Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection
Abstract
1. Clinical Manifestations of Dengue Virus Infection
2. Immunopathogenesis of DHF/DSS
3. Pathophysiology of Vascular Permeability
4. Characteristics of Plasma Leakage in DHF/DSS
5. Endothelial Cells Involved in Plasma Leakage during Dengue Virus Infection
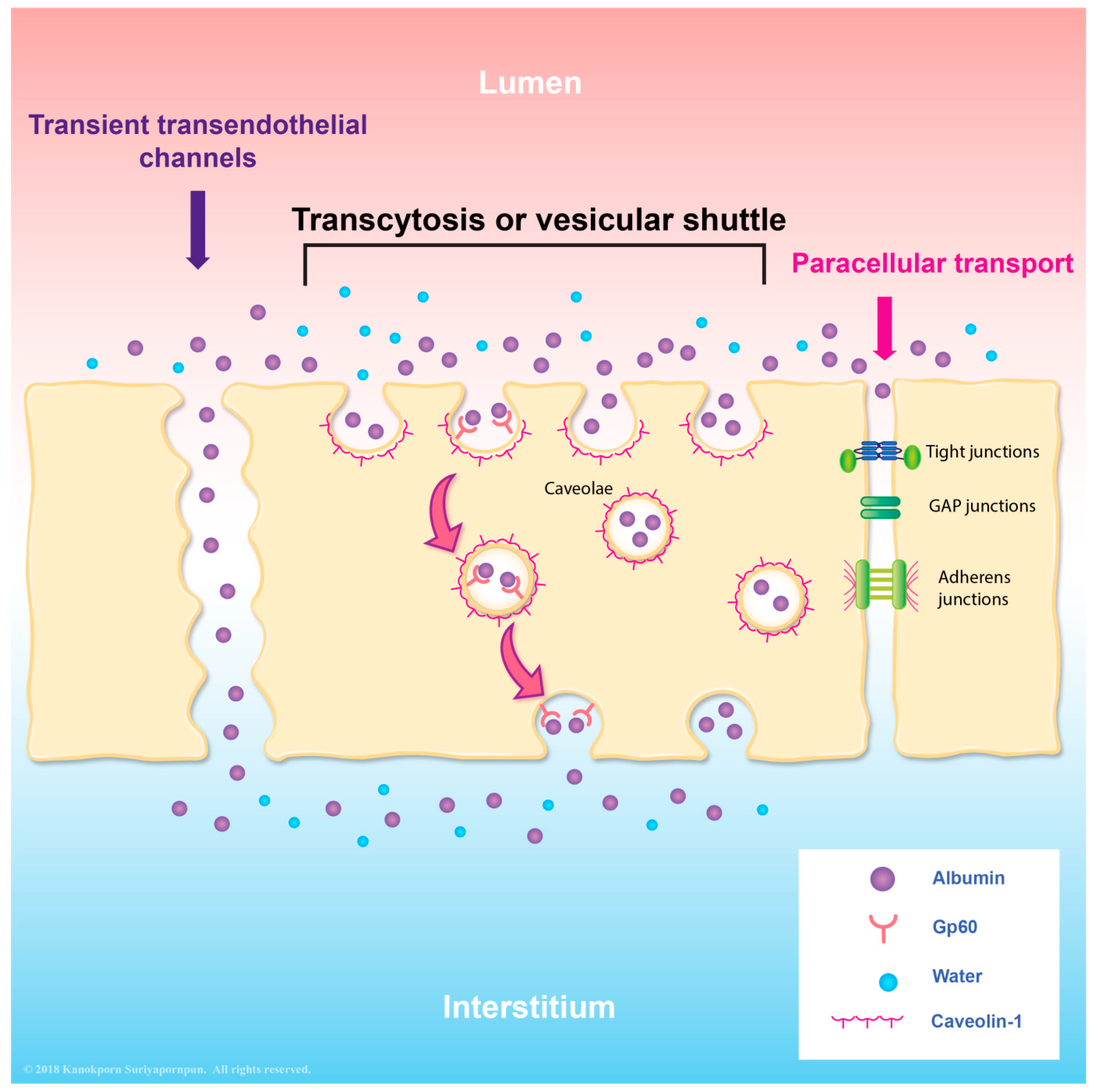
6. Transcytosis as an Alternative Transport System in Endothelial Cells
7. Conclusions
Acknowledgments
Conflicts of Interest
References
- Srikiatkhachorn, A.; Green, S. Markers of dengue disease severity. Curr. Top. Microbiol. Immunol. 2010, 338, 67–82. [Google Scholar] [PubMed]
- Gurugama, P.; Garg, P.; Perera, J.; Wijewickrama, A.; Seneviratne, S.L. Dengue viral infections. Indian J. Dermatol. 2010, 55, 68–78. [Google Scholar] [PubMed]
- Srikiatkhachorn, A.; Gibbons, R.V.; Green, S.; Libraty, D.H.; Thomas, S.J.; Endy, T.P.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; Rothman, A.L.; et al. Dengue hemorrhagic fever: The sensitivity and specificity of the world health organization definition for identification of severe cases of dengue in Thailand, 1994–2005. Clin. Infect. Dis. 2010, 50, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A. Plasma leakage in dengue haemorrhagic fever. Thromb. Haemost. 2009, 102, 1042–1049. [Google Scholar] [CrossRef] [PubMed]
- Okanurak, K.; Sornmani, S.; Indaratna, K. The cost of dengue hemorrhagic fever in Thailand. Southeast Asian J. Trop. Med. Public Health 1997, 28, 711–717. [Google Scholar] [PubMed]
- Basuki, P.S.; Puspitasari, D.; Husada, D.; Darmowandowo, W.; Soegijanto, S.; Yamanaka, A. Application of revised dengue classification criteria as a severity marker of dengue viral infection in Indonesia. Southeast Asian J. Trop. Med. Public Health 2010, 41, 1088–1094. [Google Scholar] [PubMed]
- Mathew, A.; Rothman, A.L. Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol. Rev. 2008, 225, 300–313. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Pathogenesis of dengue: Challenges to molecular biology. Science 1988, 239, 476–481. [Google Scholar] [CrossRef] [PubMed]
- Green, S.; Rothman, A. Immunopathological mechanisms in dengue and dengue hemorrhagic fever. Curr. Opin. Infect. Dis. 2006, 19, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.G. The relationship of interacting immunological components in dengue pathogenesis. Virol. J. 2009, 6, 211. [Google Scholar] [CrossRef] [PubMed]
- Kliks, S.C.; Nisalak, A.; Brandt, W.E.; Wahl, L.; Burke, D.S. Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 1989, 40, 444–451. [Google Scholar] [CrossRef] [PubMed]
- Vaughn, D.W.; Green, S.; Kalayanarooj, S.; Innis, B.L.; Nimmannitya, S.; Suntayakorn, S.; Endy, T.P.; Raengsakulrach, B.; Rothman, A.L.; Ennis, F.A.; et al. Dengue viremia titer, antibody response pattern, and virus serotype correlate with disease severity. J. Infect. Dis. 2000, 181, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.K.; Chao, D.Y.; Kao, C.L.; Wu, H.C.; Liu, Y.C.; Li, C.M.; Lin, S.C.; Ho, S.T.; Huang, J.H.; King, C.C. High levels of plasma dengue viral load during defervescence in patients with dengue hemorrhagic fever: Implications for pathogenesis. Virology 2003, 305, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Libraty, D.H.; Endy, T.P.; Houng, H.S.; Green, S.; Kalayanarooj, S.; Suntayakorn, S.; Chansiriwongs, W.; Vaughn, D.W.; Nisalak, A.; Ennis, F.A.; et al. Differing influences of virus burden and immune activation on disease severity in secondary dengue-3 virus infections. J. Infect. Dis. 2002, 185, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Halstead, S.B.; Artsob, H.; Buchy, P.; Farrar, J.; Gubler, D.J.; Hunsperger, E.; Kroeger, A.; Margolis, H.S.; Martinez, E.; et al. Dengue: A continuing global threat. Nat. Rev. Microbiol. 2010, 8 (Suppl. S12), S7–S16. [Google Scholar] [CrossRef] [PubMed]
- Mongkolsapaya, J.; Dejnirattisai, W.; Xu, X.N.; Vasanawathana, S.; Tangthawornchaikul, N.; Chairunsri, A.; Sawasdivorn, S.; Duangchinda, T.; Dong, T.; Rowland-Jones, S.; et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat. Med. 2003, 9, 921–927. [Google Scholar] [CrossRef] [PubMed]
- Kurane, I. Dengue hemorrhagic fever with special emphasis on immunopathogenesis. Comp. Immunol. Microbiol. Infect. Dis. 2007, 30, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Chaturvedi, U.C.; Agarwal, R.; Elbishbishi, E.A.; Mustafa, A.S. Cytokine cascade in dengue hemorrhagic fever: Implications for pathogenesis. FEMS Immunol. Med. Microbiol. 2000, 28, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Suharti, C.; van Gorp, E.C.; Setiati, T.E.; Dolmans, W.M.; Djokomoeljanto, R.J.; Hack, C.E.; Ten, C.H.; van der Meer, J.W. The role of cytokines in activation of coagulation and fibrinolysis in dengue shock syndrome. Thromb. Haemost. 2002, 87, 42–46. [Google Scholar] [PubMed]
- Avirutnan, P.; Malasit, P.; Seliger, B.; Bhakdi, S.; Husmann, M. Dengue virus infection of human endothelial cells leads to chemokine production, complement activation, and apoptosis. J. Immunol. 1998, 161, 6338–6346. [Google Scholar] [PubMed]
- Avirutnan, P.; Mehlhop, E.; Diamond, M.S. Complement and its role in protection and pathogenesis of flavivirus infections. Vaccine 2008, 26, I100–I107. [Google Scholar] [CrossRef] [PubMed]
- Auksornkitti, V.; Pongsiri, P.; Theamboonlers, A.; Rianthavorn, P.; Poovorawan, Y.; Manujum, K.; Luplertlop, N. Whole-genome characterisation of Chikungunya virus from Aedes albopictus collected in Thailand. Ann. Trop. Med. Parasitol. 2010, 104, 265–269. [Google Scholar] [CrossRef] [PubMed]
- Martina, B.E.; Koraka, P.; Osterhaus, A.D. Dengue virus pathogenesis: An integrated view. Clin. Microbiol. Rev. 2009, 22, 564–581. [Google Scholar] [CrossRef] [PubMed]
- Guzman, M.G.; Kouri, G.P.; Bravo, J.; Soler, M.; Vazquez, S.; Morier, L. Dengue hemorrhagic fever in Cuba, 1981: A retrospective seroepidemiologic study. Am. J. Trop. Med. Hyg. 1990, 42, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Rico-Hesse, R.; Harrison, L.M.; Salas, R.A.; Tovar, D.; Nisalak, A.; Ramos, C.; Boshell, J.; de Mesa, M.T.; Nogueira, R.M.; da Rosa, A.T. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 1997, 230, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Watts, D.M.; Porter, K.R.; Putvatana, P.; Vasquez, B.; Calampa, C.; Hayes, C.G.; Halstead, S.B. Failure of secondary infection with American genotype dengue 2 to cause dengue haemorrhagic fever. Lancet 1999, 354, 1431–1434. [Google Scholar] [CrossRef]
- Mehta, D.; Malik, A.B. Signaling mechanisms regulating endothelial permeability. Physiol. Rev. 2006, 86, 279–367. [Google Scholar] [CrossRef] [PubMed]
- Simionescu, M.; Antohe, F. Functional ultrastructure of the vascular endothelium: Changes in various pathologies. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 41–69. [Google Scholar]
- Park-Windhol, C.; D'Amore, P.A. Disorders of vascular permeability. Ann. Rev. Pathol. 2016, 11, 251–281. [Google Scholar] [CrossRef] [PubMed]
- Nagy, J.A.; Benjamin, L.; Zeng, H.; Dvorak, A.M.; Dvorak, H.F. Vascular permeability, vascular hyperpermeability and angiogenesis. Angiogenesis 2008, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Mehta, D.; Bhattacharya, J.; Matthay, M.A.; Malik, A.B. Integrated control of lung fluid balance. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L1081–L1090. [Google Scholar] [CrossRef] [PubMed]
- Minshall, R.D.; Malik, A.B. Transport across the endothelium: Regulation of endothelial permeability. Handb. Exp. Pharmacol. 2006, 176 Pt 1, 107–144. [Google Scholar]
- Simionescu, M.; Gafencu, A.; Antohe, F. Transcytosis of plasma macromolecules in endothelial cells: A cell biological survey. Microsc. Res. Tech. 2002, 57, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Predescu, S.A.; Predescu, D.N.; Malik, A.B. Molecular determinants of endothelial transcytosis and their role in endothelial permeability. Am. J. Physiol. Lung Cell. Mol. Physiol. 2007, 293, L823–L842. [Google Scholar] [CrossRef] [PubMed]
- Minshall, R.D.; Tiruppathi, C.; Vogel, S.M.; Malik, A.B. Vesicle formation and trafficking in endothelial cells and regulation of endothelial barrier function. Histochem. Cell Biol. 2002, 117, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Predescu, D.; Vogel, S.M.; Malik, A.B. Functional and morphological studies of protein transcytosis in continuous endothelia. Am. J. Physiol. Lung Cell. Mol. Physiol. 2004, 287, L895–L901. [Google Scholar] [CrossRef] [PubMed]
- Throop, J.L.; Kerl, M.E.; Cohn, L.A. Albumen in health and disease: Protein metabolism and function. Compend. Contin. Educ. Pract. Vet. 2004, 26, 932. [Google Scholar]
- Hankins, J. The role of albumin in fluid and electrolyte balance. J. Infus. Nurs. 2006, 29, 260–265. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Minshall, R.D. Regulation of transendothelial permeability by Src kinase. Microvasc. Res. 2009, 77, 21–25. [Google Scholar] [CrossRef] [PubMed]
- Schnitzer, J.E.; Oh, P. Albondin-mediated capillary permeability to albumin. Differential role of receptors in endothelial transcytosis and endocytosis of native and modified albumins. J. Biol. Chem. 1994, 269, 6072–6082. [Google Scholar] [PubMed]
- Tiruppathi, C.; Finnegan, A.; Malik, A.B. Isolation and characterization of a cell surface albumin-binding protein from vascular endothelial cells. Proc. Natl. Acad. Sci. USA 1996, 93, 250–254. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Vogel, S.M.; Schwartz, D.E.; Malik, A.B.; Minshall, R.D. Intercellular adhesion molecule-1-dependent neutrophil adhesion to endothelial cells induces caveolae-mediated pulmonary vascular hyperpermeability. Circ. Res. 2008, 102, e120–e131. [Google Scholar] [CrossRef] [PubMed]
- Ono, S.; Egawa, G.; Kabashima, K. Regulation of blood vascular permeability in the skin. Inflamm. Regen. 2017, 37, 11. [Google Scholar] [CrossRef] [PubMed]
- Rigor, R.R.; Shen, Q.; Pivetti, C.D.; Wu, M.H.; Yuan, S.Y. Myosin light chain kinase signaling in endothelial barrier dysfunction. Med. Res. Rev. 2013, 33, 911–933. [Google Scholar] [CrossRef] [PubMed]
- Komarova, Y.; Malik, A.B. Regulation of endothelial permeability via paracellular and transcellular transport pathways. Annu. Rev. Physiol. 2010, 72, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L. Vascular permeability—The essentials. Upsala J. Med. Sci. 2015, 120, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Di, A.; Mehta, D.; Malik, A.B. ROS-activated calcium signaling mechanisms regulating endothelial barrier function. Cell Calcium 2016, 60, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Komarova, Y.A.; Kruse, K.; Mehta, D.; Malik, A.B. Protein interactions at endothelial junctions and signaling mechanisms regulating endothelial permeability. Circ. Res. 2017, 120, 179–206. [Google Scholar] [CrossRef] [PubMed]
- Srikiatkhachorn, A.; Krautrachue, A.; Ratanaprakarn, W.; Wongtapradit, L.; Nithipanya, N.; Kalayanarooj, S.; Nisalak, A.; Thomas, S.J.; Gibbons, R.V.; Mammen, M.P., Jr.; et al. Natural history of plasma leakage in dengue hemorrhagic fever: A serial ultrasonographic study. Pediatr. Infect. Dis. J. 2007, 26, 283–290; discussion 291–292. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.C.; Wu, C.C.; Liu, J.W.; Lin, A.S.; Liu, S.F.; Chung, Y.H.; Su, M.C.; Lee, I.K.; Lin, M.C. Chest radiographic presentation in patients with dengue hemorrhagic Fever. Am. J. Trop. Med. Hyg. 2007, 77, 291–296. [Google Scholar] [PubMed]
- Bhamarapravati, N.; Tuchinda, P.; Boonyapaknavik, V. Pathology of Thailand haemorrhagic fever: A study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 1967, 61, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Deen, J.L.; Harris, E.; Wills, B.; Balmaseda, A.; Hammond, S.N.; Rocha, C.; Dung, N.M.; Hung, N.T.; Hien, T.T.; Farrar, J.J. The WHO dengue classification and case definitions: Time for a reassessment. Lancet 2006, 368, 170–173. [Google Scholar] [CrossRef]
- Chuang, Y.C.; Lei, H.Y.; Liu, H.S.; Lin, Y.S.; Fu, T.F.; Yeh, T.M. Macrophage migration inhibitory factor induced by dengue virus infection increases vascular permeability. Cytokine 2011, 54, 222–231. [Google Scholar] [CrossRef] [PubMed]
- Luplertlop, N.; Misse, D.; Bray, D.; Deleuze, V.; Gonzalez, J.P.; Leardkamolkarn, V.; Yssel, H.; Veas, F. Dengue-virus-infected dendritic cells trigger vascular leakage through metalloproteinase overproduction. EMBO Rep. 2006, 7, 1176–1181. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Liu, M.T.; Lei, H.Y.; Liu, C.C.; Wu, J.M.; Tung, Y.C.; Lin, Y.S.; Yeh, T.M.; Chen, S.H.; Liu, H.S. MCP-1, a highly expressed chemokine in dengue haemorrhagic fever/dengue shock syndrome patients, may cause permeability change, possibly through reduced tight junctions of vascular endothelium cells. J. Gen. Virol. 2006, 87 Pt 12, 3623–3630. [Google Scholar] [CrossRef] [PubMed]
- Inyoo, S.; Suttitheptumrong, A.; Pattanakitsakul, S.N. Synergistic Effect of TNF-α and Dengue Virus Infection on Adhesion Molecule Reorganization in Human Endothelial Cells. Jpn. J. Infect. Dis. 2017, 70, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Talavera, D.; Castillo, A.M.; Dominguez, M.C.; Gutierrez, A.E.; Meza, I. IL8 release, tight junction and cytoskeleton dynamic reorganization conducive to permeability increase are induced by dengue virus infection of microvascular endothelial monolayers. J. Gen. Virol. 2004, 85 Pt 7, 1801–1813. [Google Scholar] [CrossRef] [PubMed]
- Luplertlop, N.; Misse, D. MMP cellular responses to dengue virus infection-induced vascular leakage. Jpn. J. Infect. Dis. 2008, 61, 298–301. [Google Scholar] [PubMed]
- Azizan, A.; Fitzpatrick, K.; Signorovitz, A.; Tanner, R.; Hernandez, H.; Stark, L.; Sweat, M. Profile of time-dependent VEGF upregulation in human pulmonary endothelial cells, HPMEC-ST1.6R infected with DENV-1, -2, -3, and -4 viruses. Virol. J. 2009, 6, 49. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Woda, M.; Ennis, F.A.; Libraty, D.H. Dengue virus infection differentially regulates endothelial barrier function over time through type I interferon effects. J. Infect. Dis. 2009, 200, 191–201. [Google Scholar] [CrossRef] [PubMed]
- Dewi, B.E.; Takasaki, T.; Kurane, I. Peripheral blood mononuclear cells increase the permeability of dengue virus-infected endothelial cells in association with downregulation of vascular endothelial cadherin. J. Gen. Virol. 2008, 89 Pt 3, 642–652. [Google Scholar] [CrossRef] [PubMed]
- Myers, G.J.; Wegner, J. Endothelial glycocalyx and cardiopulmonary bypass. J. Extra-Corpor. Technol. 2017, 49, 174–181. [Google Scholar] [PubMed]
- Sieve, I.; Munster-Kuhnel, A.K.; Hilfiker-Kleiner, D. Regulation and function of endothelial glycocalyx layer in vascular diseases. Vasc. Pharmacol. 2018, 100, 26–33. [Google Scholar] [CrossRef] [PubMed]
- Tang, T.H.; Alonso, S.; Ng, L.F.; Thein, T.L.; Pang, V.J.; Leo, Y.S.; Lye, D.C.; Yeo, T.W. Increased serum hyaluronic acid and heparan sulfate in dengue fever: Association with plasma leakage and disease severity. Sci. Rep. 2017, 7, 46191. [Google Scholar] [CrossRef] [PubMed]
- Suwarto, S.; Sasmono, R.T.; Sinto, R.; Ibrahim, E.; Suryamin, M. Association of endothelial glycocalyx and tight and adherens junctions with severity of plasma leakage in dengue infection. J. Infect. Dis. 2017, 215, 992–999. [Google Scholar] [CrossRef] [PubMed]
- Puerta-Guardo, H.; Glasner, D.R.; Harris, E. Dengue virus NS1 disrupts the endothelial glycocalyx, leading to hyperpermeability. PLoS Pathog. 2016, 12, e1005738. [Google Scholar] [CrossRef] [PubMed]
- Glasner, D.R.; Ratnasiri, K.; Puerta-Guardo, H.; Espinosa, D.A.; Beatty, P.R.; Harris, E. Dengue virus NS1 cytokine-independent vascular leak is dependent on endothelial glycocalyx components. PLoS Pathog. 2017, 13, e1006673. [Google Scholar] [CrossRef] [PubMed]
- Kanlaya, R.; Pattanakitsakul, S.N.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Alterations in actin cytoskeletal assembly and junctional protein complexes in human endothelial cells induced by dengue virus infection and mimicry of leukocyte transendothelial migration. J. Proteom. Res. 2009, 8, 2551–2562. [Google Scholar] [CrossRef] [PubMed]
- Pattanakitsakul, S.N.; Poungsawai, J.; Kanlaya, R.; Sinchaikul, S.; Chen, S.T.; Thongboonkerd, V. Association of Alix with late endosomal lysobisphosphatidic acid is important for dengue virus infection in human endothelial cells. J. Proteom. Res. 2010, 9, 4640–4648. [Google Scholar] [CrossRef] [PubMed]
- Bhamarapravati, N. Hemostatic defects in dengue hemorrhagic fever. Rev. Infect. Dis. 1989, 11 (Suppl. S4), S826–S829. [Google Scholar] [CrossRef] [PubMed]
- Limonta, D.; Capo, V.; Torres, G.; Perez, A.B.; Guzman, M.G. Apoptosis in tissues from fatal dengue shock syndrome. J. Clin. Virol. 2007, 40, 50–54. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Place, A.T.; Minshall, R.D. Regulation of endothelial permeability by Src kinase signaling: Vascular leakage versus transcellular transport of drugs and macromolecules. Chem. Biol. Interact. 2008, 171, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.P.; Park, S.I.; Kopetz, S.; Gallick, G.E. Src family kinases as mediators of endothelial permeability: Effects on inflammation and metastasis. Cell Tissue Res. 2009, 335, 249–259. [Google Scholar] [CrossRef] [PubMed]
- Shajahan, A.N.; Timblin, B.K.; Sandoval, R.; Tiruppathi, C.; Malik, A.B.; Minshall, R.D. Role of Src-induced dynamin-2 phosphorylation in caveolae-mediated endocytosis in endothelial cells. J. Biol. Chem. 2004, 279, 20392–20400. [Google Scholar] [CrossRef] [PubMed]
- Tiruppathi, C.; Song, W.; Bergenfeldt, M.; Sass, P.; Malik, A.B. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J. Biol. Chem. 1997, 272, 25968–25975. [Google Scholar] [CrossRef] [PubMed]
- Minshall, R.D.; Tiruppathi, C.; Vogel, S.M.; Niles, W.D.; Gilchrist, A.; Hamm, H.E.; Malik, A.B. Endothelial cell-surface gp60 activates vesicle formation and trafficking via Gi-coupled Src kinase signaling pathway. J. Cell Biol. 2000, 150, 1057–1070. [Google Scholar] [CrossRef] [PubMed]
- Zimnicka, A.M.; Husain, Y.S.; Shajahan, A.N.; Sverdlov, M.; Chaga, O.; Chen, Z.; Toth, P.T.; Klomp, J.; Karginov, A.V.; Tiruppathi, C.; et al. Src-dependent phosphorylation of caveolin-1 Tyr-14 promotes swelling and release of caveolae. Mol. Biol. Cell 2016, 27, 2090–2106. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Hu, G.; Zhang, X.; Minshall, R.D. Phosphorylation of caveolin-1 regulates oxidant-induced pulmonary vascular permeability via paracellular and transcellular pathways. Circ. Res. 2009, 105, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Hu, G.; Schwartz, D.E.; Shajahan, A.N.; Visintine, D.J.; Salem, M.R.; Crystal, G.J.; Albrecht, R.F.; Vogel, S.M.; Minshall, R.D. Isoflurane, but not sevoflurane, increases transendothelial albumin permeability in the isolated rat lung: Role for enhanced phosphorylation of caveolin-1. Anesthesiology 2006, 104, 777–785. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Gassner, B.; Borner, S.; Nikolaev, V.O.; Schlegel, N.; Waschke, J.; Steinbronn, N.; Strasser, R.; Kuhn, M. Atrial natriuretic peptide enhances microvascular albumin permeability by the caveolae-mediated transcellular pathway. Cardiovasc. Res. 2012, 93, 141–151. [Google Scholar] [CrossRef] [PubMed]
- Kuebler, W.M.; Wittenberg, C.; Lee, W.L.; Reppien, E.; Goldenberg, N.M.; Lindner, K.; Gao, Y.; Winoto-Morbach, S.; Drab, M.; Muhlfeld, C.; et al. Thrombin stimulates albumin transcytosis in lung microvascular endothelial cells via activation of acid sphingomyelinase. Am. J. Physiol. Lung Cell. Mol. Physiol. 2016, 310, L720–L732. [Google Scholar] [CrossRef] [PubMed]
- Shang, D.; Peng, T.; Gou, S.; Li, Y.; Wu, H.; Wang, C.; Yang, Z. High mobility group box protein 1 boosts endothelial albumin transcytosis through the RAGE/Src/caveolin-1 pathway. Sci. Rep. 2016, 6, 32180. [Google Scholar] [CrossRef] [PubMed]
- Chanthick, C.; Kanlaya, R.; Kiatbumrung, R.; Pattanakitsakul, S.N.; Thongboonkerd, V. Caveolae-mediated albumin transcytosis is enhanced in dengue-infected human endothelial cells: A model of vascular leakage in dengue hemorrhagic fever. Sci. Rep. 2016, 6, 31855. [Google Scholar] [CrossRef] [PubMed]
- Sahaphong, S.; Riengrojpitak, S.; Bhamarapravati, N.; Chirachariyavej, T. Electron microscopic study of the vascular endothelial cell in dengue hemorrhagic fever. Southeast Asian J. Trop. Med. Public Health 1980, 11, 194–204. [Google Scholar] [PubMed]
- Wang, C.C.; Liu, S.F.; Liao, S.C.; Lee, I.K.; Liu, J.W.; Lin, A.S.; Wu, C.C.; Chung, Y.H.; Lin, M.C. Acute respiratory failure in adult patients with dengue virus infection. Am. J. Trop. Med. Hyg. 2007, 77, 151–158. [Google Scholar] [PubMed]
- Chavalittamrong, B.; Pidetcha, P.; Pongpipat, D.; Habanananda, S.; Tuchinda, M. Composition of pleural fluid in dengue hemorrhagic fever. J. Med. Assoc. Thail. 1979, 62, 55–58. [Google Scholar]
- Porcel, J.M.; Light, R.W. Diagnostic approach to pleural effusion in adults. Am. Fam. Phys. 2006, 73, 1211–1220. [Google Scholar]
- Kinasewitz, G.T. Transudative effusions. Eur. Respir. J. 1997, 10, 714–718. [Google Scholar] [PubMed]
- Vaz, M.A.; Vargas, F.S.; Marinho, F.C.; D’Amico, E.A.; Rocha, T.R.; Teixeira, L.R. Does the evaluation of coagulation factors contribute to etiological diagnosis of pleural effusions? Clinics 2009, 64, 891–895. [Google Scholar] [CrossRef] [PubMed]
- Mutsaers, S.E.; Prele, C.M.; Brody, A.R.; Idell, S. Pathogenesis of pleural fibrosis. Respirology 2004, 9, 428–440. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanthick, C.; Suttitheptumrong, A.; Rawarak, N.; Pattanakitsakul, S.-n. Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection. Viruses 2018, 10, 69. https://doi.org/10.3390/v10020069
Chanthick C, Suttitheptumrong A, Rawarak N, Pattanakitsakul S-n. Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection. Viruses. 2018; 10(2):69. https://doi.org/10.3390/v10020069
Chicago/Turabian StyleChanthick, Chanettee, Aroonroong Suttitheptumrong, Nantapon Rawarak, and Sa-nga Pattanakitsakul. 2018. "Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection" Viruses 10, no. 2: 69. https://doi.org/10.3390/v10020069
APA StyleChanthick, C., Suttitheptumrong, A., Rawarak, N., & Pattanakitsakul, S.-n. (2018). Transcytosis Involvement in Transport System and Endothelial Permeability of Vascular Leakage during Dengue Virus Infection. Viruses, 10(2), 69. https://doi.org/10.3390/v10020069



