Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Plasmids
2.2. Plant Materials
2.3. Agrobacterium Transformation and Agroinfiltration
2.4. Tomato Spotted Wilt Virus Mechanical Inoculation
2.5. Western Blotting
2.6. Confocal Microscopy
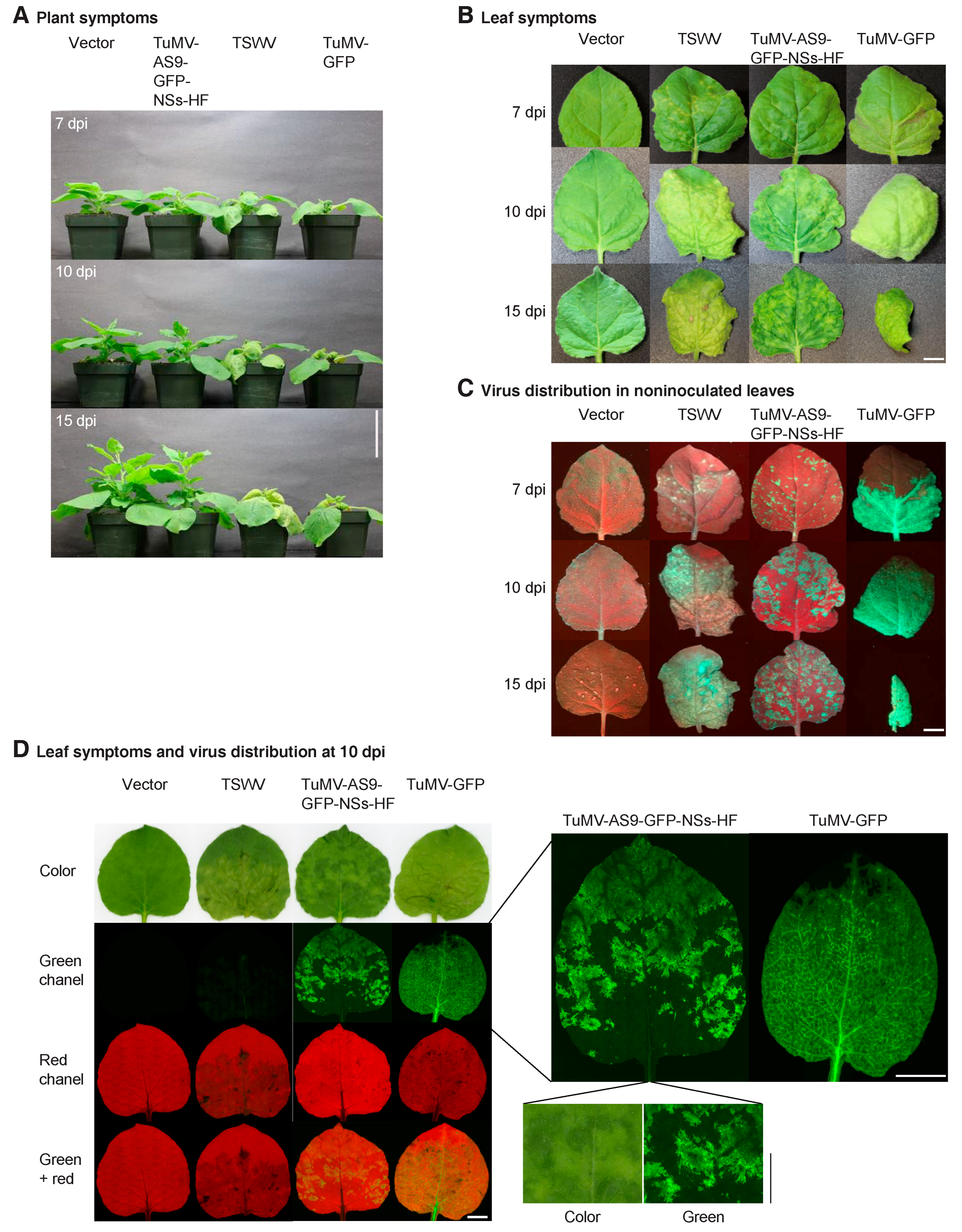
3. Results
3.1. NSs Tagging and Mutational Inactivation
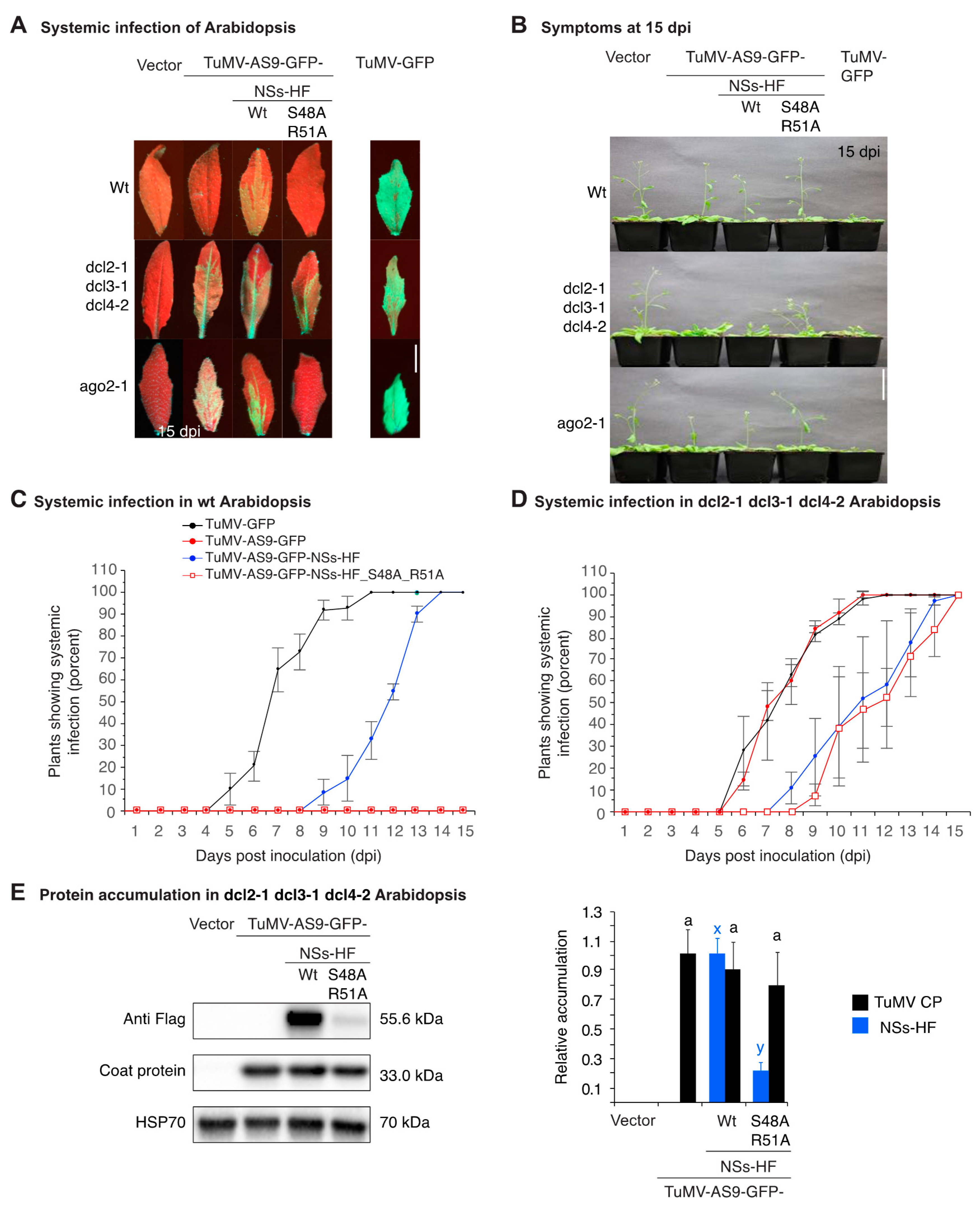
3.2. NSs Supports Systemic Movement of Suppressor-Deficient TuMV-AS9-GFP
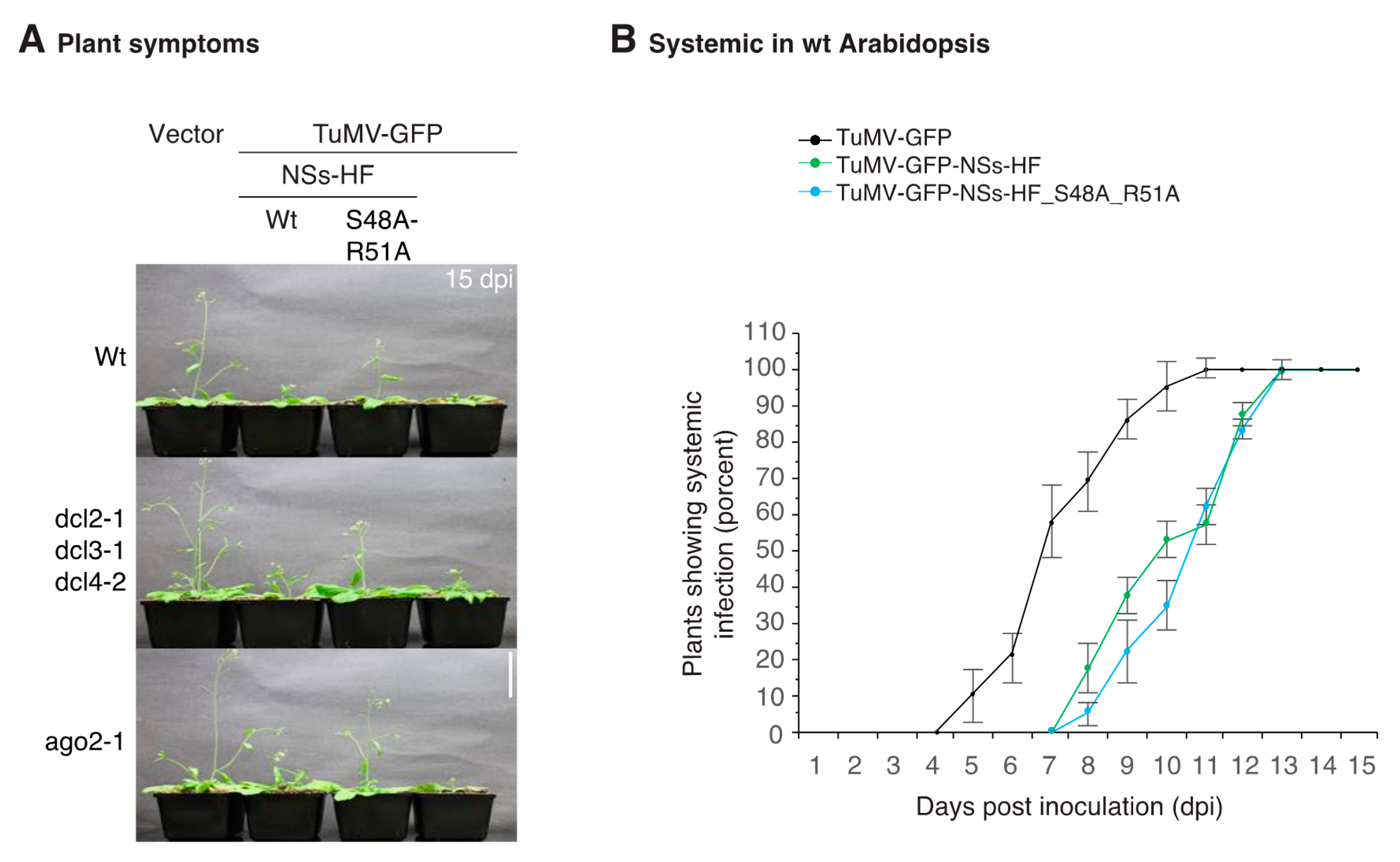
3.3. NSs Promotes Systemic Virus Movement by Suppressing Antiviral RNA Silencing
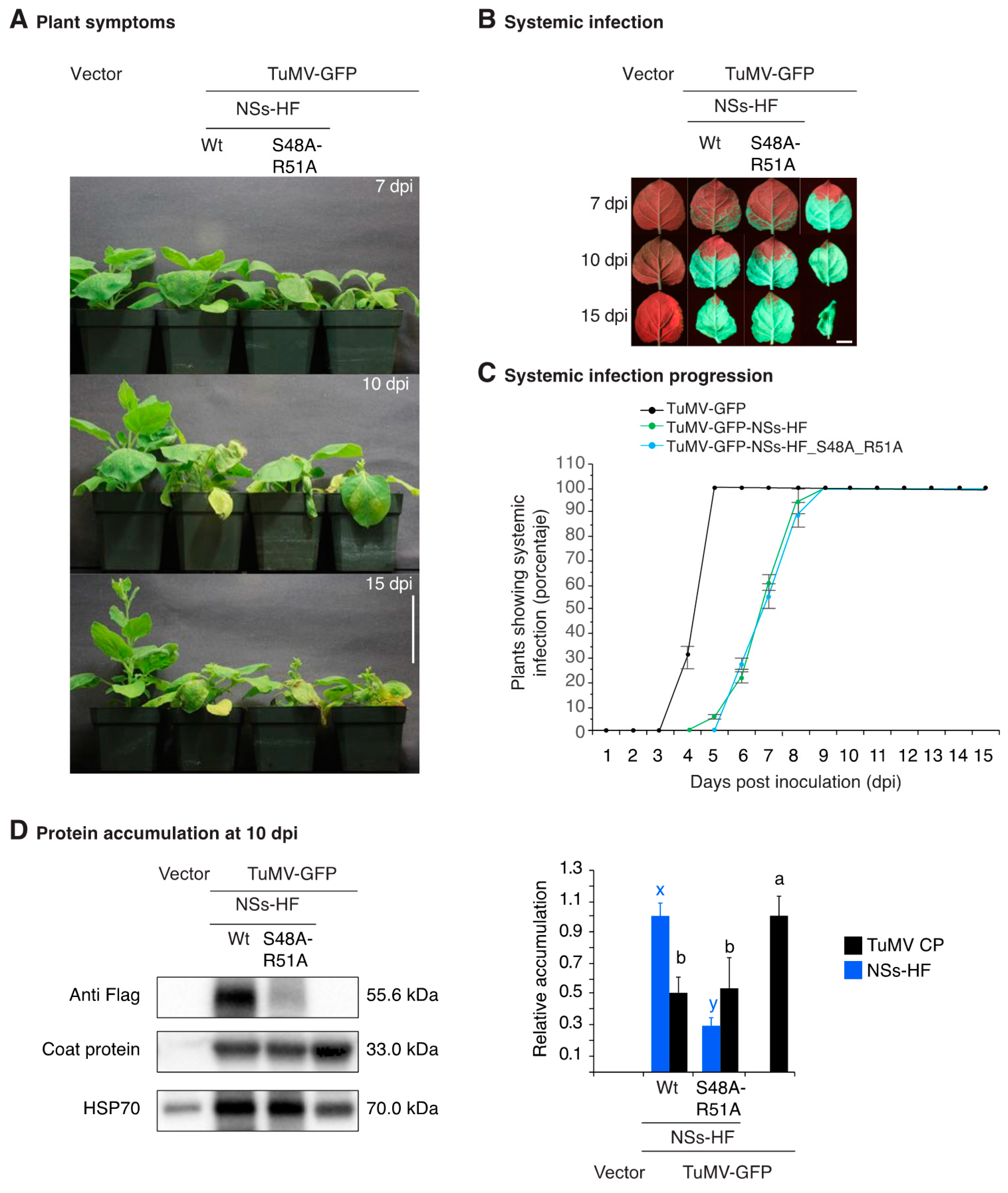
3.4. NSs Is a Symptom Determinant
3.5. NSs Silencing Suppression Activity Partially Overlaps that of HC-Pro
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ludman, M.; Burgyan, J.; Fatyol, K. CRISPR/Cas9 mediated inactivation of argonaute 2 reveals its differential involvement in antiviral responses. Sci. Rep. 2017, 7, 1010. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Carbonell, A.; Hoyer, J.S.; Fahlgren, N.; Gilbert, K.B.; Takeda, A.; Giampetruzzi, A.; Garcia Ruiz, M.T.; McGinn, M.G.; Lowery, N.; et al. Roles and programming of Arabidopsis ARGONAUTE proteins during Turnip Mosaic Virus infection. PLoS Pathog. 2015, 11, e1004755. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ruiz, H.; Takeda, A.; Chapman, E.J.; Sullivan, C.M.; Fahlgren, N.; Brempelis, K.J.; Carrington, J.C. Arabidopsis RNA-dependent RNA polymerases and dicer-like proteins in antiviral defense and small interfering RNA biogenesis during turnip mosaic virus infection. Plant Cell 2010, 22, 481–496. [Google Scholar] [CrossRef] [PubMed]
- Cao, M.; Du, P.; Wang, X.; Yu, Y.Q.; Qiu, Y.H.; Li, W.; Gal-On, A.; Zhou, C.; Li, Y.; Ding, S.W. Virus infection triggers widespread silencing of host genes by a distinct class of endogenous siRNAs in arabidopsis. Proc. Natl. Acad. Sci. USA 2014, 111, 14613–14618. [Google Scholar] [CrossRef] [PubMed]
- Blair, C.D.; Olson, K.E. The role of RNA interference (RNAi) in arbovirus-vector interactions. Viruses 2015, 7, 820–843. [Google Scholar] [CrossRef] [PubMed]
- O’Neal, S.T.; Samuel, G.H.; Adelman, Z.N.; Myles, K.M. Mosquito-borne viruses and suppressors of invertebrate antiviral RNA silencing. Viruses 2014, 6, 4314–4331. [Google Scholar] [CrossRef] [PubMed]
- Szittya, G.; Burgyan, J. RNA interference-mediated intrinsic antiviral immunity in plants. Curr. Top. Microbiol. Immunol. 2013, 371, 153–181. [Google Scholar] [PubMed]
- Ocampo, T.; Gabriel Peralta, S.M.; Bacheller, N.; Uiterwaal, S.; Knapp, A.; Hennen, A.; Ochoa-Martinez, D.L.; Garcia-Ruiz, H. Antiviral RNA silencing suppression activity of Tomato spotted wilt virus NSs protein. Genet. Mol. Res. 2016, 15, gmr–15028625. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Jovel, J.; Udomporn, P.; Wang, Y.; Wu, Q.; Li, W.X.; Gasciolli, V.; Vaucheret, H.; Ding, S.W. The 21-nucleotide, but not 22-nucleotide, viral secondary small interfering RNAs direct potent antiviral defense by two cooperative argonautes in arabidopsis thaliana. Plant Cell 2011, 23, 1625–1638. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.B.; Wu, Q.; Ito, T.; Cillo, F.; Li, W.X.; Chen, X.; Yu, J.L.; Ding, S.W. RNAi-mediated viral immunity requires amplification of virus-derived siRNAs in arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 2010, 107, 484–489. [Google Scholar] [CrossRef] [PubMed]
- Brosseau, C.; Moffett, P. Functional and genetic analysis identify a role for arabidopsis argonaute5 in antiviral RNA silencing. Plant Cell 2015, 27, 1742–1754. [Google Scholar] [CrossRef] [PubMed]
- Fatyol, K.; Ludman, M.; Burgyan, J. Functional dissection of a plant argonaute. Nucl. Acid. Res. 2016, 44, 1384–1397. [Google Scholar] [CrossRef] [PubMed]
- Karran, R.A.; Sanfacon, H. Tomato ringspot virus coat protein binds to ARGONAUTE 1 and suppresses the translation repression of a reporter gene. Mol. Plant Microbe. Interact. 2014, 27, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Kontra, L.; Burgyan, J. Viral silencing suppressors: Tools forged to fine-tune host-pathogen coexistence. Virology 2015, 479–480, 85–103. [Google Scholar] [CrossRef] [PubMed]
- Chapman, E.J.; Prokhnevsky, A.I.; Gopinath, K.; Dolja, V.V.; Carrington, J.C. Viral RNA silencing suppressors inhibit the microRNA pathway at an intermediate step. Genes Dev. 2004, 18, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Lakatos, L.; Csorba, T.; Pantaleo, V.; Chapman, E.J.; Carrington, J.C.; Liu, Y.P.; Dolja, V.V.; Calvino, L.F.; Lopez-Moya, J.J.; Burgyan, J. Small RNA binding is a common strategy to suppress RNA silencing by several viral suppressors. EMBO J. 2006, 25, 2768–2780. [Google Scholar] [CrossRef] [PubMed]
- Del Toro, F.J.; Donaire, L.; Aguilar, E.; Chung, B.N.; Tenllado, F.; Canto, T. Potato virus Y HCPro suppression of antiviral silencing in Nicotiana benthamiana plants correlates with its ability to bind In Vivo to 21- and 22-nucleotide small RNAs of viral sequence. J. Virol. 2017, 91, e00367-17. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ye, X.; Morris, T.J. Arabidopsis DRB4, AGO1, AGO7, and RDR6 participate in a DCL4-initiated antiviral RNA silencing pathway negatively regulated by DCL1. Proc. Natl. Acad. Sci. USA 2008, 105, 14732–14737. [Google Scholar] [CrossRef] [PubMed]
- Varallyay, E.; Valoczi, A.; Agyi, A.; Burgyan, J.; Havelda, Z. Plant virus-mediated induction of mir168 is associated with repression of argonaute1 accumulation. EMBO J. 2010, 29, 3507–3519. [Google Scholar] [CrossRef] [PubMed]
- Csorba, T.; Lozsa, R.; Hutvagner, G.; Burgyan, J. Polerovirus protein P0 prevents the assembly of small RNA-containing RISC complexes and leads to degradation of ARGONAUTE1. Plant J. 2010, 62, 463–472. [Google Scholar] [CrossRef] [PubMed]
- Cheng, X.; Wang, A. The potyvirus silencing suppressor protein VPg mediates degradation of SGS3 via ubiquitination and autophagy pathways. J. Virol. 2017, 91, e01478-16. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Eskelin, K.; Basic, M.; De, S.; Lohmus, A.; Varjosalo, M.; Makinen, K. Molecular insights into the function of the viral RNA silencing suppressor HCPRO. Plant J. 2016, 85, 30–45. [Google Scholar] [CrossRef] [PubMed]
- Chitwood, D.H.; Nogueira, F.T.; Howell, M.D.; Montgomery, T.A.; Carrington, J.C.; Timmermans, M.C. Pattern formation via small RNA mobility. Genes Dev. 2009, 23, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Gilbert, K.B.; Montgomery, T.A.; Nguyen, T.; Cuperus, J.T.; Carrington, J.C. Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 2012, 24, 3613–3629. [Google Scholar] [CrossRef] [PubMed]
- Montgomery, T.A.; Howell, M.D.; Cuperus, J.T.; Li, D.; Hansen, J.E.; Alexander, A.L.; Chapman, E.J.; Fahlgren, N.; Allen, E.; Carrington, J.C. Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 Trans-Acting siRNA formation. Cell 2008, 133, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Cuperus, J.T.; Carbonell, A.; Fahlgren, N.; Garcia-Ruiz, H.; Burke, R.T.; Takeda, A.; Sullivan, C.M.; Gilbert, S.D.; Montgomery, T.A.; Carrington, J.C. Unique functionality of 22-NT miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in arabidopsis. Nat. Struct. Mol. Biol. 2010, 17, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Manavella, P.A.; Koenig, D.; Weigel, D. Plant secondary siRNA production determined by microRNA-duplex structure. Proc. Nat. Acad. Sci. USA 2012, 109, 2461–2466. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Xie, Z.; Allen, E.; Llave, C.; Chapman, E.J.; Krizan, K.A.; Carrington, J.C. P1/HC-PRO, a viral suppressor of RNA silencing, interferes with Arabidopsis development and miRNA unction. Dev. Cell 2003, 4, 205–217. [Google Scholar] [CrossRef]
- Powers, J.G.; Sit, T.L.; Qu, F.; Morris, T.J.; Kim, K.H.; Lommel, S.A. A versatile assay for the identification of RNA silencing suppressors based on complementation of viral movement. Mol. Plant Microbe Interact. 2008, 21, 879–890. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jaubert, M.J.; Bhattacharjee, S.; Mello, A.F.; Perry, K.L.; Moffett, P. Ago2 mediates RNA silencing anti-viral defenses against potato virus x in Arabidopsis. Plant Phys. 2011. [Google Scholar] [CrossRef] [PubMed]
- Scholthof, H.B.; Alvarado, V.Y.; Vega-Arreguin, J.C.; Ciomperlik, J.; Odokonyero, D.; Brosseau, C.; Jaubert, M.; Zamora, A.; Moffett, P. Identification of an argonaute for antiviral RNA silencing in nicotiana benthamiana. Plant Phys. 2011, 156, 1548–1555. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.; Barton, D.; Nakasugi, K.; Jackson, C.; Kalischuk, M.; Kawchuk, L.; Vaslin, M.; Correa, R.; Waterhouse, P. The luteovirus P4 movement protein is a suppressor of systemic RNA silencing. Viruses 2017, 9, 294. [Google Scholar] [CrossRef] [PubMed]
- Hedil, M.; Sterken, M.G.; de Ronde, D.; Lohuis, D.; Kormelink, R. Analysis of tospovirus NSs proteins in suppression of systemic silencing. PLoS ONE 2015, 10, e0134517. [Google Scholar] [CrossRef] [PubMed]
- Takeda, A.; Sugiyama, K.; Nagano, H.; Mori, M.; Kaido, M.; Mise, K.; Tsuda, S.; Okuno, T. Identification of a novel RNA silencing suppressor, NSs protein of tomato spotted wilt virus. FEBS Lett. 2002, 532, 75–79. [Google Scholar] [CrossRef]
- Bucher, E.; Sijen, T.; de Haan, P.; Goldbach, R.W.; Prins, M. Negative-strand tospoviruses and tenuiviruses carry a gene for a suppressor of gene silencing at analogous genomic positions. J. Virol. 2003, 77, 1329–1336. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Bosco, L.; Vallino, M.; Ciuffo, M.; Mautino, G.C.; Tavella, L.; Turina, M. The NSs protein of Tomato spotted wilt virus is required for persistent infection and transmission by Frankliniella occidentalis. J. Virol. 2014, 88, 5788–5802. [Google Scholar] [CrossRef] [PubMed]
- Whitfield, A.E.; Ullman, D.E.; German, T.L. Tospovirus-thrips interactions. Ann. Rev. Phytopathol. 2005, 43, 459–489. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Pasquier, A.; Ying, S.; Butterbach, P.; Lohuis, D.; Kormelink, R. Analysis of tomato spotted wilt virus NSs protein indicates the importance of the n-terminal domain for avirulence and RNA silencing suppression. Mol. Plant Pathol. 2014, 15, 185–195. [Google Scholar] [CrossRef] [PubMed]
- De Ronde, D.; Butterbach, P.; Lohuis, D.; Hedil, M.; van Lent, J.W.; Kormelink, R. Tsw gene-based resistance is triggered by a functional RNA silencing suppressor protein of the tomato spotted wilt virus. Mol. Plant Pathol. 2013, 14, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Parrella, G.; Gognalons, P.; Gebre-Selassie, K.; Vovlas, C.; Marchoux, G. An update of the host range of tomato spotted wilt virus. J. Plant Pathol. 2003, 85, 227–264. [Google Scholar]
- Hedil, M.; Kormelink, R. Viral RNA silencing suppression: The enigma of bunyavirus NSs proteins. Viruses 2016, 8, 208. [Google Scholar] [CrossRef] [PubMed]
- Curtis, M.D.; Grossniklaus, U. A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol. 2003, 133, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Qi, D.; Scholthof, K.B. A one-step PCR-based method for rapid and efficient site-directed fragment deletion, insertion, and substitution mutagenesis. J. Virol. Method. 2008, 149, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Bag, S.; Mitter, N.; Turina, M.; Pappu, H.R. Mutational analysis of two highly conserved motifs in the silencing suppressor encoded by tomato spotted wilt virus (genus tospovirus, family bunyaviridae). Arch. Virol. 2014, 159, 1499–1504. [Google Scholar] [CrossRef] [PubMed]
- Deleris, A.; Gallego-Bartolome, J.; Bao, J.; Kasschau, K.D.; Carrington, J.C.; Voinnet, O. Hierarchical action and inhibition of plant dicer-like proteins in antiviral defense. Science 2006, 313, 68. [Google Scholar] [CrossRef] [PubMed]
- Lobbes, D.; Rallapalli, G.; Schmidt, D.D.; Martin, C.; Clarke, J. Serrate: A new player on the plant microRNA scene. EMBO Rep. 2006, 7, 1052–1058. [Google Scholar] [CrossRef] [PubMed]
- Johansen, L.K.; Carrington, J.C. Silencing on the spot. Induction and suppression of RNA silencing in the agrobacterium-mediated transient expression system. Plant Physiol. 2001, 126, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Qi, D.; Ashfield, T.; Helm, M.; Innes, R.W. Using decoys to expand the recognition specificity of a plant disease resistance protein. Science 2016, 351, 684. [Google Scholar] [CrossRef] [PubMed]
- Diaz-Pendon, J.A.; Li, F.; Li, W.X.; Ding, S.W. Suppression of antiviral silencing by cucumber mosaic virus 2b protein in arabidopsis is associated with drastically reduced accumulation of three classes of viral small interfering RNAs. Plant Cell 2007, 19, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, K.I.; Eskelin, K.; Lohmus, A.; Makinen, K. Molecular and cellular mechanisms underlying potyvirus infection. J. Gen. Virol. 2014, 95, 1415–1429. [Google Scholar] [CrossRef] [PubMed]
- Cardinale, D.J.; DeRosa, K.; Duffy, S. Base composition and translational selection are insufficient to explain codon usage bias in plant viruses. Viruses 2013, 5, 162–181. [Google Scholar] [CrossRef] [PubMed]
- German, T.L.; Adkins, S.; Witherell, A.; Richmond, K.E.; Knaack, W.R.; Willis, D.K. Infection of Arabidopsis thaliana ecotype columbia by tomato spotted wilt virus. Plant Mol. Biol. Rep. 1995, 13, 110–117. [Google Scholar] [CrossRef]
- Molnar, A.; Melnyk, C.W.; Bassett, A.; Hardcastle, T.J.; Dunn, R.; Baulcombe, D.C. Small silencing RNAs in plants are mobile and direct epigenetic modification in recipient cells. Science 2010, 328, 872–875. [Google Scholar] [CrossRef] [PubMed]
- Schnettler, E.; Hemmes, H.; Huismann, R.; Goldbach, R.; Prins, M.; Kormelink, R. Diverging affinity of tospovirus RNA silencing suppressor proteins, NSs, for various RNA duplex molecules. J. Virol. 2010, 84, 11542–11554. [Google Scholar] [CrossRef] [PubMed]
- Widana Gamage, S.M.K.; Dietzgen, R.G. Intracellular localization, interactions and functions of capsicum chlorosis virus proteins. Front. Microbiol. 2017, 8, 612. [Google Scholar] [CrossRef] [PubMed]
- Goswami, S.; Sahana, N.; Pandey, V.; Doblas, P.; Jain, R.K.; Palukaitis, P.; Canto, T.; Praveen, S. Interference in plant defense and development by non-structural protein NSs of groundnut bud necrosis virus. Virus Res. 2012, 163, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Bag, S.; Mitter, N.; Eid, S.; Pappu, H.R. Complementation between two tospoviruses facilitates the systemic movement of a plant virus silencing suppressor in an otherwise restrictive host. PLoS ONE 2012, 7, e44803. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Johansen, L.K.; Gustafson, A.M.; Kasschau, K.D.; Lellis, A.D.; Zilberman, D.; Jacobsen, S.E.; Carrington, J.C. Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2004, 2, E104. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Bao, F.S.; Xie, Z. Small RNA deep sequencing reveals role for arabidopsis thaliana RNA-dependent RNA polymerases in viral siRNA biogenesis. PLoS ONE 2009, 4, e4971. [Google Scholar] [CrossRef]
- Blevins, T.; Rajeswaran, R.; Shivaprasad, P.V.; Beknazariants, D.; Si-Ammour, A.; Park, H.S.; Vazquez, F.; Robertson, D.; Meins, F., Jr.; Hohn, T.; et al. Four plant dicers mediate viral small RNA biogenesis and DNA virus induced silencing. Nucl. Acid. Res. 2006, 34, 6233–6246. [Google Scholar] [CrossRef] [PubMed]
- Qu, F.; Ye, X.; Hou, G.; Sato, S.; Clemente, T.E.; Morris, T.J. RDR6 has a broad-spectrum but temperature-dependent antiviral defense role in nicotiana benthamiana. J. Virol. 2005, 79, 15209–15217. [Google Scholar] [CrossRef] [PubMed]
- Tatineni, S.; Qu, F.; Li, R.; Morris, T.J.; French, R. Triticum mosaic poacevirus enlists P1 rather than HC-pro to suppress RNA silencing-mediated host defense. Virology 2012, 433, 104–115. [Google Scholar] [CrossRef] [PubMed]
- Ryabov, E.V.; Robinson, D.J.; Taliansky, M.E. A plant virus-encoded protein facilitates long-distance movement of heterologous viral RNA. Proc. Nat. Acad. Sci. USA 1999, 96, 1212–1217. [Google Scholar] [CrossRef] [PubMed]
- Schuck, J.; Gursinsky, T.; Pantaleo, V.; Burgyan, J.; Behrens, S.E. Ago/RISC-Mediated antiviral RNA silencing in a plant in vitro system. Nucl. Acid. Res. 2013, 41, 5090–5103. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, S.J.; Shrestha, A.; Peters, J.R.; Carroll, B.J.; Srinivasan, R.; Pappu, H.R.; Mitter, N. The tomato spotted wilt virus genome is processed differentially in its plant host Arachis Hypogaea and its thrips vector Frankliniella fusca. Front. Plant Sci. 2016, 7, 1349. [Google Scholar] [CrossRef] [PubMed]
- Mitter, N.; Koundal, V.; Williams, S.; Pappu, H. Differential expression of tomato spotted wilt virus-derived viral small RNAs in infected commercial and experimental host plants. PLoS ONE 2013, 8, e76276. [Google Scholar] [CrossRef] [PubMed]
- Hedil, M.; Hassani-Mehraban, A.; Lohuis, D.; Kormelink, R. Analysis of the A-U rich hairpin from the intergenic region of tospovirus s RNA as target and inducer of RNA silencing. PLoS ONE 2014, 9, e106027. [Google Scholar] [CrossRef] [PubMed]
- Margaria, P.; Miozzi, L.; Rosa, C.; Axtell, M.J.; Pappu, H.R.; Turina, M. Small RNA profiles of wild-type and silencing suppressor-deficient tomato spotted wilt virus infected Nicotiana benthamiana. Virus Res. 2015, 208, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Kasschau, K.D.; Carrington, J.C. A counterdefensive strategy of plant viruses: Suppression of posttranscriptional gene silencing. Cell 1998, 95, 461–470. [Google Scholar] [CrossRef]

| Host and Virus | Plants Inoculated | Plants Systemically Infected | |||
|---|---|---|---|---|---|
| 5 dpi | 7 dpi | 10 dpi | 15 dpi | ||
| Nicotiana benthamiana | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP-NSs-HF | 36 | 2 | 18 | 31 | 36 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 0 | 0 |
| TuMV-GFP | 36 | 36 | 36 | 36 | 36 |
| Wt Arabidopsis thaliana (Col-0) 2 | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP-NSs-HF | 36 | 0 | 0 | 5 | 36 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 0 | 0 |
| TuMV-GFP | 36 | 4 | 23 | 34 | 36 |
| A. thaliana dcl2-1 dcl3-1 dcl4-2 triple mutant 2 | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 0 | 16 | 32 | 36 |
| TuMV-AS9-GFP-NSs-HF | 36 | 0 | 0 | 15 | 36 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 13 | 36 |
| TuMV-GFP | 36 | 0 | 14 | 32 | 36 |
| Host and Virus | Plants Inoculated | Plants Systemically Infected at 15 dpi 2 | |||
|---|---|---|---|---|---|
| Cauline Leaves | Inflorescence | ||||
| Number | Percent | Number | Percent | ||
| A. thaliana ago2-1 single mutant 2 | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 36 | 100 | 0 | 0 |
| TuMV-AS9-GFP-NSs-HF | 36 | 36 | 100 | 36 | 100 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 0 | 0 |
| TuMV-GFP | 36 | 36 | 100 | 36 | 100 |
| A. thaliana rdr1-1 single mutant 3 | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 36 | 100 | 0 | 0 |
| TuMV-AS9-GFP-NSs-HF | 36 | 36 | 100 | 36 | 100 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 0 | 0 |
| TuMV-GFP | 36 | 36 | 100 | 36 | 100 |
| A. thaliana rdr6-15 single mutant 3 | |||||
| Empty vector | 36 | 0 | 0 | 0 | 0 |
| TuMV-AS9-GFP | 36 | 36 | 100 | 0 | 0 |
| TuMV-AS9-GFP-NSs-HF | 36 | 28 | 78 | 28 | 78 |
| TuMV-AS9-GFP-NSs-S48A-R51A-HF | 36 | 0 | 0 | 0 | 0 |
| TuMV-GFP | 36 | 36 | 100 | 36 | 100 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garcia-Ruiz, H.; Gabriel Peralta, S.M.; Harte-Maxwell, P.A. Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant. Viruses 2018, 10, 129. https://doi.org/10.3390/v10030129
Garcia-Ruiz H, Gabriel Peralta SM, Harte-Maxwell PA. Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant. Viruses. 2018; 10(3):129. https://doi.org/10.3390/v10030129
Chicago/Turabian StyleGarcia-Ruiz, Hernan, Sergio M. Gabriel Peralta, and Patricia A. Harte-Maxwell. 2018. "Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant" Viruses 10, no. 3: 129. https://doi.org/10.3390/v10030129
APA StyleGarcia-Ruiz, H., Gabriel Peralta, S. M., & Harte-Maxwell, P. A. (2018). Tomato Spotted Wilt Virus NSs Protein Supports Infection and Systemic Movement of a Potyvirus and Is a Symptom Determinant. Viruses, 10(3), 129. https://doi.org/10.3390/v10030129