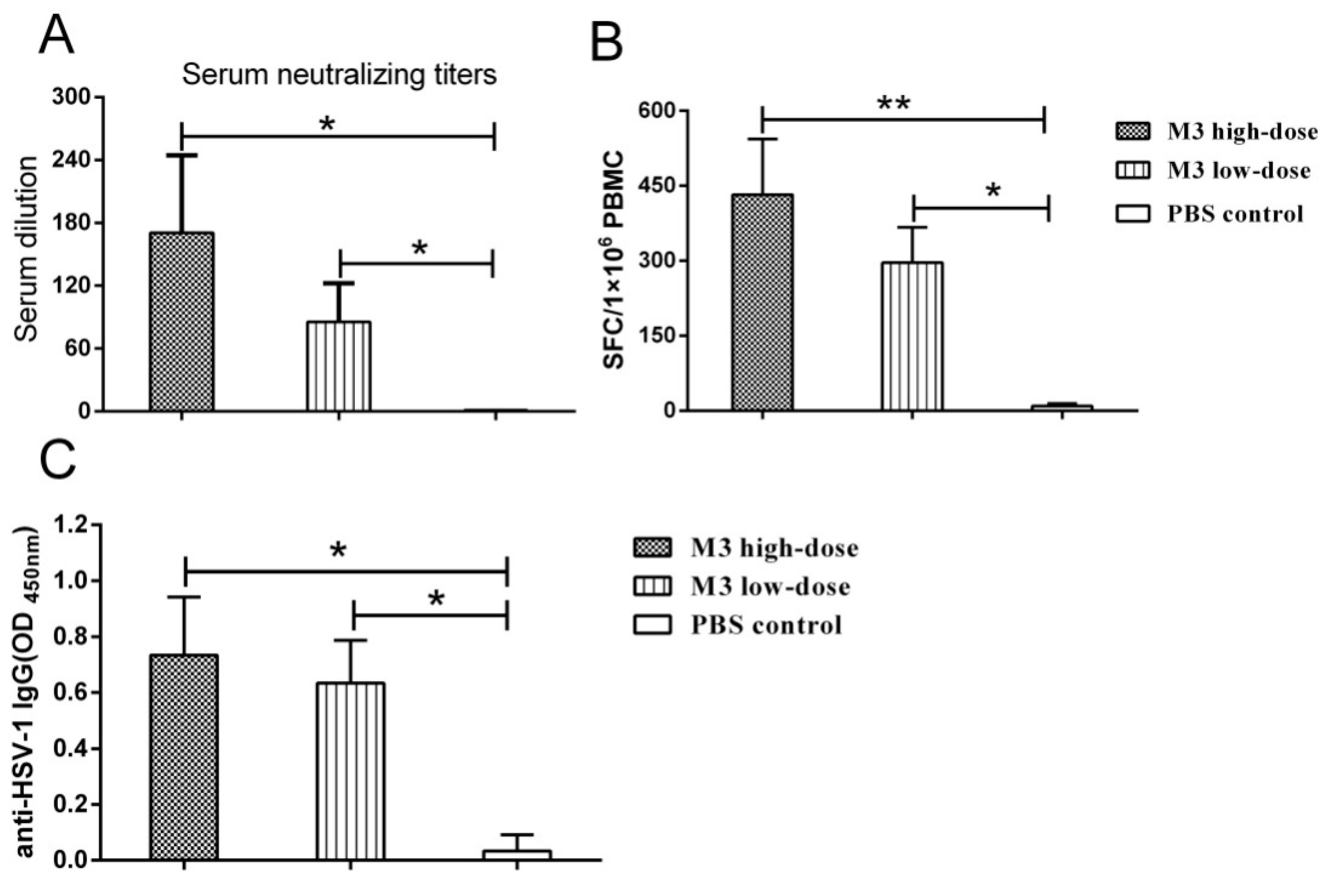
3.4. The M3 Strain-Induced Antibody and Cell-Mediated Responses in Inoculated Macaques
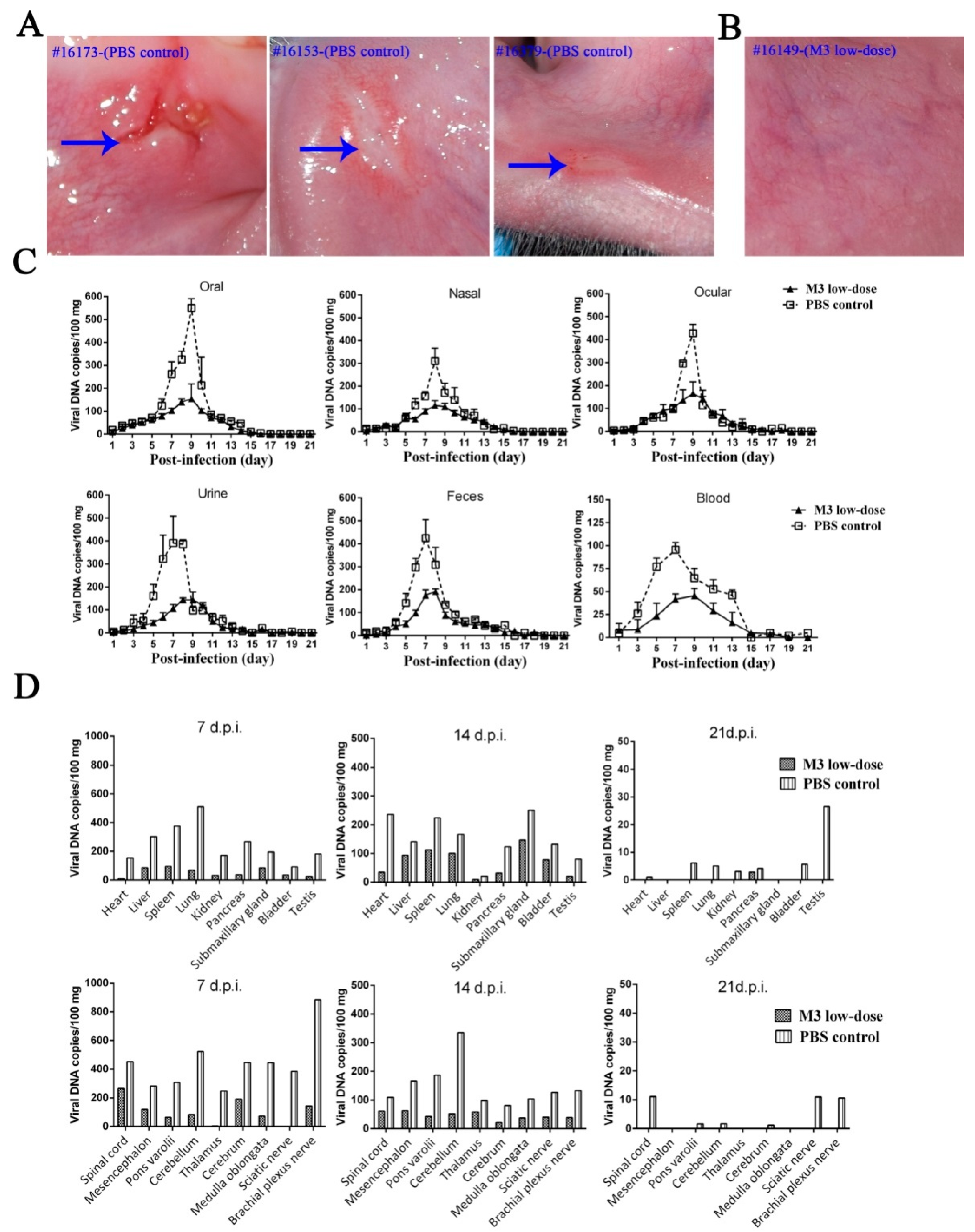
3.5. M3-Induced Immunity Defended against the 17+ Strain Challenge
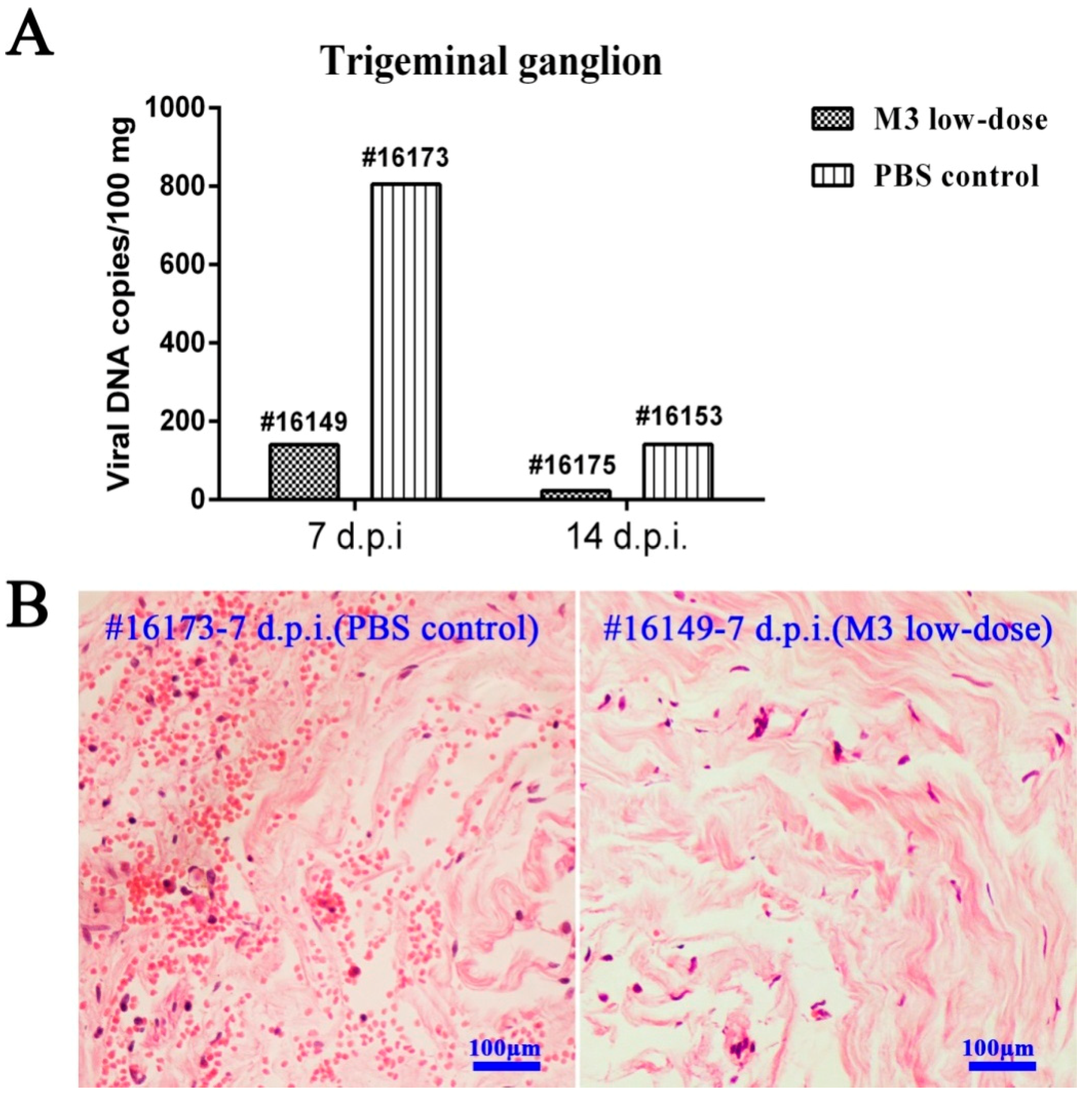
3.6. M3-Induced Immunity Restricted the Entry of the Wild-Type Strain into the TG
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Kaeley, N.; Bansal, S.; Bhatia, R.; Ahmad, S. Herpes simplex encephalitis: An uncommon presentation. J. Clin. Diagn. Res. JCDR 2016, 10, OD25–OD26. [Google Scholar] [CrossRef] [PubMed]
- Steiner, I.; Benninger, F. Update on herpes virus infections of the nervous system. Curr. Neurol. Neurosci. Rep. 2013, 13, 414. [Google Scholar] [CrossRef] [PubMed]
- Looker, K.J.; Magaret, A.S.; May, M.T.; Turner, K.M.; Vickerman, P.; Gottlieb, S.L.; Newman, L.M. Global and regional estimates of prevalent and incident herpes simplex virus type 1 infections in 2012. PLoS ONE 2015, 10, e0140765. [Google Scholar] [CrossRef] [PubMed]
- Smith, J.S.; Robinson, N.J. Age-specific prevalence of infection with herpes simplex virus types 2 and 1: A global review. J. Infect. Dis. 2002, 186 (Suppl. 1), S3–S28. [Google Scholar] [CrossRef] [PubMed]
- Davola, M.E.; Mazaira, G.I.; Galigniana, M.D.; Alche, L.E.; Ramirez, J.A.; Barquero, A.A. Synthetic pregnenolone derivatives as antiviral agents against acyclovir-resistant isolates of herpes simplex virus type 1. Antivir. Res. 2015, 122, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Chiuppesi, F.; Vannucci, L.; De Luca, A.; Lai, M.; Matteoli, B.; Freer, G.; Manservigi, R.; Ceccherini-Nelli, L.; Maggi, F.; Bendinelli, M.; et al. A lentiviral vector-based, herpes simplex virus 1 (HSV-1) glycoprotein B vaccine affords cross-protection against HSV-1 and HSV-2 genital infections. J. Virol. 2012, 86, 6563–6574. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.; Goodman, K.N.; Li, D.Y.; Raffeld, M.; Chavez, M.; Cohen, J.I. A herpes simplex virus 2 (HSV-2) gD mutant impaired for neural tropism is superior to an HSV-2 gD subunit vaccine to protect animals from challenge with HSV-2. J. Virol. 2015, 90, 562–574. [Google Scholar] [CrossRef] [PubMed]
- Skoberne, M.; Cardin, R.; Lee, A.; Kazimirova, A.; Zielinski, V.; Garvie, D.; Lundberg, A.; Larson, S.; Bravo, F.J.; Bernstein, D.I.; et al. An adjuvanted herpes simplex virus 2 subunit vaccine elicits a T cell response in mice and is an effective therapeutic vaccine in guinea pigs. J. Virol. 2013, 87, 3930–3942. [Google Scholar] [CrossRef] [PubMed]
- Group, H.S.V.S.; Abu-Elyazeed, R.R.; Heineman, T.; Dubin, G.; Fourneau, M.; Leroux-Roels, I.; Leroux-Roels, G.; Richardus, J.H.; Ostergaard, L.; Diez-Domingo, J.; et al. Safety and immunogenicity of a glycoprotein D genital herpes vaccine in healthy girls 10–17 years of age: Results from a randomised, controlled, double-blind trial. Vaccine 2013, 31, 6136–6143. [Google Scholar]
- Gorfinkel, I.S.; Aoki, F.; McNeil, S.; Dionne, M.; Shafran, S.D.; Zickler, P.; Halperin, S.; Langley, J.; Bellamy, A.; Schulte, J.; et al. Seroprevalence of HSV-1 and HSV-2 antibodies in Canadian women screened for enrolment in a herpes simplex virus vaccine trial. Int. J. STD AIDS 2013, 24, 345–349. [Google Scholar] [CrossRef] [PubMed]
- Belshe, R.B.; Leone, P.A.; Bernstein, D.I.; Wald, A.; Levin, M.J.; Stapleton, J.T.; Gorfinkel, I.; Morrow, R.L.; Ewell, M.G.; Stokes-Riner, A.; et al. Efficacy results of a trial of a herpes simplex vaccine. N. Engl. J. Med. 2012, 366, 34–43. [Google Scholar] [CrossRef] [PubMed]
- Iyer, A.V.; Pahar, B.; Chouljenko, V.N.; Walker, J.D.; Stanfield, B.; Kousoulas, K.G. Single dose of glycoprotein K (gK)-deleted HSV-1 live-attenuated virus protects mice against lethal vaginal challenge with HSV-1 and HSV-2 and induces lasting t cell memory immune responses. Virol. J. 2013, 10, 317. [Google Scholar] [CrossRef] [PubMed]
- Visalli, R.J.; Natuk, R.J.; Kowalski, J.; Guo, M.; Blakeney, S.; Gangolli, S.; Cooper, D. Vaccination with a HSV-2 UL24 mutant induces a protective immune response in murine and guinea pig vaginal infection models. Vaccine 2014, 32, 1398–1406. [Google Scholar] [CrossRef] [PubMed]
- Walker, J.; Leib, D.A. Protection from primary infection and establishment of latency by vaccination with a herpes simplex virus type 1 recombinant deficient in the virion host shutoff (vhs) function. Vaccine 1998, 16, 1–5. [Google Scholar] [CrossRef]
- Prichard, M.N.; Kaiwar, R.; Jackman, W.T.; Quenelle, D.C.; Collins, D.J.; Kern, E.R.; Kemble, G.M.; Spaete, R.R. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine 2005, 23, 5424–5431. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Thompson, R.L.; Sawtell, N.M. The herpes simplex virus type 1 latency-associated transcript gene regulates the establishment of latency. J. Virol. 1997, 71, 5432–5440. [Google Scholar] [PubMed]
- Nicoll, M.P.; Proenca, J.T.; Efstathiou, S. The molecular basis of herpes simplex virus latency. FEMS Microbiol. Rev. 2012, 36, 684–705. [Google Scholar] [CrossRef] [PubMed]
- Gershon, A.A. Varicella zoster vaccines and their implications for development of HSV vaccines. Virology 2013, 435, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, M. Development and characterization of a live varicella vaccine (Oka strain). Biken J. 1984, 27, 31–36. [Google Scholar] [PubMed]
- Luo, C.; Goshima, F.; Kamakura, M.; Mutoh, Y.; Iwata, S.; Kimura, H.; Nishiyama, Y. Immunization with a highly attenuated replication-competent herpes simplex virus type 1 mutant, HF10, protects mice from genital disease caused by herpes simplex virus type 2. Front. Microbiol. 2012, 3, 158. [Google Scholar] [CrossRef] [PubMed]
- Hunter, W.D.; Martuza, R.L.; Feigenbaum, F.; Todo, T.; Mineta, T.; Yazaki, T.; Toda, M.; Newsome, J.T.; Platenberg, R.C.; Manz, H.J.; et al. Attenuated, replication-competent herpes simplex virus type 1 mutant G207: Safety evaluation of intracerebral injection in nonhuman primates. J. Virol. 1999, 73, 6319–6326. [Google Scholar] [PubMed]
- Xu, X.; Fan, S.; Zhou, J.; Zhang, Y.; Che, Y.; Cai, H.; Wang, L.; Guo, L.; Liu, L.; Li, Q. The mutated tegument protein UL7 attenuates the virulence of herpes simplex virus 1 by reducing the modulation of alpha-4 gene transcription. Virol. J. 2016, 13, 152. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Guo, Y.; Fan, S.; Cui, P.; Feng, M.; Wang, L.; Zhang, Y.; Liao, Y.; Zhang, X.; Li, Q. Attenuated phenotypes and analysis of a herpes simplex virus 1 strain with partial deletion of the UL7, UL41 and LAT genes. Virol. Sin. 2017, 32, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Pesola, J.M.; Zhu, J.; Knipe, D.M.; Coen, D.M. Herpes simplex virus 1 immediate-early and early gene expression during reactivation from latency under conditions that prevent infectious virus production. J. Virol. 2005, 79, 14516–14525. [Google Scholar] [CrossRef] [PubMed]
- Fan, S.; Cai, H.; Xu, X.; Feng, M.; Wang, L.; Liao, Y.; Zhang, Y.; He, Z.; Yang, F.; Yu, W.; et al. The characteristics of herpes simplex virus type 1 infection in rhesus macaques and the associated pathological features. Viruses 2017, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- National Research Council (US) Institute for Laboratory Animal Research. Guidance for the Description of Animal Research in Scientific Publications; National Academies Press: Washington, DC, USA, 2011. [Google Scholar]
- Ryncarz, A.J.; Goddard, J.; Wald, A.; Huang, M.L.; Roizman, B.; Corey, L. Development of a high-throughput quantitative assay for detecting herpes simplex virus DNA in clinical samples. J. Clin. Microbiol. 1999, 37, 1941–1947. [Google Scholar] [PubMed]
- Lee, S.; Ives, A.M.; Bertke, A.S. Herpes simplex virus 1 reactivates from autonomic ciliary ganglia independently from sensory trigeminal ganglia to cause recurrent ocular disease. J. Virol. 2015, 89, 8383–8391. [Google Scholar] [CrossRef] [PubMed]
- Petro, C.; Gonzalez, P.A.; Cheshenko, N.; Jandl, T.; Khajoueinejad, N.; Benard, A.; Sengupta, M.; Herold, B.C.; Jacobs, W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. eLife 2015, 4. [Google Scholar] [CrossRef] [PubMed]
- Bull, M.; Lee, D.; Stucky, J.; Chiu, Y.L.; Rubin, A.; Horton, H.; McElrath, M.J. Defining blood processing parameters for optimal detection of cryopreserved antigen-specific responses for HIV vaccine trials. J. Immunol. Methods 2007, 322, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Melendez, L.V.; Espana, C.; Hunt, R.D.; Daniel, M.D.; Garcia, F.G. Natural herpes simplex infection in the owl monkey (Aotus trivirgatus). Lab. Anim. Care 1969, 19, 38–45. [Google Scholar] [PubMed]
- Katzin, D.S.; Connor, J.D.; Wilson, L.A.; Sexton, R.S. Experimental herpes simplex infection in the owl monkey. Proc. Soc. Exp. Biol. Med. 1967, 125, 391–398. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Fan, S.; Wang, X.; Hu, Y.; Feng, M.; Wang, L.; Zhang, Y.; Liao, Y.; Zhang, X.; Li, Q. Analysis of the protective immunity induced by herpes simplex virus 1 strain M3 with an attenuated phenotype due to mutations in the viral UL7, UL41, and LAT genes. Front. Microbiol. 2017, 8, 1958. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.C.; Cassady, K.A.; Cody, J.J.; Parker, J.N.; Price, K.H.; Coleman, J.M.; Peggins, J.O.; Noker, P.E.; Powers, N.W.; Grimes, S.D.; et al. Evaluation of the safety and biodistribution of M032, an attenuated herpes simplex virus type 1 expressing hIL-12, after intracerebral administration to aotus nonhuman primates. Hum. Gene Ther. Clin. Dev. 2014, 25, 16–27. [Google Scholar] [CrossRef] [PubMed]
- Awasthi, S.; Hook, L.M.; Shaw, C.E.; Pahar, B.; Stagray, J.A.; Liu, D.; Veazey, R.S.; Friedman, H.M. An HSV-2 trivalent vaccine is immunogenic in rhesus macaques and highly efficacious in guinea pigs. PLoS Pathog. 2017, 13, e1006141. [Google Scholar] [CrossRef] [PubMed]
- Dauber, B.; Pelletier, J.; Smiley, J.R. The herpes simplex virus 1 vhs protein enhances translation of viral true late mRNAs and virus production in a cell type-dependent manner. J. Virol. 2011, 85, 5363–5373. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Mott, K.R.; Matsuura, Y.; Moriishi, K.; Kousoulas, K.G.; Ghiasi, H. Binding of hsv-1 glycoprotein K (gK) to signal peptide peptidase (SPP) is required for virus infectivity. PLoS ONE 2014, 9, e85360. [Google Scholar] [CrossRef] [PubMed]
- Barzilai, A.; Zivony-Elbom, I.; Sarid, R.; Noah, E.; Frenkel, N. The herpes simplex virus type 1 vhs-UL41 gene secures viral replication by temporarily evading apoptotic cellular response to infection: Vhs-UL41 activity might require interactions with elements of cellular mRNA degradation machinery. J. Virol. 2006, 80, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, X.J.; Morrison, L.A.; Knipe, D.M. Comparison of different forms of herpes simplex replication-defective mutant viruses as vaccines in a mouse model of HSV-2 genital infection. Virology 2001, 288, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Brittle, E.E.; Wang, F.; Lubinski, J.M.; Bunte, R.M.; Friedman, H.M. A replication-competent, neuronal spread-defective, live attenuated herpes simplex virus type 1 vaccine. J. Virol. 2008, 82, 8431–8441. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Richards, A.L.; Sollars, P.J.; Pitts, J.D.; Stults, A.M.; Heldwein, E.E.; Pickard, G.E.; Smith, G.A. The pUL37 tegument protein guides alpha-herpesvirus retrograde axonal transport to promote neuroinvasion. PLoS Pathog. 2017, 13, e1006741. [Google Scholar] [CrossRef] [PubMed]
- Halford, W.P.; Puschel, R.; Rakowski, B. Herpes simplex virus 2 ICP0 mutant viruses are avirulent and immunogenic: Implications for a genital herpes vaccine. PLoS ONE 2010, 5, e12251. [Google Scholar] [CrossRef] [PubMed]
- Ozaki, T.; Nishimura, N.; Kajita, Y. Experience with live attenuated varicella vaccine (oka strain) in healthy japanese subjects; 10-year survey at pediatric clinic. Vaccine 2000, 18, 2375–2380. [Google Scholar] [CrossRef]
- Wang, K.; Ni, L.; Wang, S.; Zheng, C. Herpes simplex virus 1 protein kinase US3 hyperphosphorylates p65/RelA and dampens NF-kappab activation. J. Virol. 2014, 88, 7941–7951. [Google Scholar] [CrossRef] [PubMed]
- Jerome, K.R.; Chen, Z.; Lang, R.; Torres, M.R.; Hofmeister, J.; Smith, S.; Fox, R.; Froelich, C.J.; Corey, L. HSV and glycoprotein J inhibit caspase activation and apoptosis induced by granzyme B or Fas. J. Immunol. 2001, 167, 3928–3935. [Google Scholar] [CrossRef] [PubMed]
- Gobeil, P.A.; Leib, D.A. Herpes simplex virus gamma34.5 interferes with autophagosome maturation and antigen presentation in dendritic cells. mBio 2012, 3, e00267-12. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Ma, Y.; Prabhakar, B.S.; Feng, Z.; Valyi-Nagy, T.; Yan, Z.; Verpooten, D.; Zhang, C.; Cao, Y.; He, B. The γ134.5 protein of herpes simplex virus 1 is required to interfere with dendritic cell maturation during productive infection. J. Virol. 2009, 83, 4984–4994. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chentoufi, A.A.; Kritzer, E.; Yu, D.M.; Nesburn, A.B.; Benmohamed, L. Towards a rational design of an asymptomatic clinical herpes vaccine: The old, the new, and the unknown. Clin. Dev. Immunol. 2012, 2012, 187585. [Google Scholar] [CrossRef] [PubMed]


© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fan, S.; Xu, X.; Liao, Y.; Wang, Y.; Wang, J.; Feng, M.; Wang, L.; Zhang, Y.; He, Z.; Yang, F.; et al. Attenuated Phenotype and Immunogenic Characteristics of a Mutated Herpes Simplex Virus 1 Strain in the Rhesus Macaque. Viruses 2018, 10, 234. https://doi.org/10.3390/v10050234
Fan S, Xu X, Liao Y, Wang Y, Wang J, Feng M, Wang L, Zhang Y, He Z, Yang F, et al. Attenuated Phenotype and Immunogenic Characteristics of a Mutated Herpes Simplex Virus 1 Strain in the Rhesus Macaque. Viruses. 2018; 10(5):234. https://doi.org/10.3390/v10050234
Chicago/Turabian StyleFan, Shengtao, Xingli Xu, Yun Liao, Yongrong Wang, Jianbin Wang, Min Feng, Lichun Wang, Ying Zhang, Zhanlong He, Fengmei Yang, and et al. 2018. "Attenuated Phenotype and Immunogenic Characteristics of a Mutated Herpes Simplex Virus 1 Strain in the Rhesus Macaque" Viruses 10, no. 5: 234. https://doi.org/10.3390/v10050234
APA StyleFan, S., Xu, X., Liao, Y., Wang, Y., Wang, J., Feng, M., Wang, L., Zhang, Y., He, Z., Yang, F., Fraser, N. W., & Li, Q. (2018). Attenuated Phenotype and Immunogenic Characteristics of a Mutated Herpes Simplex Virus 1 Strain in the Rhesus Macaque. Viruses, 10(5), 234. https://doi.org/10.3390/v10050234



