Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets
Abstract
1. Introduction
2. Materials and Methods
2.1. Virus, Bacterium, and Cell Line
2.2. Vaccination Programs
2.3. Enzyme-Linked Immunosorbent Assay
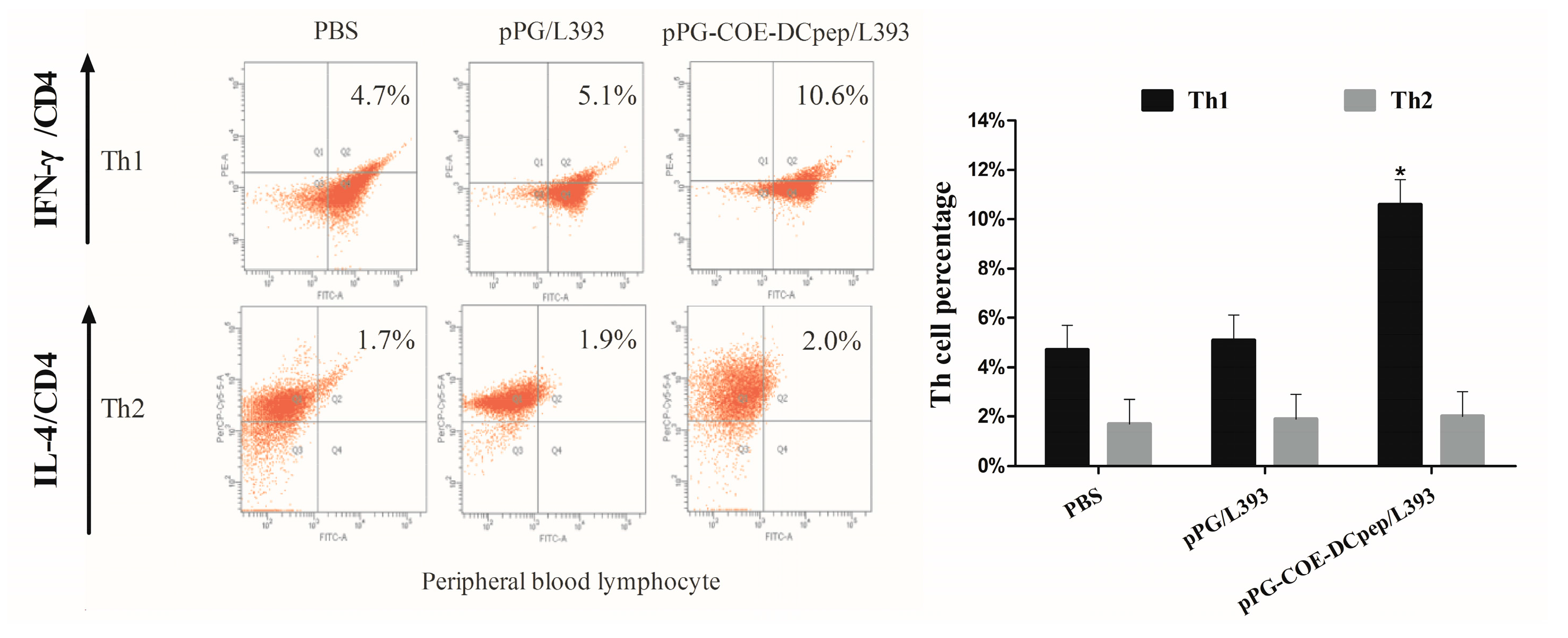
2.4. Th Cell Analysis before and after Infection
2.5. Protective Efficacy
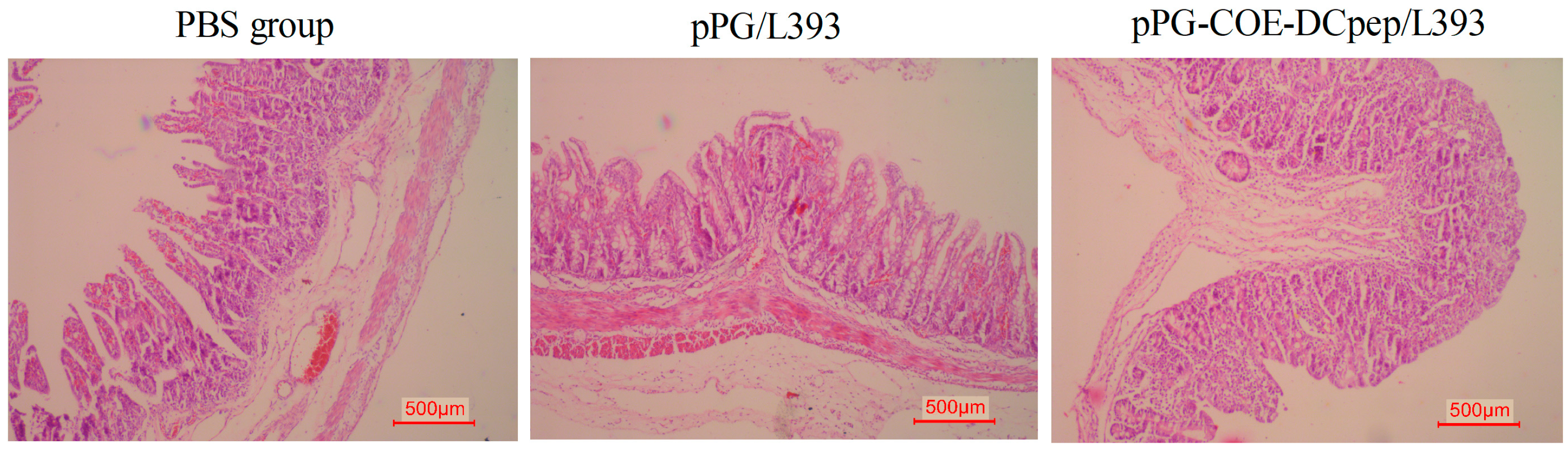
2.6. Gross Lesion and Histopathological Examinations
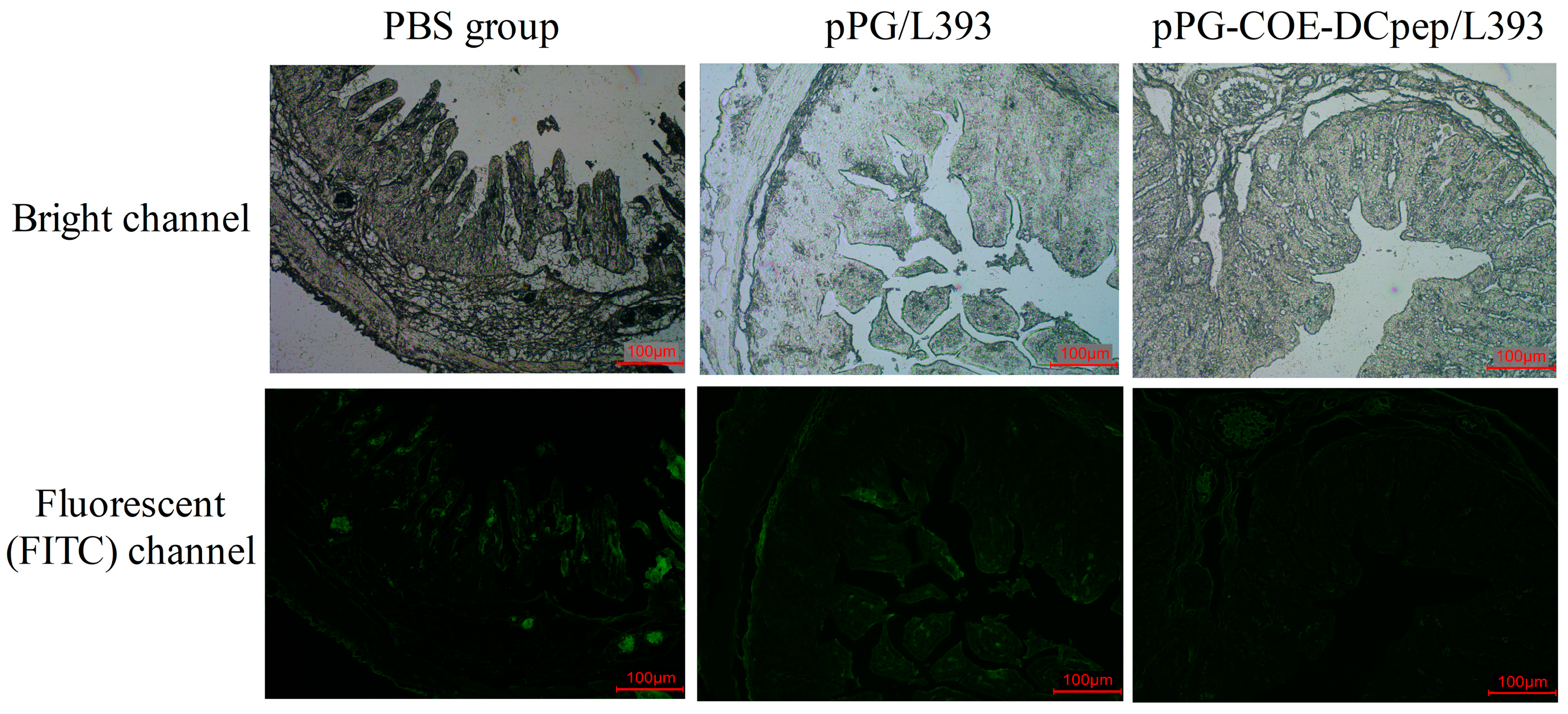
2.7. Immunofluorescence Assay (IFA)
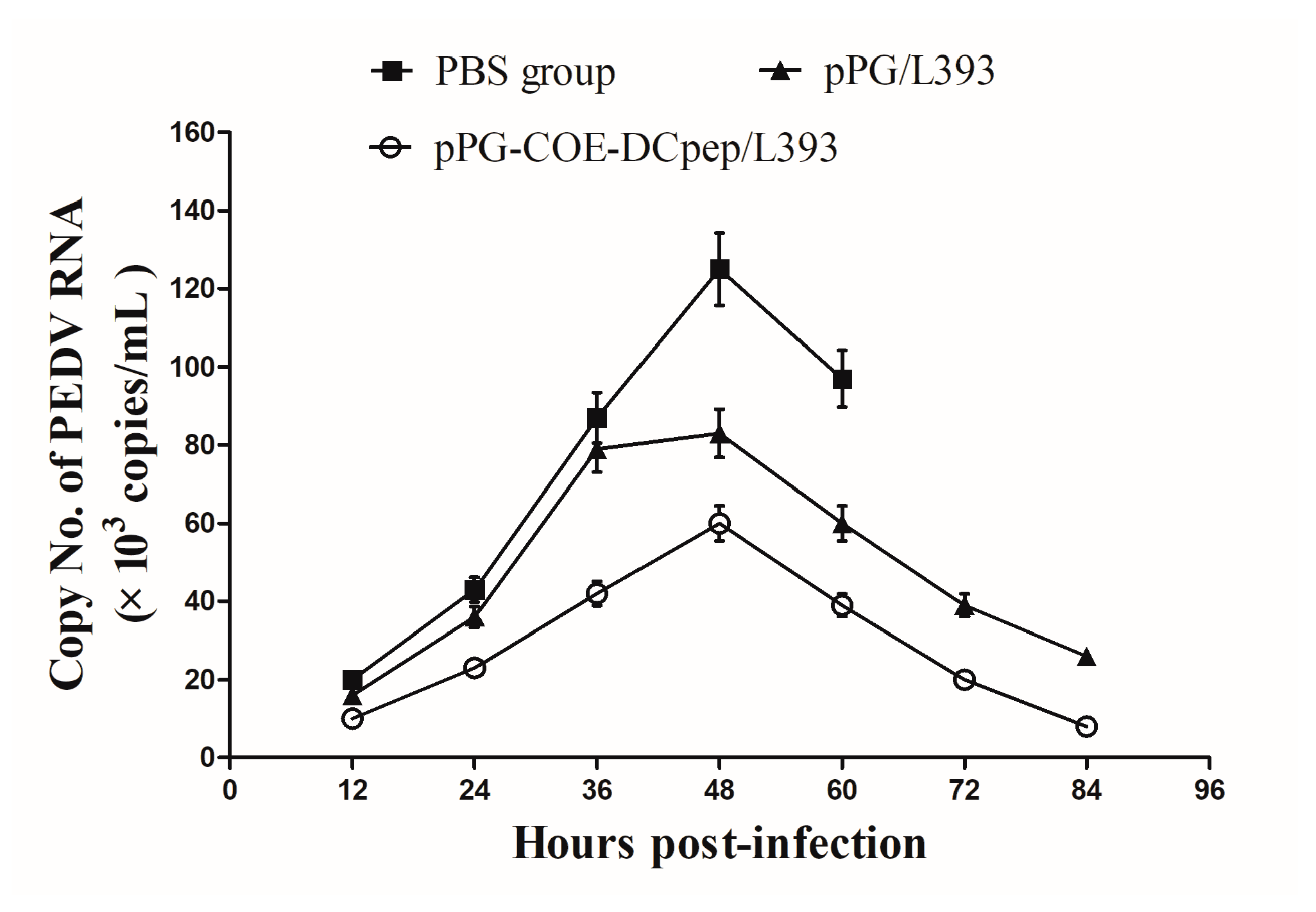
2.8. Real-Time RT-PCR (qRT-PCR) Analysis
2.9. Statistical Analysis
3. Results
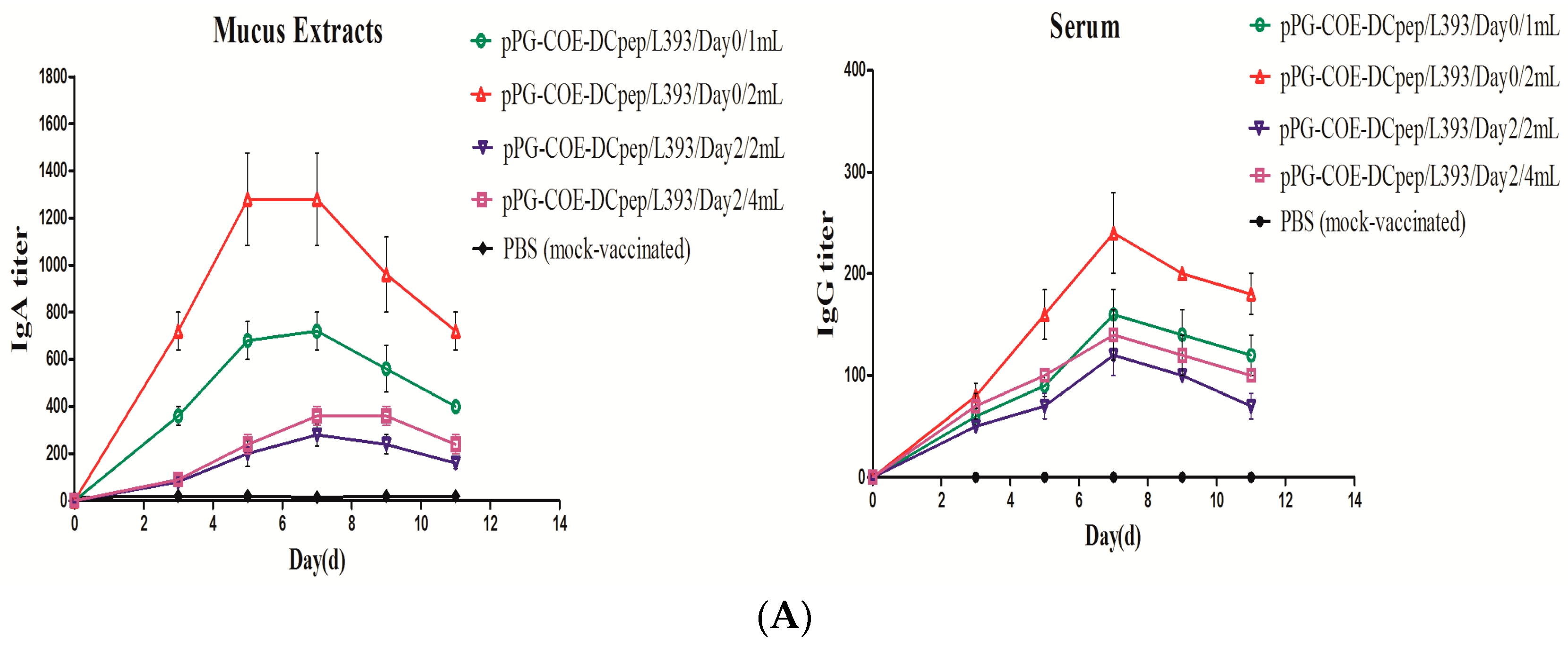
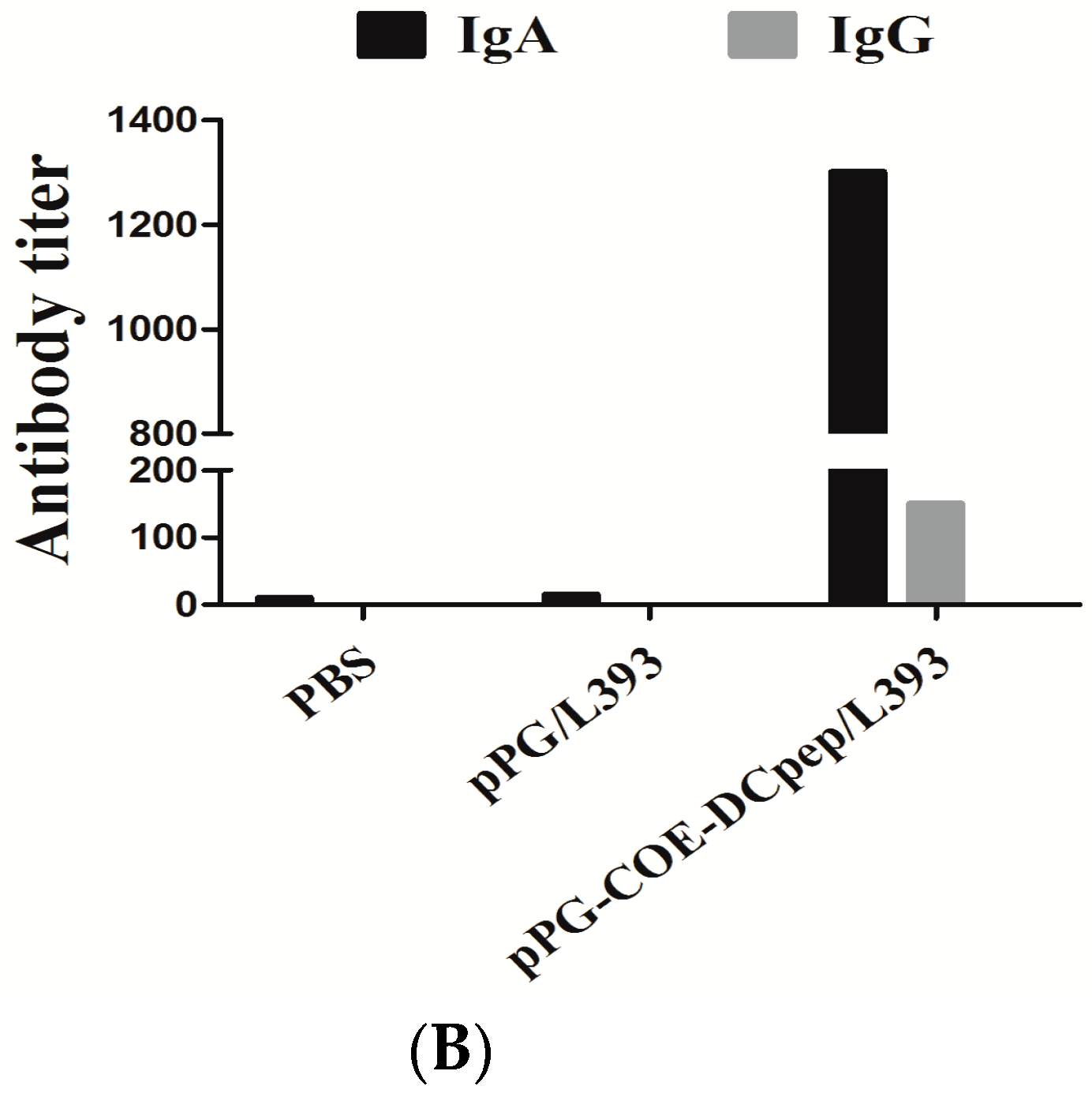
3.1. Production of Mucosal and Humoral Immunity
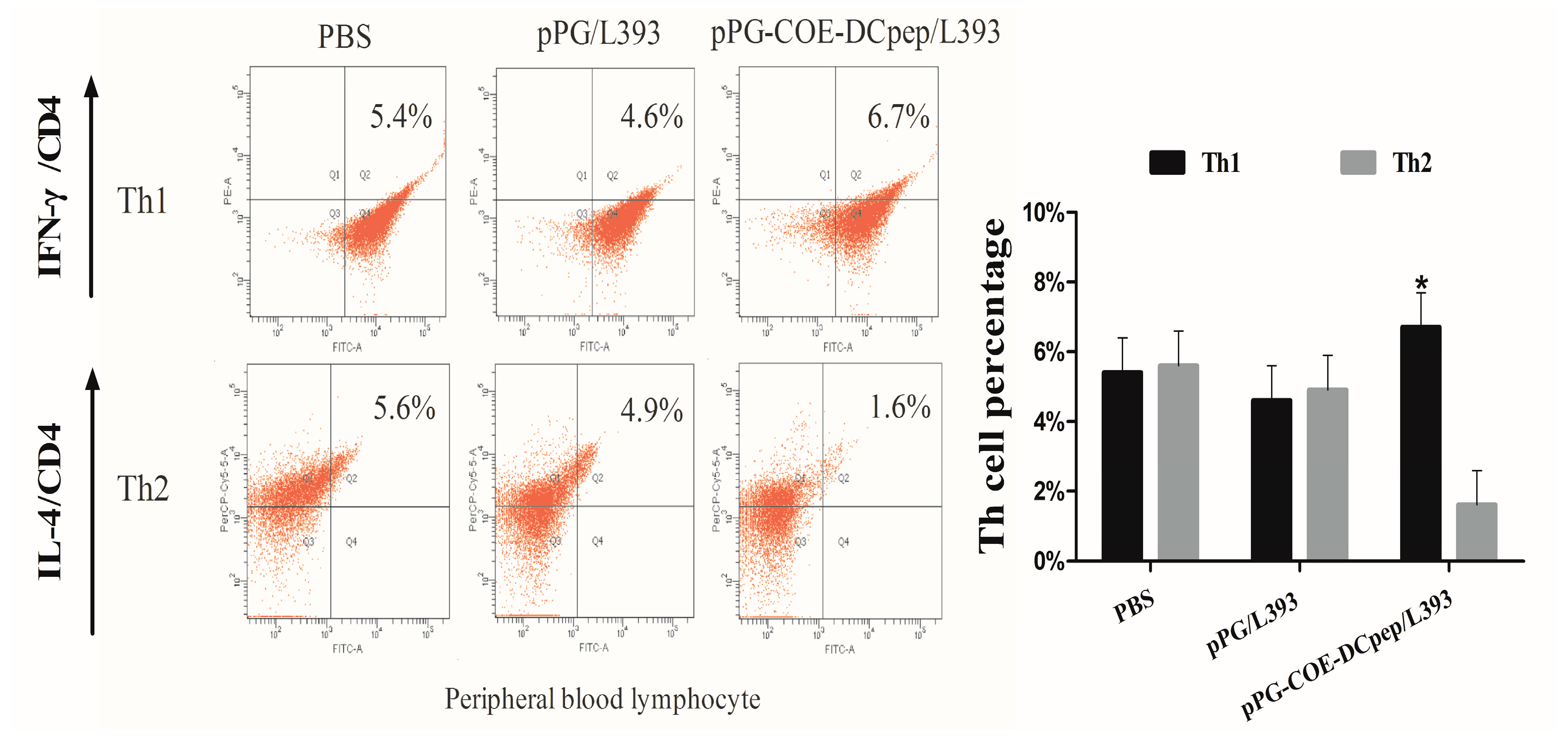
3.2. The Percentage of T-Helper Cells by pPG-COE-DCpep/L393
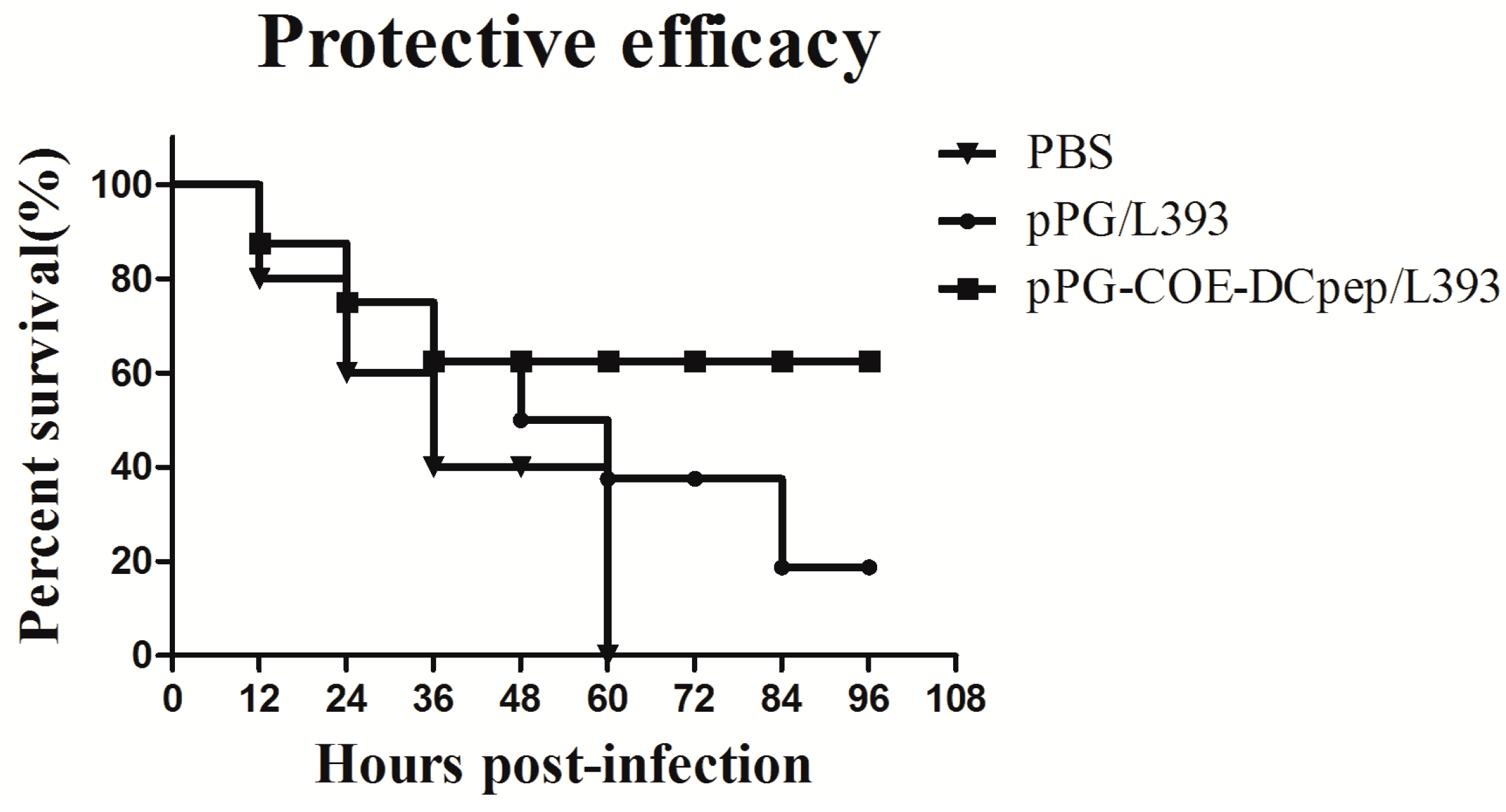
3.3. Protective Efficacy
3.4. Gross Lesion and Histopathological Examinations
3.5. Application of Real-Time RT-PCR to Clinical Samples
3.6. Immunofluorescence Assay (IFA)
3.7. The Percentage of T-Helper Cells after PEDV Infection
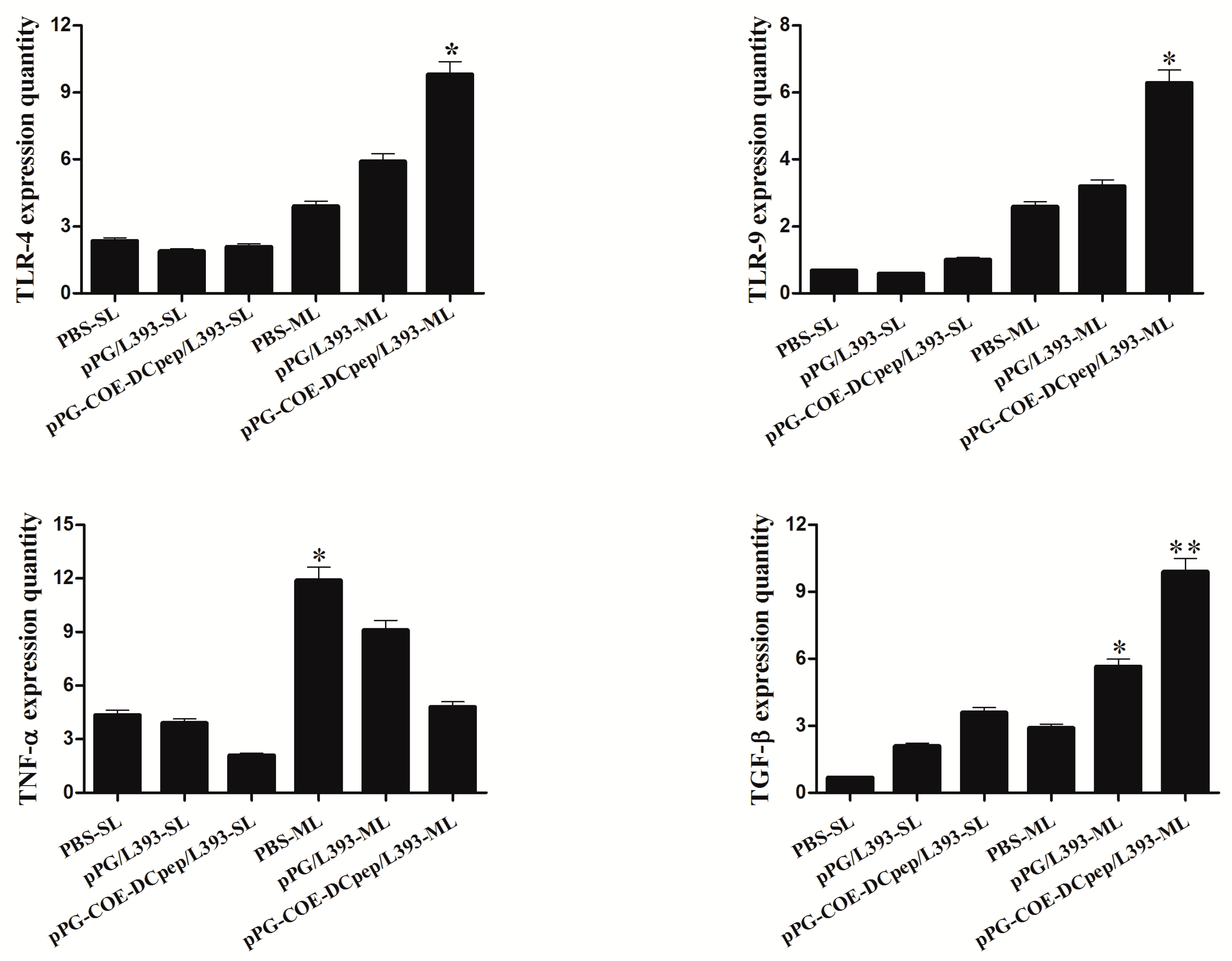
3.8. Cytokine Expression
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Stevenson, G.W.; Hoang, H.; Schwartz, K.J.; Burrough, E.R.; Sun, D.; Madson, D.; Cooper, V.L.; Pillatzki, A.; Gauger, P.; Schmitt, B.J.; et al. Emergence of porcine epidemic diarrhea virus in the united states: Clinical signs, lesions, and viral genomic sequences. J. Vet. Diagn. Investig. 2013, 25, 649–654. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.L.; Yu, L.Y.; Liu, J.; Wang, G.H. Surface-displayed porcine epidemic diarrhea viral (pedv) antigens on lactic acid bacteria. Vaccine 2007, 26, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.L.; Lee, J.G.; Kang, T.J.; Jang, H.S.; Jang, Y.S.; Yang, M.S. Induction of antigen-specific systemic and mucosal immune responses by feeding animals transgenic plants expressing the antigen. Vaccine 2003, 21, 4052–4058. [Google Scholar] [CrossRef]
- Song, D.S.; Oh, J.S.; Kang, B.K.; Yang, J.S.; Moon, H.J.; Yoo, H.S.; Jang, Y.S.; Park, B.K. Oral efficacy of vero cell attenuated porcine epidemic diarrhea virus dr13 strain. Res. Vet. Sci. 2007, 82, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, H.; van Reit, E.; Kida, H. Mucosal immunization and adjuvants. Curr. Top. Microbiol. Immunol. 2015, 386, 371–380. [Google Scholar] [PubMed]
- Mota, R.M.; Moreira, J.L.; Souza, M.R.; Horta, M.F.; Teixeira, S.M.; Neumann, E.; Nicoli, J.R.; Nunes, A.C. Genetic transformation of novel isolates of chicken lactobacillus bearing probiotic features for expression of heterologous proteins: A tool to develop live oral vaccines. BMC Biotechnol. 2006, 6, 2. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Song, D.; Moon, H.; Kang, B. Porcine epidemic diarrhea: A review of current epidemiology and available vaccines. Clin. Exp. Vaccine Res. 2015, 4, 166–176. [Google Scholar] [CrossRef] [PubMed]
- Chirdo, F.G.; Millington, O.R.; Beacock-Sharp, H.; Mowat, A.M. Immunomodulatory dendritic cells in intestinal lamina propria. Eur. J. Immunol. 2005, 35, 1831–1840. [Google Scholar] [CrossRef] [PubMed]
- Mowat, A.M. Anatomical basis of tolerance and immunity to intestinal antigens. Nat. Rev. Immunol. 2003, 3, 331–341. [Google Scholar] [CrossRef] [PubMed]
- Wilson, N.S.; El-Sukkari, D.; Belz, G.T.; Smith, C.M.; Steptoe, R.J.; Heath, W.R.; Shortman, K.; Villadangos, J.A. Most lymphoid organ dendritic cell types are phenotypically and functionally immature. Blood 2003, 102, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Pouwels, P.H.; Leer, R.J.; Boersma, W.J. The potential of lactobacillus as a carrier for oral immunization: Development and preliminary characterization of vector systems for targeted delivery of antigens. J. Biotechnol. 1996, 44, 183–192. [Google Scholar] [CrossRef]
- Tsai, Y.T.; Cheng, P.C.; Pan, T.M. The immunomodulatory effects of lactic acid bacteria for improving immune functions and benefits. Appl. Microbiol. Biotechnol. 2012, 96, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Wang, L.; Huang, X.; Ma, S.; Yu, M.; Shi, W.; Qiao, X.; Tang, L.; Xu, Y.; Li, Y. Oral delivery of probiotics expressing dendritic cell-targeting peptide fused with porcine epidemic diarrhea virus coe antigen: A promising vaccine strategy against pedv. Viruses 2017, 9, 312. [Google Scholar] [CrossRef] [PubMed]
- Jinghui, F.; Yijing, L. Cloning and sequence analysis of the m gene of porcine epidemic diarrhea virus ljb/03. Virus Genes 2005, 30, 69–73. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Hou, X.; Tang, L.; Jiang, Y.; Ma, G.; Li, Y. A phase trial of the oral lactobacillus casei vaccine polarizes th2 cell immunity against transmissible gastroenteritis coronavirus infection. Appl. Microbiol. Biotechnol. 2016, 100, 7457–7469. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Shi, N.; Suo, S.; Cui, J.; Zarlenga, D.; Ren, X. Vaccination of mice with orf5 plasmid DNA of prrsv; enhanced effects by co-immunizing with porcine il-15. Immunol. Investig. 2012, 41, 231–248. [Google Scholar] [CrossRef] [PubMed]
- Arce, C.; Ramirez-Boo, M.; Lucena, C.; Garrido, J.J. Innate immune activation of swine intestinal epithelial cell lines (ipec-j2 and ipi-2i) in response to lps from salmonella typhimurium. Comp. Immunol. Microbiol. Infect. Dis. 2010, 33, 161–174. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Wang, A.; Zeng, X.; Hou, C.; Liu, H.; Qiao, S. Lactobacillus reuteri i5007 modulates tight junction protein expression in ipec-j2 cells with lps stimulation and in newborn piglets under normal conditions. BMC Microbiol. 2015, 15, 32. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Zhang, T.; Song, D.; Huang, T.; Peng, Q.; Chen, Y.; Li, A.; Zhang, F.; Wu, Q.; Ye, Y.; et al. Comparison and evaluation of conventional rt-pcr, sybr green i and taqman real-time rt-pcr assays for the detection of porcine epidemic diarrhea virus. Mol. Cell. Probes 2017, 33, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.M.; Annamalai, T.; Liu, X.; Gao, X.; Lu, Z.; El-Tholoth, M.; Hu, H.; Saif, L.J.; Wang, Q. Experimental infection of a us spike-insertion deletion porcine epidemic diarrhea virus in conventional nursing piglets and cross-protection to the original us pedv infection. Vet. Res. 2015, 46, 134. [Google Scholar] [CrossRef] [PubMed]
- Ducatelle, R.; Coussement, W.; Debouck, P.; Hoorens, J. Pathology of experimental cv777 coronavirus enteritis in piglets. Ii. Electron microscopic study. Vet. Pathol. 1982, 19, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Madson, D.M.; Magstadt, D.R.; Arruda, P.H.; Hoang, H.; Sun, D.; Bower, L.P.; Bhandari, M.; Burrough, E.R.; Gauger, P.C.; Pillatzki, A.E.; et al. Pathogenesis of porcine epidemic diarrhea virus isolate (us/iowa/18984/2013) in 3-week-old weaned pigs. Vet. Microbiol. 2014, 174, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Lee, C.Y.; Kim, S.J.; Han, J.H.; Choi, K.H. Medicinal herb extracts ameliorate impaired growth performance and intestinal lesion of newborn piglets challenged with the virulent porcine epidemic diarrhea virus. J. Anim. Sci. Technol. 2015, 57, 33. [Google Scholar] [CrossRef] [PubMed]
- Hirahara, K.; Nakayama, T. CD4+ T-cell subsets in inflammatory diseases: Beyond the th1/th2 paradigm. Int. Immunol. 2016, 28, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [PubMed]
- Meng, F.; Ren, Y.; Suo, S.; Sun, X.; Li, X.; Li, P.; Yang, W.; Li, G.; Li, L.; Schwegmann-Wessels, C.; et al. Evaluation on the efficacy and immunogenicity of recombinant DNA plasmids expressing spike genes from porcine transmissible gastroenteritis virus and porcine epidemic diarrhea virus. PLoS ONE 2013, 8, e57468. [Google Scholar] [CrossRef] [PubMed]
- Annamalai, T.; Saif, L.J.; Lu, Z.; Jung, K. Age-dependent variation in innate immune responses to porcine epidemic diarrhea virus infection in suckling versus weaned pigs. Vet. Immunol. Immunopathol. 2015, 168, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, G.; Wang, J.; Man, K.; Yang, Q. Cell attenuated porcine epidemic diarrhea virus strain zhejiang08 provides effective immune protection attributed to dendritic cell stimulation. Vaccine 2017, 35, 7033–7041. [Google Scholar] [CrossRef] [PubMed]
- Belardelli, F. Role of interferons and other cytokines in the regulation of the immune response. APMIS 1995, 103, 161–179. [Google Scholar] [CrossRef] [PubMed]
- Ramshaw, I.A.; Ramsay, A.J.; Karupiah, G.; Rolph, M.S.; Mahalingam, S.; Ruby, J.C. Cytokines and immunity to viral infections. Immunol. Rev. 1997, 159, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Woo, S.R.; Corrales, L.; Gajewski, T.F. Innate immune recognition of cancer. Annu. Rev. Immunol. 2015, 33, 445–474. [Google Scholar] [CrossRef] [PubMed]
- Uenishi, H.; Shinkai, H. Porcine toll-like receptors: The front line of pathogen monitoring and possible implications for disease resistance. Dev. Comp. Immunol. 2009, 33, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Thada, S.; Valluri, V.L.; Gaddam, S.L. Influence of toll-like receptor gene polymorphisms to tuberculosis susceptibility in humans. Scand. J. Immunol. 2013, 78, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Kaczanowska, S.; Joseph, A.M.; Davila, E. Tlr agonists: Our best frenemy in cancer immunotherapy. J. Leukoc. Biol. 2013, 93, 847–863. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.H.; Taylor, D.K.; Turka, L.A. The contribution of direct tlr signaling to t cell responses. Immunol. Res. 2009, 45, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, A.; Osorio, N.S.; Saraiva, M.; Cunha, C.; Almeida, A.J.; Teixeira-Coelho, M.; Ludovico, P.; Pedrosa, J.; Pitzurra, L.; Aversa, F.; et al. The c allele of rs5743836 polymorphism in the human tlr9 promoter links il-6 and tlr9 up-regulation and confers increased b-cell proliferation. PLoS ONE 2011, 6, e28256. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Roulis, M.; Neurath, M.F.; Kollias, G.; Becker, C. Pleiotropic functions of tnf-alpha in the regulation of the intestinal epithelial response to inflammation. Int. Immunol. 2014, 26, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Wahl, S.M.; Hunt, D.A.; Wakefield, L.M.; McCartney-Francis, N.; Wahl, L.M.; Roberts, A.B.; Sporn, M.B. Transforming growth factor type beta induces monocyte chemotaxis and growth factor production. Proc. Natl. Acad. Sci. USA 1987, 84, 5788–5792. [Google Scholar] [CrossRef] [PubMed]
- Maspi, N.; Abdoli, A.; Ghaffarifar, F. Pro- and anti-inflammatory cytokines in cutaneous leishmaniasis: A review. Pathog. Glob. Health 2016, 110, 247–260. [Google Scholar] [CrossRef] [PubMed]
| Gene | Sequence (5′-3′) Forward | Sequence (5′-3′) Reverse | Accession No. a |
|---|---|---|---|
| TLR4 | CTCTGCCTTCACTACAGAGA | CTGAGTCGTCTCCAGAAGAT | AB078418 |
| TLR9 | GTGGAACTGTTTTGGCATC | CACAGCACTCTGAGCTTTGT | AB071394 |
| TGF-β | CACGTGGAGCTATACCAGAA | TCCGGTGACATCAAAGGACA | Y00111 |
| TNF-α | ATTCAGGGATGTGTGGCCTG | CCAGATGTCCCAGGTTGCAT | JF831365.1 |
| β-actin | CATCACCATCGGCAACGA | GCGTAGAGGTCCTTCCTGATGT | U07786 |
| PEDV-M | GGTTCTATTCCCGTTGATGAGGT | AACACAAGAGGCCAAAGTATCCAT | AF353511.1 |
| Group | Mean Weight Gain after Vaccination (kg) | Mean Weight Gain after Infection (kg) |
|---|---|---|
| PBS | 1.39 ± 0.13 | −0.65 ± 0.08 |
| pPG-COE-DCpep/L393 | 1.91 ± 0.27 * | −0.18 ± 0.12 ** |
| pPG/L393 | 1.77 ± 0.14 * | −0.43±0.17 * |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hou, X.; Jiang, X.; Jiang, Y.; Tang, L.; Xu, Y.; Qiao, X.; Liu, M.; Cui, W.; Ma, G.; Li, Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses 2018, 10, 106. https://doi.org/10.3390/v10030106
Hou X, Jiang X, Jiang Y, Tang L, Xu Y, Qiao X, Liu M, Cui W, Ma G, Li Y. Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses. 2018; 10(3):106. https://doi.org/10.3390/v10030106
Chicago/Turabian StyleHou, Xingyu, Xinpeng Jiang, Yanping Jiang, Lijie Tang, Yigang Xu, Xinyuan Qiao, Min Liu, Wen Cui, Guangpeng Ma, and Yijing Li. 2018. "Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets" Viruses 10, no. 3: 106. https://doi.org/10.3390/v10030106
APA StyleHou, X., Jiang, X., Jiang, Y., Tang, L., Xu, Y., Qiao, X., Liu, M., Cui, W., Ma, G., & Li, Y. (2018). Oral Immunization against PEDV with Recombinant Lactobacillus casei Expressing Dendritic Cell-Targeting Peptide Fusing COE Protein of PEDV in Piglets. Viruses, 10(3), 106. https://doi.org/10.3390/v10030106





