A Simple Mechanism Based on Amino Acid Substitutions is not a Critical Determinant of High Mortality of Japanese Encephalitis Virus Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Viruses and Cells
2.3. Mice
2.4. Statistical Analyses
3. Results
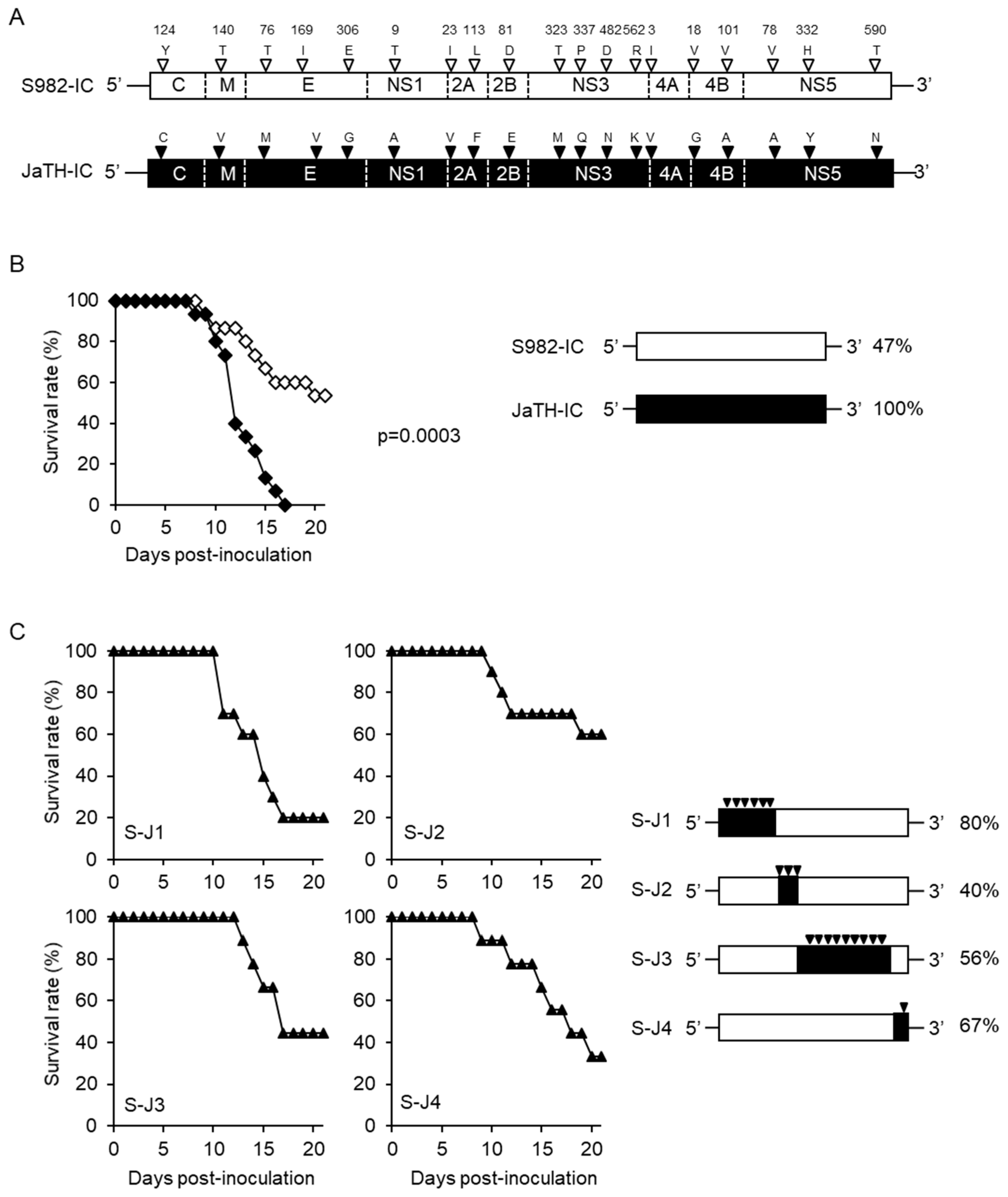
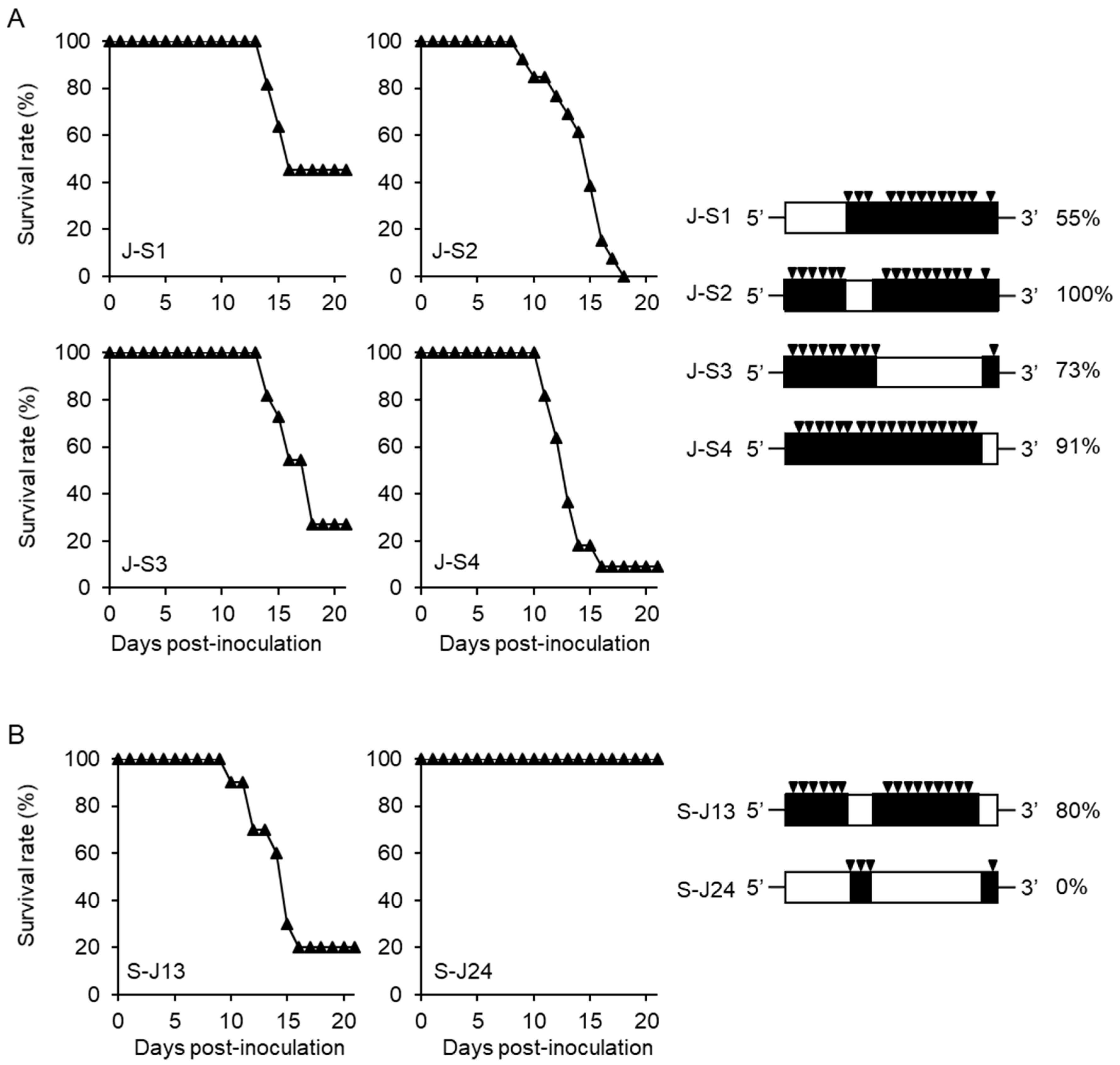
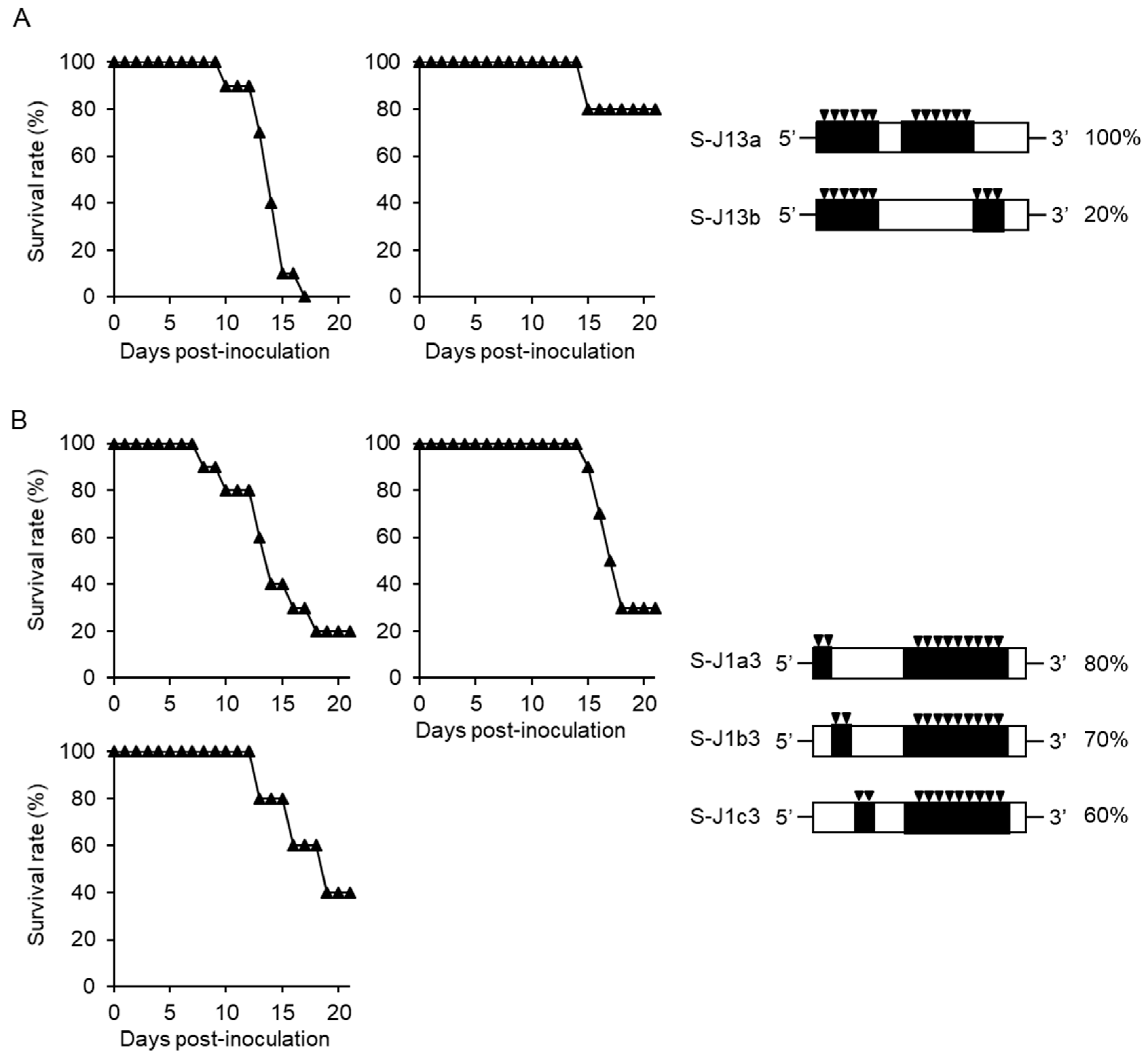
3.1. Separate Regions of Viral Genome Affect the High Mortality in Mice
3.2. Amino Acids C124 and NS3482 of JaTH160 May Affect the High Mortality in Mice
3.3. Simultaneous Substitutions of C124 and NS3482 are Not Enough to Cause High Mortality
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ghosh, D.; Basu, A. Japanese encephalitis—A pathological and clinical perspective. PLoS Negl. Trop. Dis. 2009, 3, e437. [Google Scholar] [CrossRef] [PubMed]
- Solomon, T. Flavivirus encephalitis. N. Engl. J. Med. 2004, 351, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Westaway, E.G.; Brinton, M.A.; Gaidamovich, S.; Horzinek, M.C.; Igarashi, A.; Kaariainen, L.; Lvov, D.K.; Porterfield, J.S.; Russell, P.K.; Trent, D.W. Flaviviridae. Intervirology 1985, 24, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Gubler, D.J.; Kuno, G.; Markoff, L. Field's Virology, 5th ed.; Wolters Kluwer Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2007; pp. 1153–1252. [Google Scholar]
- Sumiyoshi, H.; Hoke, C.H.; Trent, D.W. Infectious Japanese encephalitis virus RNA can be synthesized from in vitro-ligated cDNA templates. J. Virol. 1992, 66, 5425–5431. [Google Scholar] [PubMed]
- Tsai, T.F. New initiatives for the control of Japanese encephalitis by vaccination: Minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 2000, 18 (Suppl. 2), 1–25. [Google Scholar] [CrossRef]
- Misra, U.K.; Kalita, J. Overview: Japanese encephalitis. Prog. Neurobiol. 2010, 91, 108–120. [Google Scholar] [CrossRef] [PubMed]
- Aoki, K.; Shimada, S.; Simantini, D.S.; Tun, M.M.; Buerano, C.C.; Morita, K.; Hayasaka, D. Type-I interferon response affects an inoculation dose-independent mortality in mice following Japanese encephalitis virus infection. Virol. J. 2014, 11, 105. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, D.; Shirai, K.; Aoki, K.; Nagata, N.; Simantini, D.S.; Kitaura, K.; Takamatsu, Y.; Gould, E.; Suzuki, R.; Morita, K. TNF-α acts as an immunoregulator in the mouse brain by reducing the incidence of severe disease following Japanese encephalitis virus infection. PLoS ONE 2013, 8, e71643. [Google Scholar] [CrossRef] [PubMed]
- Fujii, Y.; Kitaura, K.; Nakamichi, K.; Takasaki, T.; Suzuki, R.; Kurane, I. Accumulation of T-cells with selected T-cell receptors in the brains of Japanese encephalitis virus-infected mice. Jpn. J. Infect. Dis. 2008, 61, 40–48. [Google Scholar] [PubMed]
- Lee, E.; Hall, R.A.; Lobigs, M. Common E protein determinants for attenuation of glycosaminoglycan-binding variants of Japanese encephalitis and west nile viruses. J. Virol. 2004, 78, 8271–8280. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Burns, N.J.; Chang, G.J.; Zhang, M.J.; Wills, M.R.; Trent, D.W.; Sanders, P.G.; Barrett, A.D. Comparison of nucleotide and deduced amino acid sequence of the 5′ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J. Gen. Virol. 1994, 75 Pt 6, 1505–1510. [Google Scholar] [CrossRef] [PubMed]
- Tajima, S.; Nerome, R.; Nukui, Y.; Kato, F.; Takasaki, T.; Kurane, I. A single mutation in the Japanese encephalitis virus E protein (S123R) increases its growth rate in mouse neuroblastoma cells and its pathogenicity in mice. Virology 2010, 396, 298–304. [Google Scholar] [CrossRef] [PubMed]
- Ni, H.; Chang, G.J.; Xie, H.; Trent, D.W.; Barrett, A.D. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J. Gen. Virol 1995, 76 Pt 2, 409–413. [Google Scholar] [CrossRef] [PubMed]
- Mori, Y.; Okabayashi, T.; Yamashita, T.; Zhao, Z.; Wakita, T.; Yasui, K.; Hasebe, F.; Tadano, M.; Konishi, E.; Moriishi, K.; et al. Nuclear localization of Japanese encephalitis virus core protein enhances viral replication. J. Virol. 2005, 79, 3448–3458. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.M.; Yun, S.I.; Song, B.H.; Hahn, Y.S.; Lee, C.H.; Oh, H.W.; Lee, Y.M. A single N-linked glycosylation site in the Japanese encephalitis virus prm protein is critical for cell type-specific prm protein biogenesis, virus particle release, and pathogenicity in mice. J. Virol. 2008, 82, 7846–7862. [Google Scholar] [CrossRef] [PubMed]
- Melian, E.B.; Hinzman, E.; Nagasaki, T.; Firth, A.E.; Wills, N.M.; Nouwens, A.S.; Blitvich, B.J.; Leung, J.; Funk, A.; Atkins, J.F.; et al. NS1’ of flaviviruses in the Japanese encephalitis virus serogroup is a product of ribosomal frameshifting and plays a role in viral neuroinvasiveness. J. Virol. 2010, 84, 1641–1647. [Google Scholar] [CrossRef] [PubMed]
- Yoshii, K.; Sunden, Y.; Yokozawa, K.; Igarashi, M.; Kariwa, H.; Holbrook, M.R.; Takashima, I. A critical determinant of neurological disease associated with highly pathogenic tick-borne flavivirus in mice. J. Virol. 2014, 88, 5406–5420. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Okamoto, K.; Dinh, D.T.; Yu, F.; Hayasaka, D.; Uchida, L.; Nabeshima, T.; Buerano, C.C.; Morita, K. NS1’ protein expression facilitates production of Japanese encephalitis virus in avian cells and embryonated chicken eggs. J. Gen. Virol. 2014, 95, 373–383. [Google Scholar] [CrossRef] [PubMed]
- Ye, Q.; Li, X.F.; Zhao, H.; Li, S.H.; Deng, Y.Q.; Cao, R.Y.; Song, K.Y.; Wang, H.J.; Hua, R.H.; Yu, Y.X.; et al. A single nucleotide mutation in NS2A of Japanese encephalitis-live vaccine virus (SA14-14-2) ablates NS1’ formation and contributes to attenuation. J. Gen. Virol. 2012, 93, 1959–1964. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Raekiansyah, M.; Morita, K.; Hayasaka, D. NS1’ protein expression in the JaOArS982 strain of Japanese encephalitis virus does not enhance virulence in mice. Trop. Med. Health 2015, 43, 233–237. [Google Scholar] [CrossRef] [PubMed]
- Takamatsu, Y.; Morita, K.; Hayasaka, D. A single amino acid substitution in the NS2A protein of Japanese encephalitis virus affects virus propagation in vitro but not in vivo. J. Virol. 2015, 89, 6126–6130. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, D.; Gritsun, T.S.; Yoshii, K.; Ueki, T.; Goto, A.; Mizutani, T.; Kariwa, H.; Iwasaki, T.; Gould, E.A.; Takashima, I. Amino acid changes responsible for attenuation of virus neurovirulence in an infectious cDNA clone of the oshima strain of tick-borne encephalitis virus. J. Gen. Virol. 2004, 85, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Hayasaka, D.; Nagata, N.; Fujii, Y.; Hasegawa, H.; Sata, T.; Suzuki, R.; Gould, E.A.; Takashima, I.; Koike, S. Mortality following peripheral infection with tick-borne encephalitis virus results from a combination of central nervous system pathology, systemic inflammatory and stress responses. Virology 2009, 390, 139–150. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takamatsu, Y.; Uchida, L.; Raekiansyah, M.; Luz, M.A.; Morita, K.; Hayasaka, D. A Simple Mechanism Based on Amino Acid Substitutions is not a Critical Determinant of High Mortality of Japanese Encephalitis Virus Infection in Mice. Viruses 2018, 10, 62. https://doi.org/10.3390/v10020062
Takamatsu Y, Uchida L, Raekiansyah M, Luz MA, Morita K, Hayasaka D. A Simple Mechanism Based on Amino Acid Substitutions is not a Critical Determinant of High Mortality of Japanese Encephalitis Virus Infection in Mice. Viruses. 2018; 10(2):62. https://doi.org/10.3390/v10020062
Chicago/Turabian StyleTakamatsu, Yuki, Leo Uchida, Muhareva Raekiansyah, Mark Anthony Luz, Kouichi Morita, and Daisuke Hayasaka. 2018. "A Simple Mechanism Based on Amino Acid Substitutions is not a Critical Determinant of High Mortality of Japanese Encephalitis Virus Infection in Mice" Viruses 10, no. 2: 62. https://doi.org/10.3390/v10020062
APA StyleTakamatsu, Y., Uchida, L., Raekiansyah, M., Luz, M. A., Morita, K., & Hayasaka, D. (2018). A Simple Mechanism Based on Amino Acid Substitutions is not a Critical Determinant of High Mortality of Japanese Encephalitis Virus Infection in Mice. Viruses, 10(2), 62. https://doi.org/10.3390/v10020062




