Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy
Abstract
1. Introduction
2. Materials and Methods
2.1. Production and Purification of BuV1, BuV2, and BuV3 Virus-Like Particles
2.2. VLP Sample Purity and Integrity
2.3. Stability of BuV VLPs
2.4. Cryo-Electron Microscopy Data Collection and Image Reconstruction
2.5. Model Building and Structure Refinement
2.6. Structure Comparison among the BuV Serotypes
2.7. Comparison of BuV1 to Other Parvovirus Capsid Structures at the Genus and Subfamily Levels
2.8. Structure Accession Numbers
3. Results and Discussion
3.1. Purified BuV VLPs Are Suitable for Atomic Resolution Structure Determination
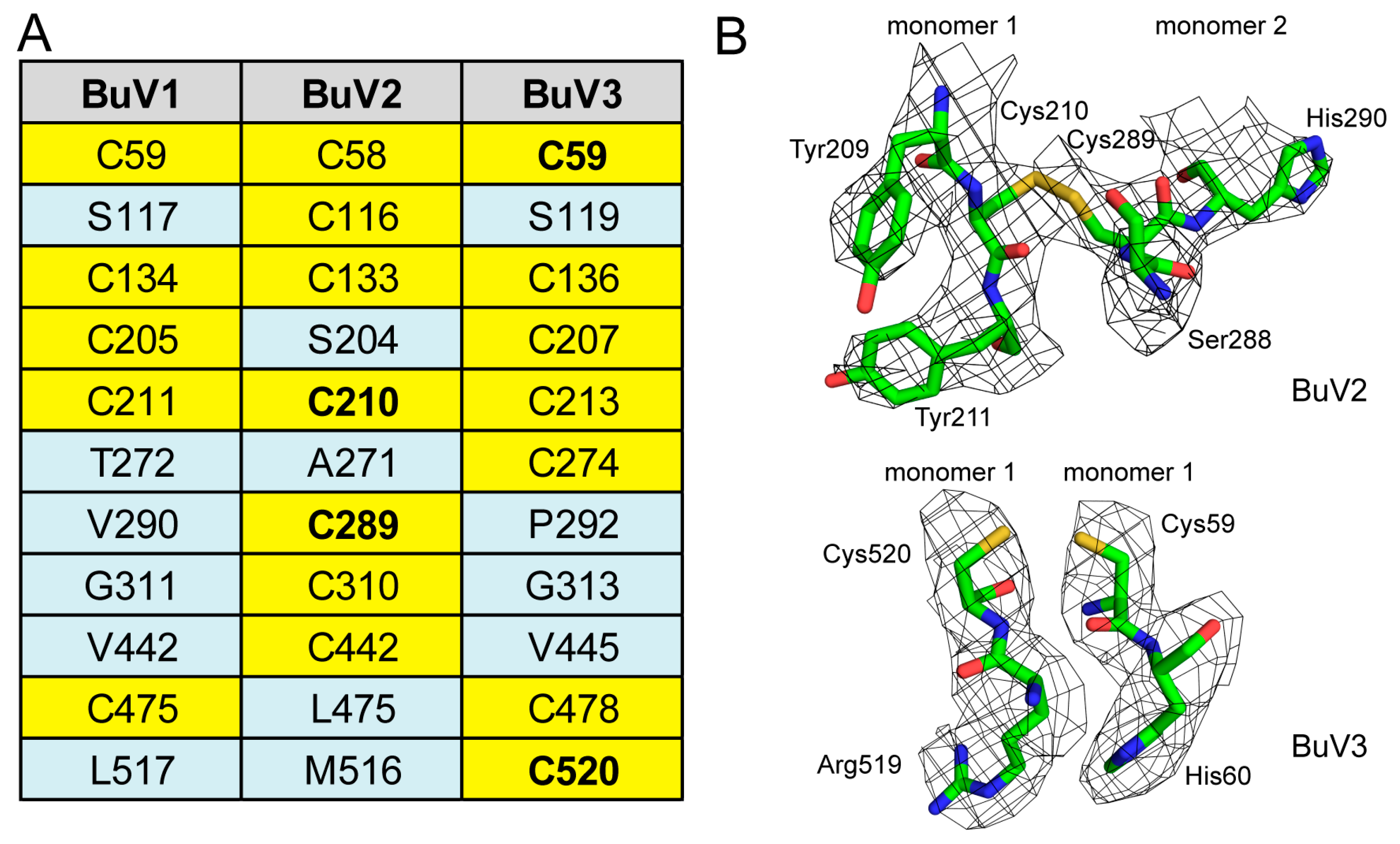
3.2. A Unique Disulfide Bond in BuV2 May Confer Increased Capsid Stability
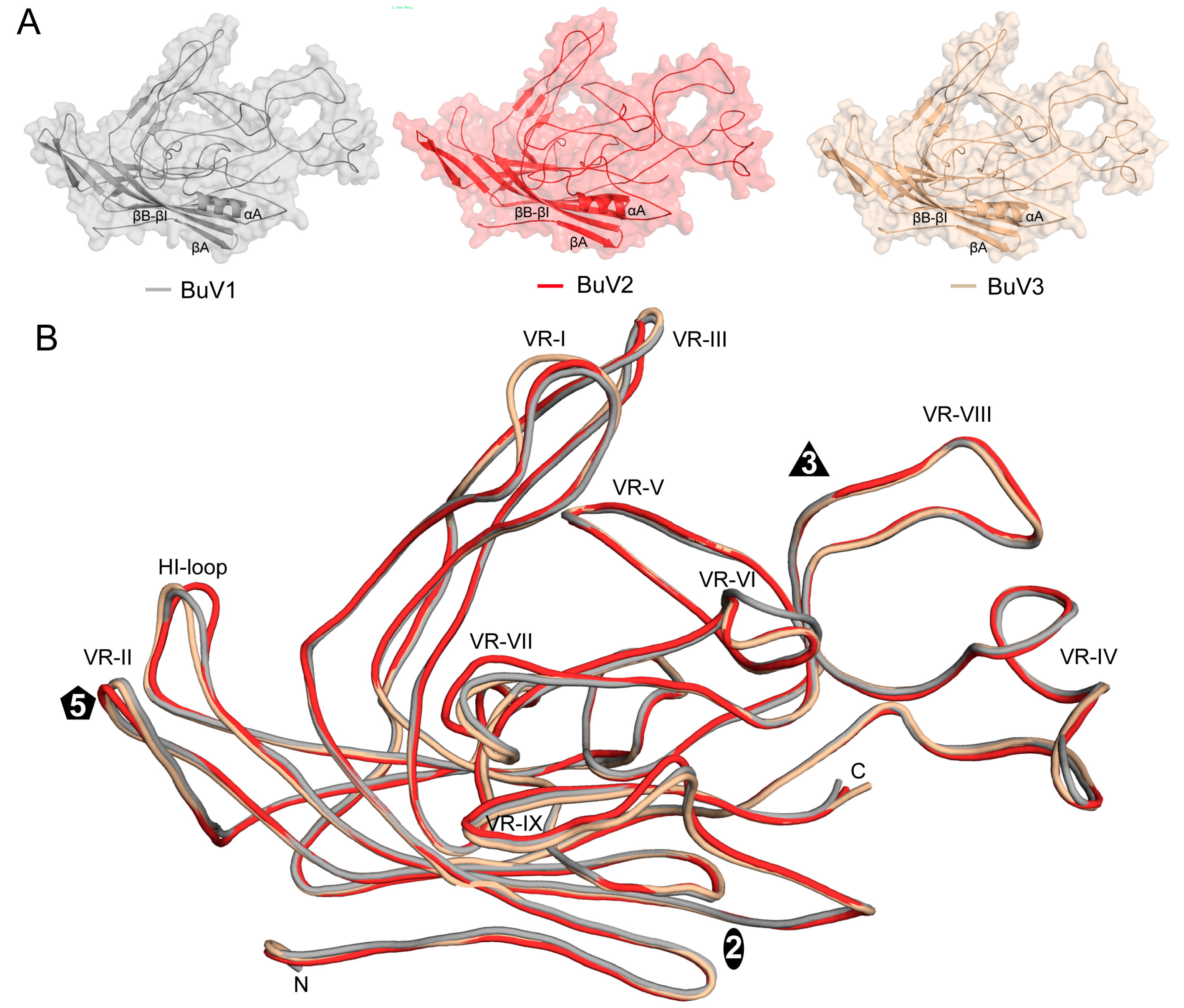
3.3. BuV1 VP2 and Capsids Conserve Protoparvovirus Features Despite Low Sequence Identity But Have a Unique 3-Fold Morphology
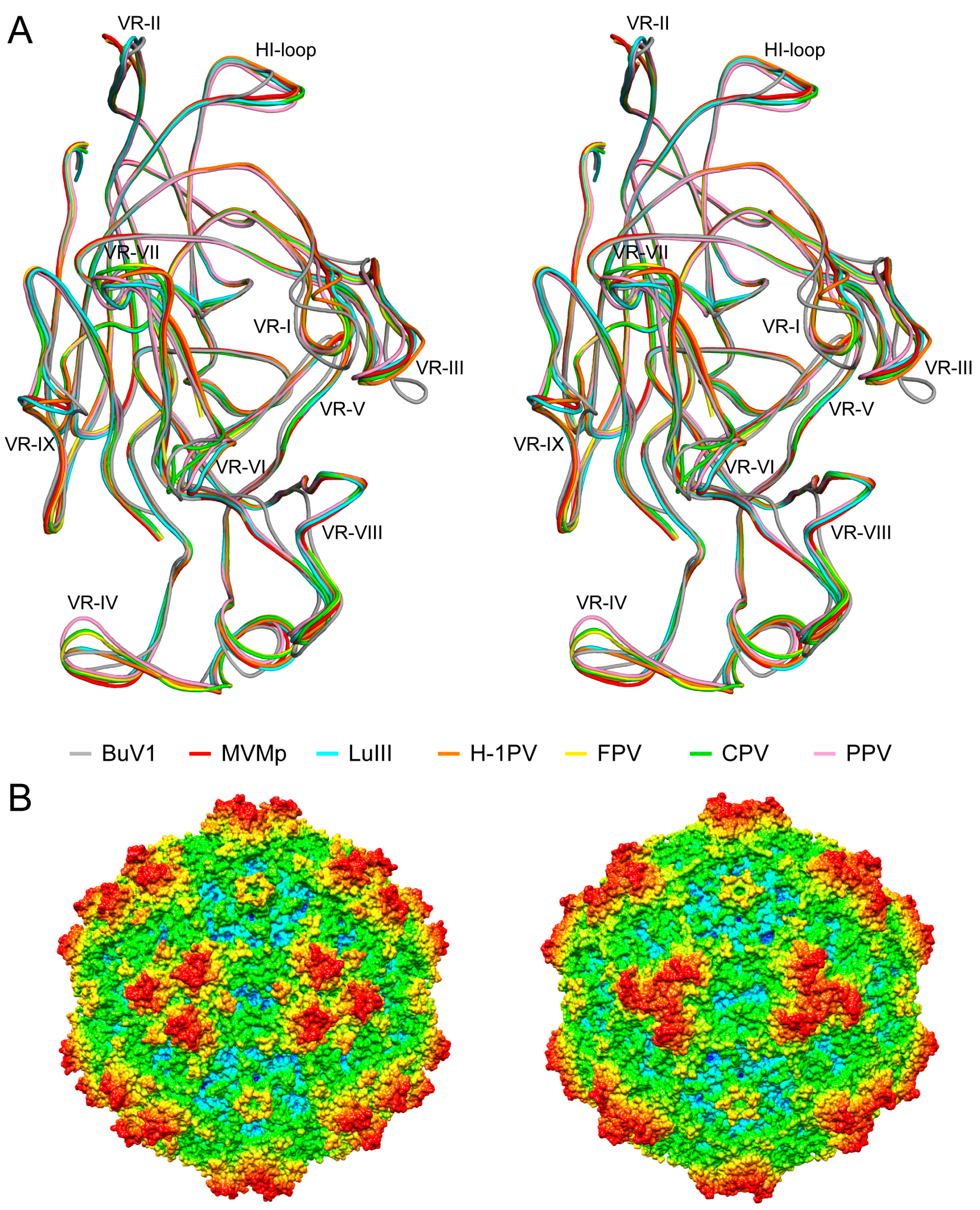
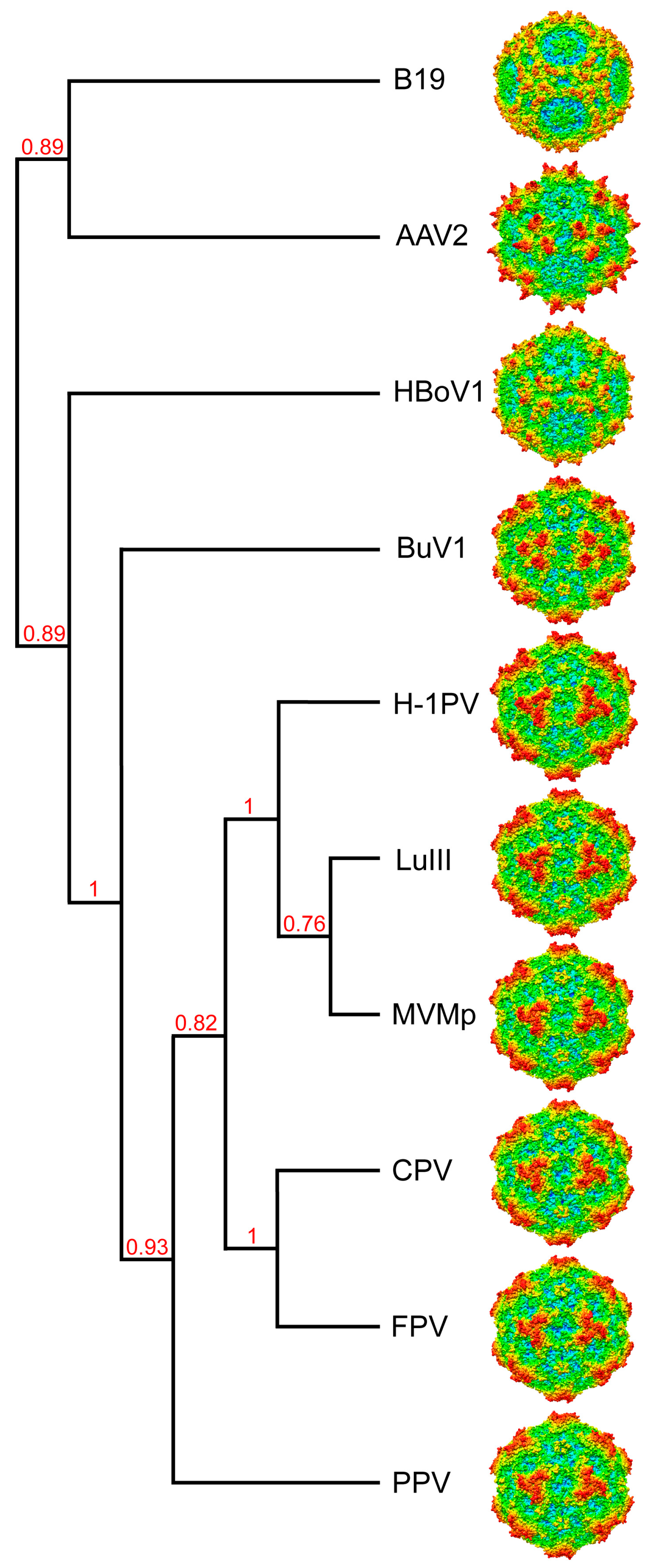
3.4. BuV1 Comparison to Other Human Parvoviruses Suggests Host Specific Capsid Structural Evolution
4. BuVs Structures Conserve Functional Parvovirus Features
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Allander, T.; Tammi, M.T.; Eriksson, M.; Bjerkner, A.; Tiveljung-Lindell, A.; Andersson, B. Cloning of a human parvovirus by molecular screening of respiratory tract samples. Proc. Natl. Acad. Sci. USA 2005, 102, 12891–12896. [Google Scholar] [CrossRef] [PubMed]
- Arthur, J.L.; Higgins, G.D.; Davidson, G.P.; Givney, R.C.; Ratcliff, R.M. A novel bocavirus associated with acute gastroenteritis in Australian children. PLoS Pathog. 2009, 5. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Simmonds, P.; Slikas, E.; Li, L.; Bodhidatta, L.; Sethabutr, O.; Triki, H.; Bahri, O.; Oderinde, B.S.; Baba, M.M.; et al. Human bocaviruses are highly diverse, dispersed, recombination prone, and prevalent in enteric infections. J. Infect. Dis. 2010, 201, 1633–1643. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Vo, N.P.; Bonkoungou, I.J.; Kapoor, A.; Barro, N.; O’Ryan, M.; Kapusinszky, B.; Wang, C.; Delwart, E. Acute diarrhea in west African children: Diverse enteric viruses and a novel parvovirus genus. J. Virol. 2012, 86, 11024–11030. [Google Scholar] [CrossRef] [PubMed]
- Jones, M.S.; Kapoor, A.; Lukashov, V.V.; Simmonds, P.; Hecht, F.; Delwart, E. New DNA viruses identified in patients with acute viral infection syndrome. J. Virol. 2005, 79, 8230–8236. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Sdiri-Loulizi, K.; Aouni, M.; Ambert-Balay, K.; Pothier, P.; Deng, X.; Delwart, E. New parvovirus in child with unexplained diarrhea, Tunisia. Emerg. Infect. Dis. 2014, 20, 1911–1913. [Google Scholar] [CrossRef] [PubMed]
- Mollerup, S.; Fridholm, H.; Vinner, L.; Kjartansdottir, K.R.; Friis-Nielsen, J.; Asplund, M.; Herrera, J.A.; Steiniche, T.; Mourier, T.; Brunak, S.; et al. Cutavirus in cutaneous malignant melanoma. Emerg. Infect. Dis. 2017, 23, 363–365. [Google Scholar] [CrossRef] [PubMed]
- Cotmore, S.F.; Agbandje-McKenna, M.; Chiorini, J.A.; Mukha, D.V.; Pintel, D.J.; Qiu, J.; Soderlund-Venermo, M.; Tattersall, P.; Tijssen, P.; Gatherer, D.; et al. The family parvoviridae. Arch. Virol. 2014, 159, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.L.; Schapendonk, C.M.; van Beek, J.; Vennema, H.; Schurch, A.C.; Schipper, D.; Bodewes, R.; Haagmans, B.L.; Osterhaus, A.D.; Koopmans, M.P. New viruses in idiopathic human diarrhea cases, the Netherlands. Emerg. Infect. Dis. 2014, 20, 1218–1222. [Google Scholar] [CrossRef] [PubMed]
- Yahiro, T.; Wangchuk, S.; Tshering, K.; Bandhari, P.; Zangmo, S.; Dorji, T.; Tshering, K.; Matsumoto, T.; Nishizono, A.; Soderlund-Venermo, M.; et al. Novel human bufavirus genotype 3 in children with severe diarrhea, Bhutan. Emerg. Infect. Dis. 2014, 20, 1037–1039. [Google Scholar] [CrossRef] [PubMed]
- Vaisanen, E.; Kuisma, I.; Phan, T.G.; Delwart, E.; Lappalainen, M.; Tarkka, E.; Hedman, K.; Soderlund-Venermo, M. Bufavirus in feces of patients with gastroenteritis, Finland. Emerg. Infect. Dis. 2014, 20, 1077–1079. [Google Scholar] [CrossRef] [PubMed]
- Vaisanen, E.; Paloniemi, M.; Kuisma, I.; Lithovius, V.; Kumar, A.; Franssila, R.; Ahmed, K.; Delwart, E.; Vesikari, T.; Hedman, K.; et al. Epidemiology of two human protoparvoviruses, bufavirus and tusavirus. Sci. Rep. 2016, 6, 39267. [Google Scholar] [CrossRef] [PubMed]
- Väisänen, E.; Fu, Y.; Hedman, K.; Söderlund-Venermo, M. Human protoparvoviruses. Viruses 2017, 9, 354. [Google Scholar] [CrossRef] [PubMed]
- Zadori, Z.; Szelei, J.; Tijssen, P. SAT: A late NS protein of porcine parvovirus. J. Virol. 2005, 79, 13129–13138. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Ng, R.; Agbandje-McKenna, M. Parvoviruses: Structure and infection. Future Virol. 2012, 7, 253–278. [Google Scholar] [CrossRef]
- Wu, H.; Rossmann, M.G. The canine parvovirus empty capsid structure. J. Mol. Biol. 1993, 233, 231–244. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.A.; Chandrasekar, V.; Hebert, B.; Sullivan, G.M.; Rossmann, M.G.; Parrish, C.R. Host range and variability of calcium binding by surface loops in the capsids of canine and feline parvoviruses. J. Mol. Biol. 2000, 300, 597–610. [Google Scholar] [CrossRef] [PubMed]
- Halder, S.; Nam, H.J.; Govindasamy, L.; Vogel, M.; Dinsart, C.; Salome, N.; McKenna, R.; Agbandje-McKenna, M. Structural characterization of H-1 parvovirus: Comparison of infectious virions to empty capsids. J. Virol. 2013, 87, 5128–5140. [Google Scholar] [CrossRef] [PubMed]
- Pittman, N.; Misseldine, A.; Geilen, L.; Halder, S.; Smith, J.K.; Kurian, J.; Chipman, P.; Janssen, M.; McKenna, R.; Baker, T.S.; et al. Atomic resolution structure of the oncolytic parvovirus LuIII by electron microscopy and 3D image reconstruction. Viruses 2017, 9, 321. [Google Scholar] [CrossRef] [PubMed]
- Kontou, M.; Govindasamy, L.; Nam, H.J.; Bryant, N.; Llamas-Saiz, A.L.; Foces-Foces, C.; Hernando, E.; Rubio, M.P.; McKenna, R.; Almendral, J.M.; et al. Structural determinants of tissue tropism and in vivo pathogenicity for the parvovirus minute virus of mice. J. Virol. 2005, 79, 10931–10943. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.A.; Hebert, B.; Sullivan, G.M.; Parrish, C.R.; Zadori, Z.; Tijssen, P.; Rossmann, M.G. The structure of porcine parvovirus: Comparison with related viruses. J. Mol. Biol. 2002, 315, 1189–1198. [Google Scholar] [CrossRef] [PubMed]
- Xie, Q.; Bu, W.; Bhatia, S.; Hare, J.; Somasundaram, T.; Azzi, A.; Chapman, M.S. The atomic structure of adeno-associated virus (AAV-2), a vector for human gene therapy. Proc. Natl. Acad. Sci. USA 2002, 99, 10405–10410. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Simpson, A.A.; Rossmann, M.G. The structure of human parvovirus B19. Proc. Natl. Acad. Sci. USA 2004, 101, 11628–11633. [Google Scholar] [CrossRef] [PubMed]
- Mietzsch, M.; Kailasan, S.; Garrison, J.; Ilyas, M.; Chipman, P.; Kantola, K.; Janssen, M.E.; Spear, J.; Sousa, D.; McKenna, R.; et al. Structural Insights into Human Bocaparvoviruses. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed]
- Bennett, A.; Patel, S.; Mietzsch, M.; Jose, A.; Lins-Austin, B.; Yu, J.C.; Bothner, B.; McKenna, R.; Agbandje-McKenna, M. Thermal stability as a determinant of AAV serotype identity. Mol. Ther. Methods Clin. Dev. 2017, 6, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Suloway, C.; Pulokas, J.; Fellmann, D.; Cheng, A.; Guerra, F.; Quispe, J.; Stagg, S.; Potter, C.S.; Carragher, B. Automated molecular microscopy: The new Leginon system. J. Struct. Biol. 2005, 151, 41–60. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.Q.; Palovcak, E.; Armache, J.P.; Verba, K.A.; Cheng, Y.; Agard, D.A. MotionCor2: Anisotropic correction of beam-induced motion for improved cryo-electron microscopy. Nat. Methods 2017, 14, 331–332. [Google Scholar] [CrossRef] [PubMed]
- Spear, J.M.; Noble, A.J.; Xie, Q.; Sousa, D.R.; Chapman, M.S.; Stagg, S.M. The influence of frame alignment with dose compensation on the quality of single particle reconstructions. J. Struct. Biol. 2015, 192, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Sinkovits, R.S.; Baker, T.S. AUTO3DEM—An automated and high throughput program for image reconstruction of icosahedral particles. J. Struct. Biol. 2007, 157, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Tang, G.; Peng, L.; Baldwin, P.R.; Mann, D.S.; Jiang, W.; Rees, I.; Ludtke, S.J. EMAN2: An extensible image processing suite for electron microscopy. J. Struct. Biol. 2007, 157, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Rohou, A.; Grigorieff, N. CTFFIND4: Fast and accurate defocus estimation from electron micrographs. J. Struct. Biol. 2015, 192, 216–221. [Google Scholar] [CrossRef] [PubMed]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [PubMed]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed]
- Bordoli, L.; Kiefer, F.; Arnold, K.; Benkert, P.; Battey, J.; Schwede, T. Protein structure homology modeling using SWISS-MODEL workspace. Nat. Protoc. 2008, 4, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Tripp, M.; Shepherd, C.M.; Borelli, I.A.; Venkataraman, S.; Lander, G.; Natarajan, P.; Johnson, J.E.; Brooks, C.L., 3rd; Reddy, V.S. VIPERdb2: An enhanced and web API enabled relational database for structural virology. Nucleic Acids Res. 2009, 37, D436–D442. [Google Scholar] [CrossRef] [PubMed]
- Kleywegt, G.J.; Jones, T.A. xdlMAPMAN and xdlDATAMAN—Programs for reformatting, analysis and manipulation of biomacromolecular electron-density maps and reflection data sets. Acta Crystallogr. D 1996, 52, 826–828. [Google Scholar] [CrossRef] [PubMed]
- Adams, P.D.; Afonine, P.V.; Bunkoczi, G.; Chen, V.B.; Davis, I.W.; Echols, N.; Headd, J.J.; Hung, L.W.; Kapral, G.J.; Grosse-Kunstleve, R.W.; et al. PHENIX: A comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 213–221. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Halder, S.; Gurda, B.; Bladek, H.; Chipman, P.R.; McKenna, R.; Brown, K.; Agbandje-McKenna, M. Structure of an enteric pathogen, bovine parvovirus. J. Virol. 2015, 89, 2603–2614. [Google Scholar] [CrossRef] [PubMed]
- Krissinel, E.; Henrick, K. Secondary-structure matching (SSM), a new tool for fast protein structure alignment in three dimensions. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2256–2268. [Google Scholar] [CrossRef] [PubMed]
- Kaufmann, B.; Chipman, P.R.; Kostyuchenko, V.A.; Modrow, S.; Rossmann, M.G. Visualization of the externalized VP2 N termini of infectious human parvovirus B19. J. Virol. 2008, 82, 7306–7312. [Google Scholar] [CrossRef] [PubMed]
- Hafenstein, S.; Palermo, L.M.; Kostyuchenko, V.A.; Xiao, C.; Morais, M.C.; Nelson, C.D.; Bowman, V.D.; Battisti, A.J.; Chipman, P.R.; Parrish, C.R.; et al. Asymmetric binding of transferrin receptor to parvovirus capsids. Proc. Natl. Acad. Sci. USA 2007, 104, 6585–6589. [Google Scholar] [CrossRef] [PubMed]
- Gurda, B.L.; DiMattia, M.A.; Miller, E.B.; Bennett, A.; McKenna, R.; Weichert, W.S.; Nelson, C.D.; Chen, W.J.; Muzyczka, N.; Olson, N.H.; et al. Capsid antibodies to different adeno-associated virus serotypes bind common regions. J. Virol. 2013, 87, 9111–9124. [Google Scholar] [CrossRef] [PubMed]
- Tseng, Y.S.; Gurda, B.L.; Chipman, P.; McKenna, R.; Afione, S.; Chiorini, J.A.; Muzyczka, N.; Olson, N.H.; Baker, T.S.; Kleinschmidt, J.; et al. Adeno-associated virus serotype 1 (AAV1)- and AAV5-antibody complex structures reveal evolutionary commonalities in parvovirus antigenic reactivity. J. Virol. 2015, 89, 1794–1808. [Google Scholar] [CrossRef] [PubMed]
- Kailasan, S.; Garrison, J.; Ilyas, M.; Chipman, P.; McKenna, R.; Kantola, K.; Soderlund-Venermo, M.; Kucinskaite-Kodze, I.; Zvirbliene, A.; Agbandje-McKenna, M. Mapping antigenic epitopes on the human bocavirus capsid. J. Virol. 2016, 90, 4670–4680. [Google Scholar] [CrossRef] [PubMed]
- Carreira, A.; Menendez, M.; Reguera, J.; Almendral, J.M.; Mateu, M.G. In vitro disassembly of a parvovirus capsid and effect on capsid stability of heterologous peptide insertions in surface loops. J. Biol. Chem. 2004, 279, 6517–6525. [Google Scholar] [CrossRef] [PubMed]
- López-Bueno, A.; Rubio, M.P.; Bryant, N.; McKenna, R.; Agbandje-McKenna, M.; Almendral, J.M. Host-selected amino acid changes at the sialic acid binding pocket of the parvovirus capsid modulate cell binding affinity and determine virulence. J. Virol. 2006, 80, 1563–1573. [Google Scholar] [CrossRef] [PubMed]
- Plevka, P.; Hafenstein, S.; Li, L.; D’Abrgamo, A., Jr.; Cotmore, S.F.; Rossmann, M.G.; Tattersall, P. Structure of a packaging-defective mutant of minute virus of mice indicates that the genome is packaged via a pore at a 5-fold axis. J. Virol. 2011, 85, 4822–4827. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, J.; Taylor, K.A.; Chapman, M.S. Adeno-associatedvirus-2andits primary cellular receptor—Cryo-EM structure of a heparin complex. Virology 2009, 385, 434–443. [Google Scholar] [CrossRef] [PubMed]
- Chipman, P.R.; Agbandje-McKenna, M.; Kajigaya, S.; Brown, K.E.; Young, N.S.; Baker, T.S.; Rossmann, M.G. Cryo-electron microscopy studies of empty capsids of human parvovirus B19 complexed with its cellular receptor. Proc. Natl. Acad. Sci. USA 1996, 93, 7502–7506. [Google Scholar] [CrossRef] [PubMed]
- Levy, H.C.; Bowman, V.D.; Govindasamy, L.; McKenna, R.; Nash, K.; Warrington, K.; Chen, W.; Muzyczka, N.; Yan, X.; Baker, T.S.; et al. Heparin binding induces conformational changes in Adeno-associated virus serotype 2. J. Struct. Biol. 2009, 165, 146–156. [Google Scholar] [CrossRef] [PubMed]
- McCraw, D.M.; O’Donnell, J.K.; Taylor, K.A.; Stagg, S.M.; Chapman, M.S. Structure of adeno-associated virus-2 in complex with neutralizing monoclonal antibody A20. Virology 2012, 431, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Gurda, B.L.; Raupp, C.; Popa-Wagner, R.; Naumer, M.; Olson, N.H.; Ng, R.; McKenna, R.; Baker, T.S.; Kleinschmidt, J.A.; Agbandje-McKenna, M. Mapping a neutralizing epitope onto the capsid of adeno-associated virus serotype 8. J. Virol. 2012, 86, 7739–7751. [Google Scholar] [CrossRef] [PubMed]
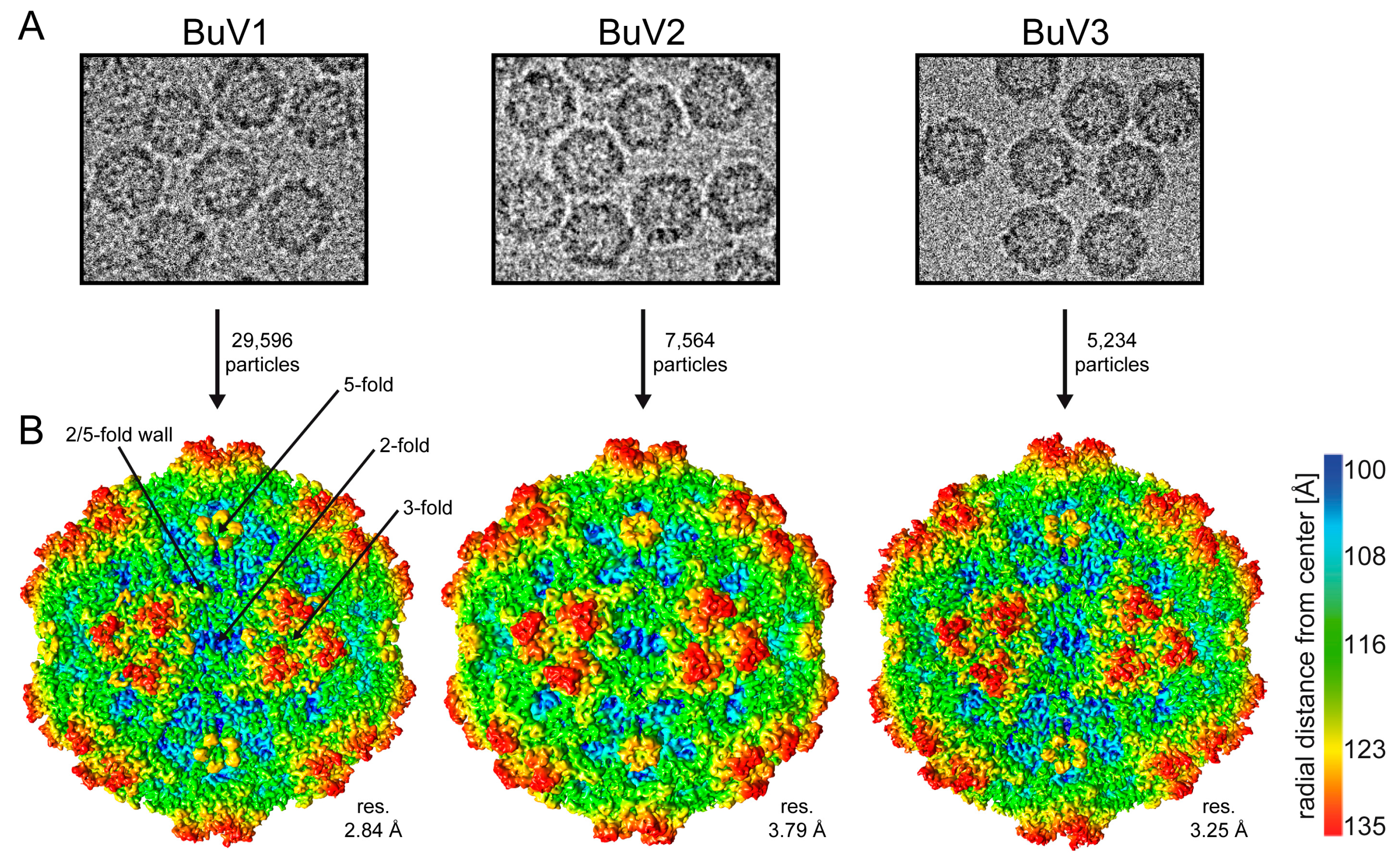
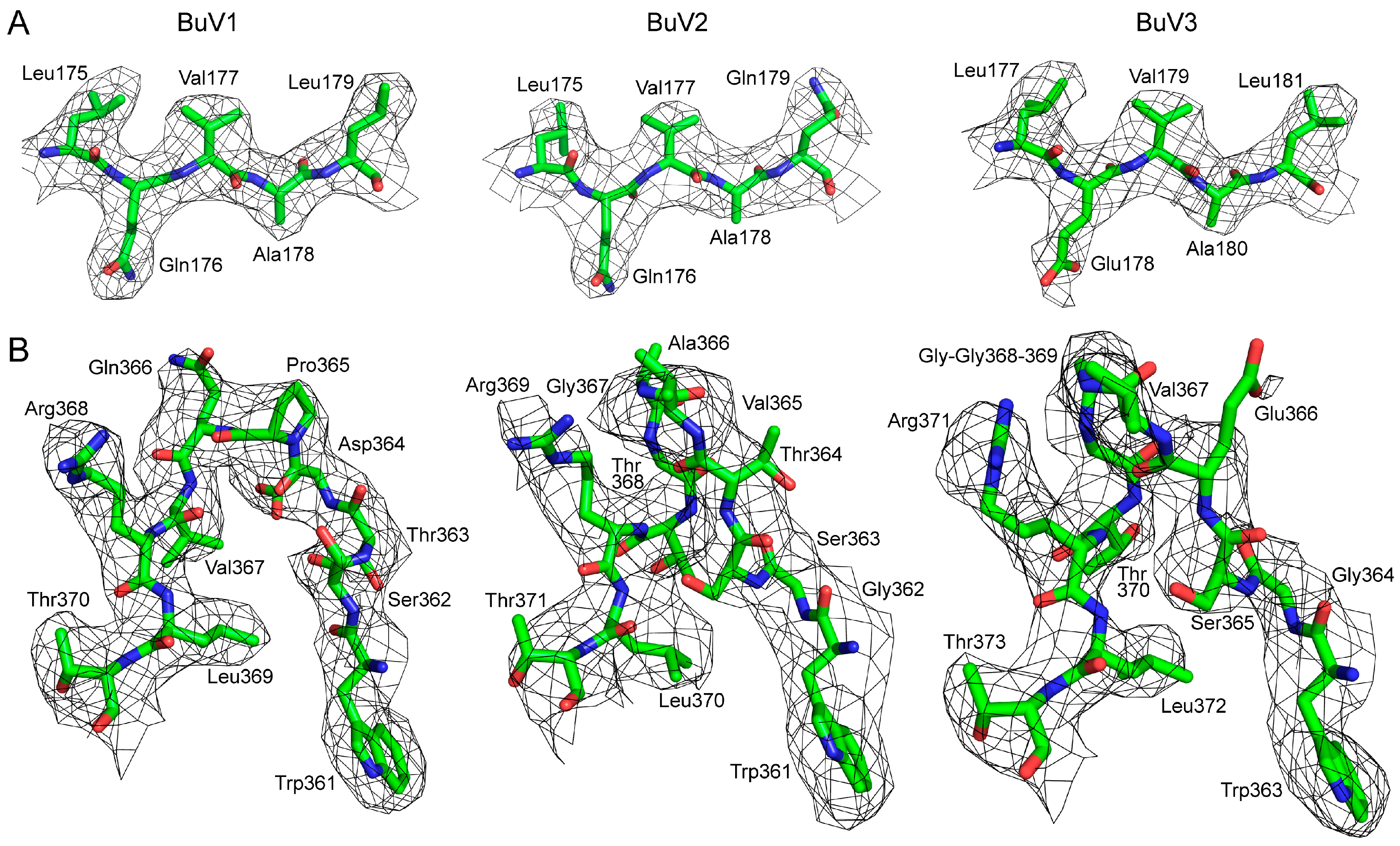


| Processing and Refinement Parameters | BuV1 | BuV2 | BuV3 |
|---|---|---|---|
| Total number of micrographs | 1961 | 429 | 689 |
| Defocus range (µm) | 0.79–4.42 | 0.90–3.87 | 1.82–3.34 |
| Electron dose (e−/Å2) | 59.5 | 57.2 | 75 |
| Frames/micrograph | 60 | 34 | 50 |
| Pixel size (Å/pixel) | 1.07 | 1.22 | 1.06 |
| Startin gnumber of particles | 59,170 | 8404 | 6543 |
| Particles used for final map | 29,596 | 7564 | 5234 |
| B-factor used for final map (Å2) | −50 | −100 | −100 |
| Resolution of final map (Å) | 2.84 | 3.79 | 3.25 |
| PHENIX Model Refinement Statistics | |||
| Residue range | 33–568 | 33–567 | 33–572 |
| Map correlation coefficient | 0.863 | 0.678 | 0.852 |
| RMSD (root-mean-square deviation) [bonds] (Å) | 0.01 | 0.01 | 0.01 |
| RMSD [angles] (Å) | 0.91 | 1.20 | 0.97 |
| All-atom clash score | 10.41 | 18.44 | 11.79 |
| Ramachandran Plot | |||
| Favored (%) | 95.5 | 94.0 | 94.8 |
| Allowed (%) | 4.5 | 6.0 | 5.2 |
| Outliers (%) | 0 | 0 | 0 |
| Rotameroutliers (%) | 0 | 0 | 0 |
| C-β deviations | 0 | 0 | 0 |
| BuV1 | MVMp | LuIII | H-1PV | CPV | FPV | PPV | ||
|---|---|---|---|---|---|---|---|---|
| BuV1 | 75.0 | 76.7 | 74.1 | 74.3 | 74.8 | 75.7 | Structural similarity(%) | |
| MVMp | 29.5 | 97.8 | 97.8 | 90.7 | 90.5 | 91.4 | ||
| LuIII | 31.4 | 73.2 | 97.1 | 89.3 | 88.0 | 91.7 | ||
| H-1PV | 29.9 | 67.3 | 67.7 | 90.9 | 90.4 | 92.0 | ||
| CPV | 30.8 | 51.9 | 50.7 | 51.2 | 96.7 | 91.1 | ||
| FPV | 31.0 | 51.9 | 50.7 | 51.2 | 99.7 | 90.0 | ||
| PPV | 31.3 | 49.4 | 50.3 | 49.3 | 57.2 | 57.2 | ||
| VP2/3 sequence identity (%) | ||||||||
| BuV1 | AAV2 | B19 | HBoV1 | ||
|---|---|---|---|---|---|
| BuV1 | 54.7 | 44.8 | 48.3 | Structural similarity (%) | |
| AAV2 | 18.2 | 62.8 | 63.2 | ||
| B19 | 15.5 | 23.7 | 51.6 | ||
| HBoV1 | 19.0 | 24.0 | 20.6 | ||
| VP2/3 sequence identity (%) | |||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ilyas, M.; Mietzsch, M.; Kailasan, S.; Väisänen, E.; Luo, M.; Chipman, P.; Smith, J.K.; Kurian, J.; Sousa, D.; McKenna, R.; et al. Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy. Viruses 2018, 10, 22. https://doi.org/10.3390/v10010022
Ilyas M, Mietzsch M, Kailasan S, Väisänen E, Luo M, Chipman P, Smith JK, Kurian J, Sousa D, McKenna R, et al. Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy. Viruses. 2018; 10(1):22. https://doi.org/10.3390/v10010022
Chicago/Turabian StyleIlyas, Maria, Mario Mietzsch, Shweta Kailasan, Elina Väisänen, Mengxiao Luo, Paul Chipman, J. Kennon Smith, Justin Kurian, Duncan Sousa, Robert McKenna, and et al. 2018. "Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy" Viruses 10, no. 1: 22. https://doi.org/10.3390/v10010022
APA StyleIlyas, M., Mietzsch, M., Kailasan, S., Väisänen, E., Luo, M., Chipman, P., Smith, J. K., Kurian, J., Sousa, D., McKenna, R., Söderlund-Venermo, M., & Agbandje-McKenna, M. (2018). Atomic Resolution Structures of Human Bufaviruses Determined by Cryo-Electron Microscopy. Viruses, 10(1), 22. https://doi.org/10.3390/v10010022





