Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cell Lines
2.3. Viruses
2.4. Flow Cytometric Detection of Cell-Surface Molecules
2.5. Transduction of Lymphoid Cell Lines
2.6. Transduction of Adherent Cell Lines
2.7. Fluorescent Labelling of Adenoviruses
2.8. Virus: Cell Binding
2.9. Isolation of Peripheral Blood Mononuclear Cells (PBMC)
2.10. Transduction of PBMC
3. Results
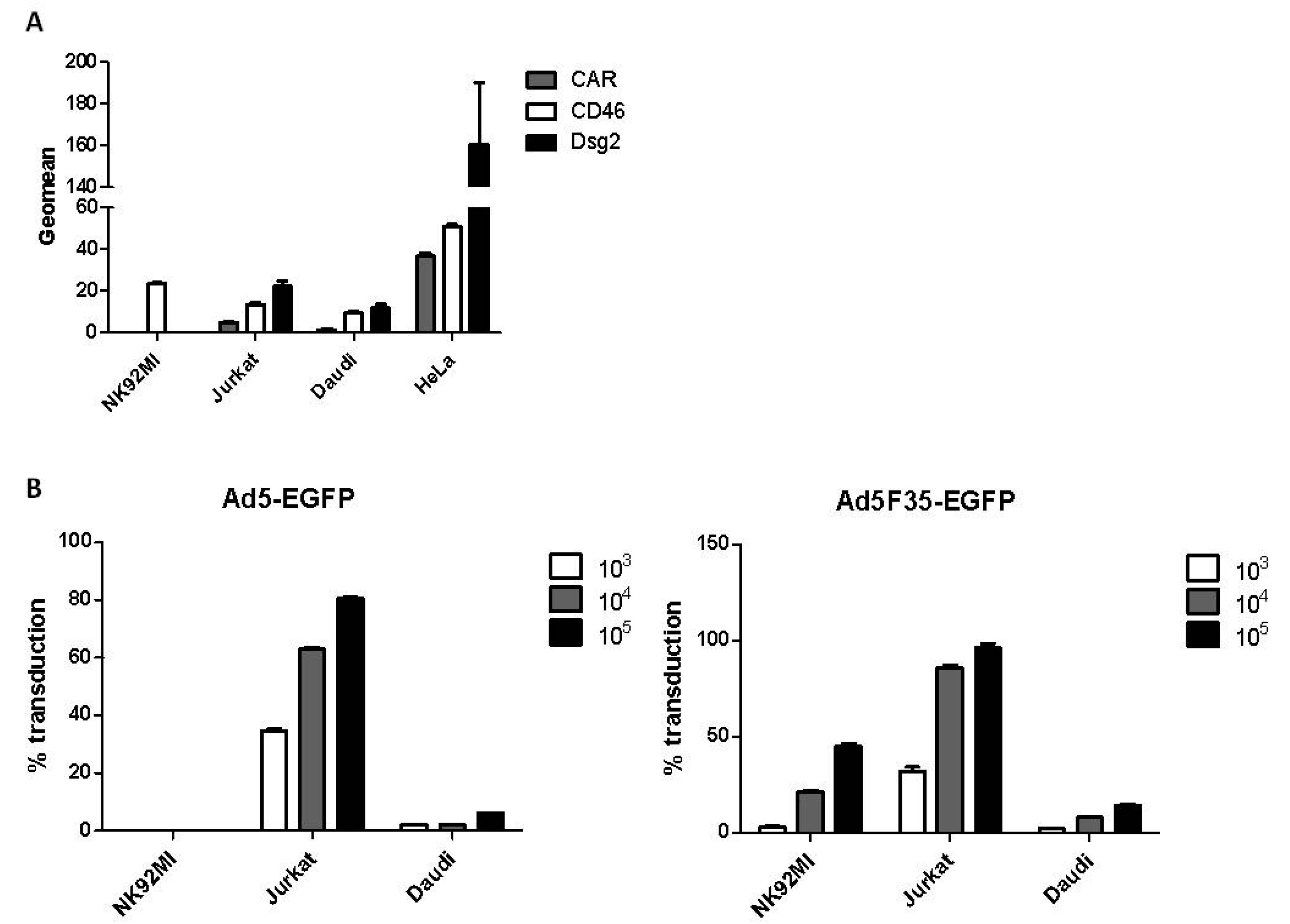
3.1. Adenovirus Transduction of Lymphoid Cell Lines
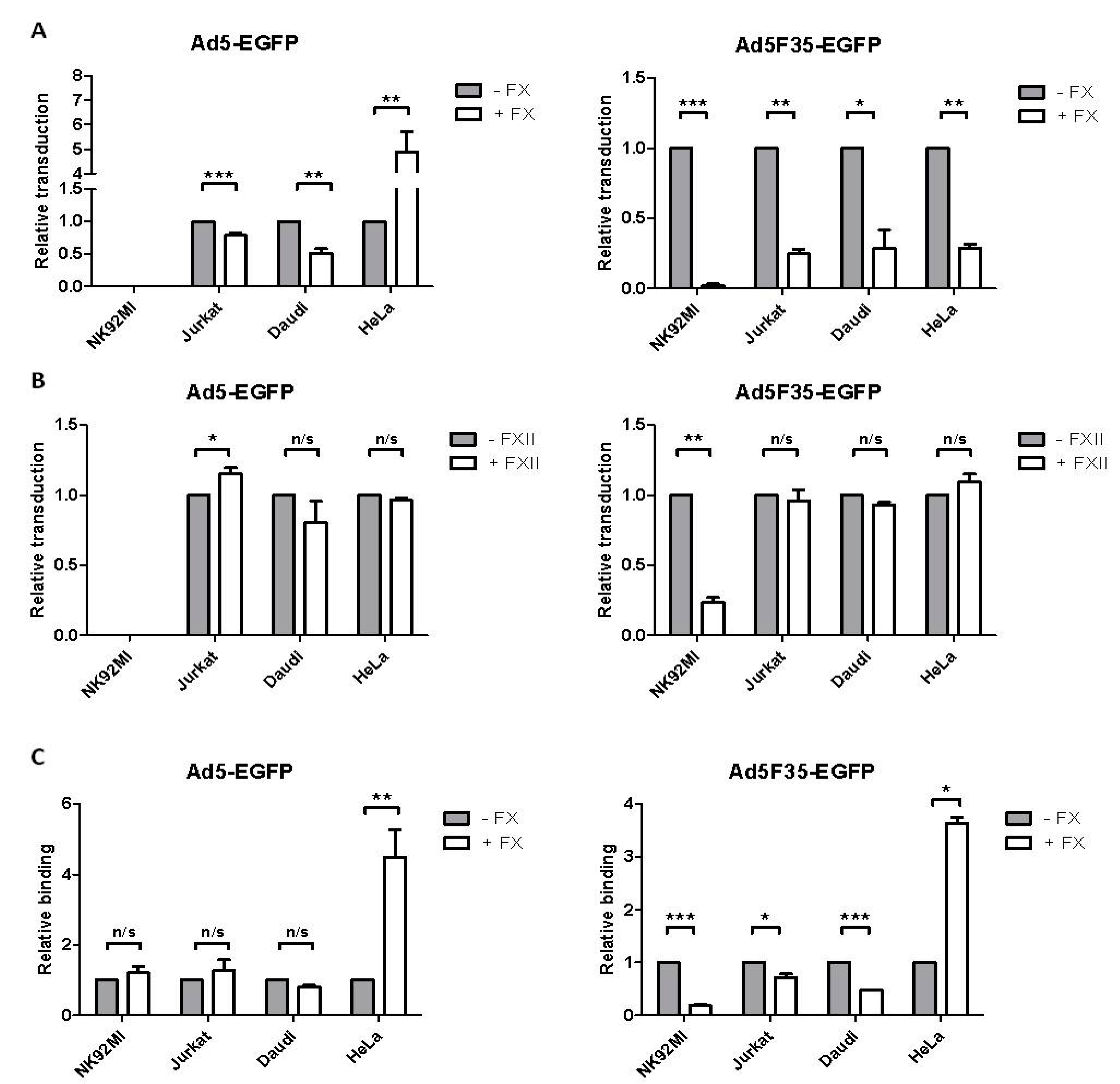
3.2. Adenovirus Interactions with Lymphoid and Epithelial Cells in the Presence of Factor X
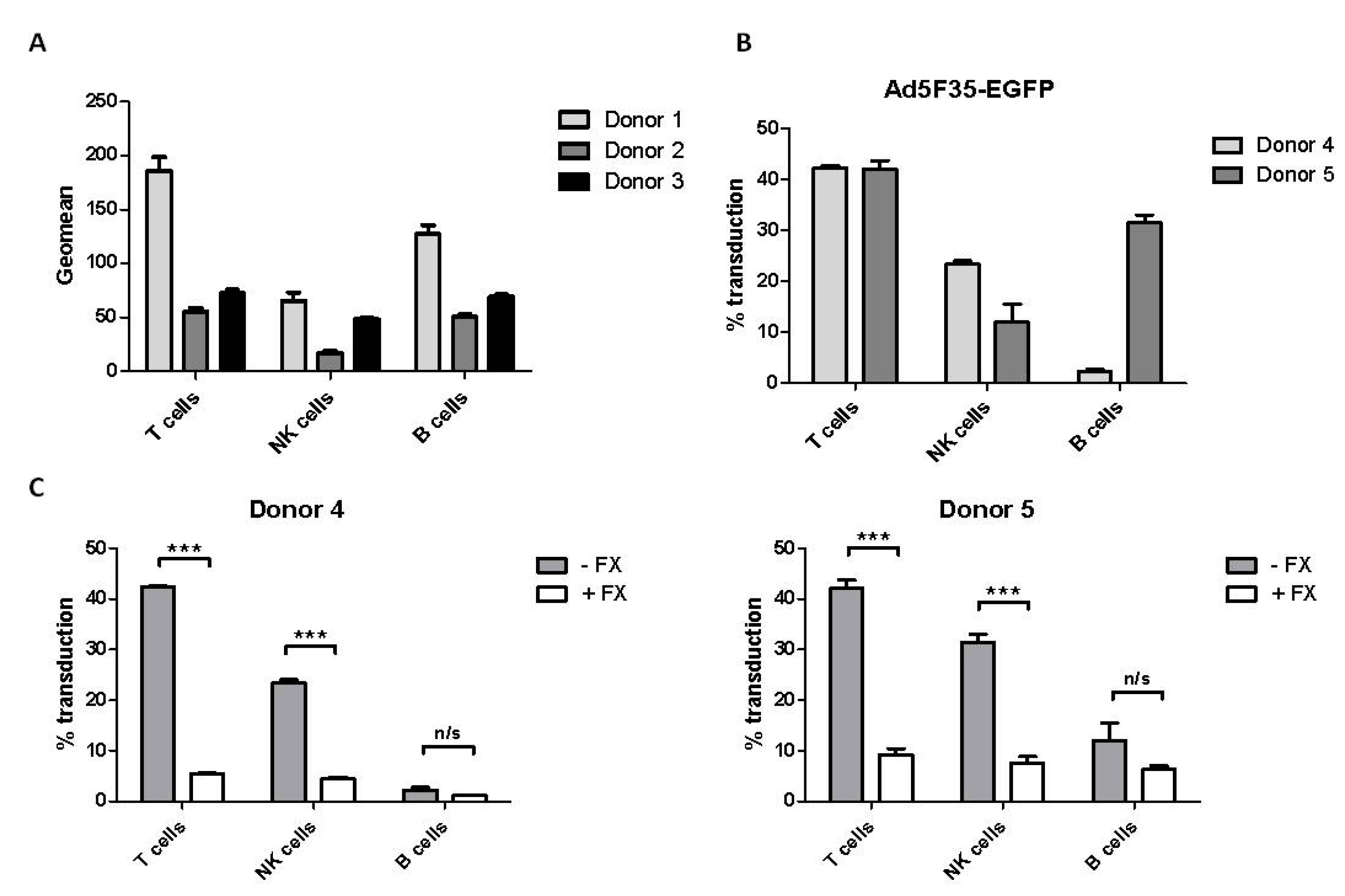
3.3. Effect of Factor X on Adenovirus Transduction of Primary Blood Lymphoid Cells
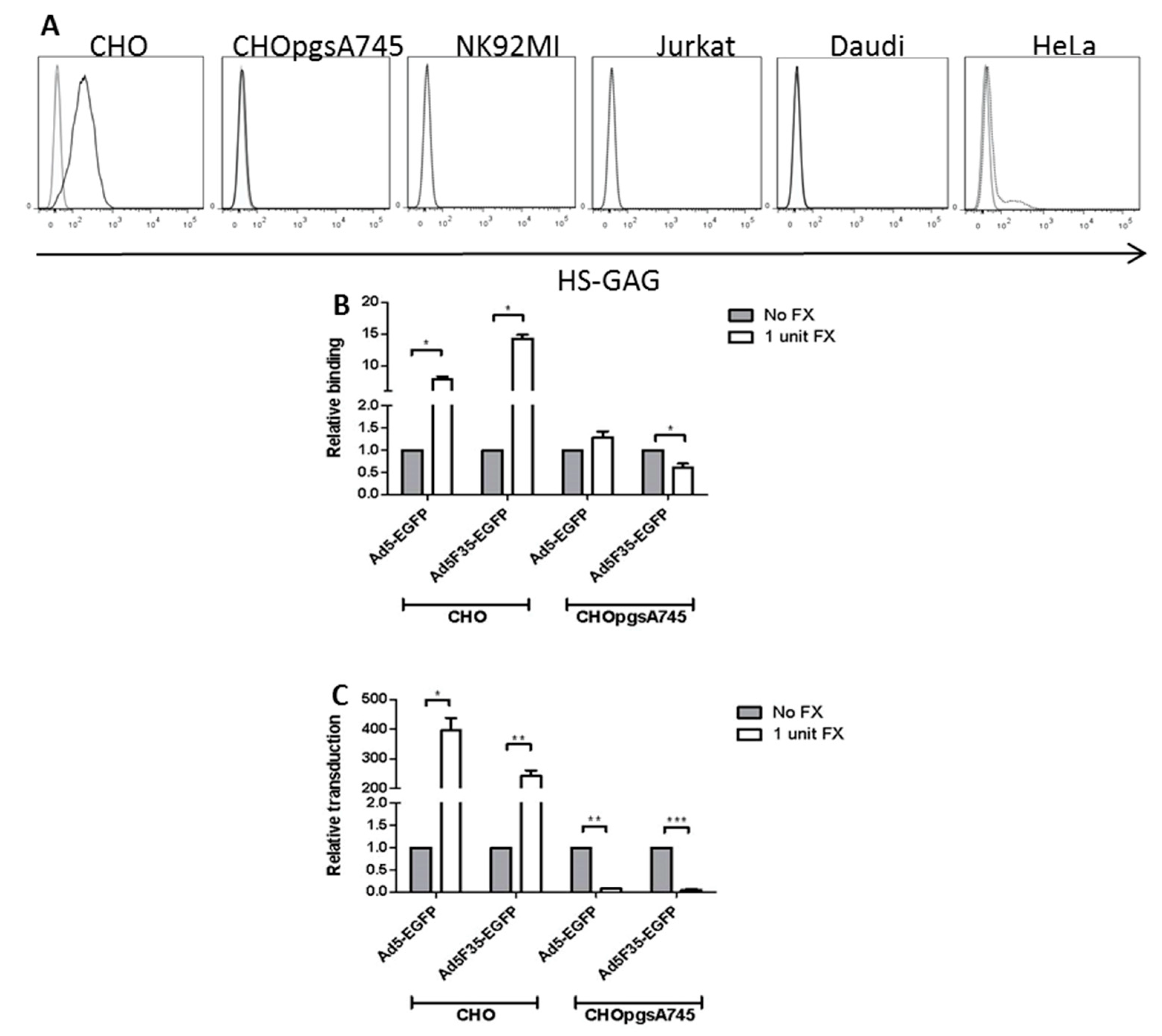
3.4. Role of HSPG Expression in FX-Mediated Adenovirus Transduction of Lymphoid Cells
4. Discussion
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Roelvink, P.W.; Lizonova, A.; Lee, J.G.; Li, Y.; Bergelson, J.M.; Finberg, R.W.; Brough, D.E.; Kovesdi, I.; Wickham, T.J. The coxsackievirus-adenovirus receptor protein can function as a cellular attachment protein for adenovirus serotypes from subgroups A, C, D, E, and F. J. Virol. 1998, 72, 7909–7915. [Google Scholar] [PubMed]
- Gaggar, A.; Shayakhmetov, D.M.; Lieber, A. CD46 is a cellular receptor for group B adenoviruses. Nat. Med. 2003, 9, 1408–1412. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, Z.Y.; Liu, Y.; Persson, J.; Beyer, I.; Möller, T.; Koyuncu, D.; Drescher, M.R.; Strauss, R.; Zhang, X.B.; et al. Desmoglein 2 is a receptor for adenovirus serotypes 3, 7, 11 and 14. Nat. Med. 2011, 17, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Fechner, H.; Haack, A.; Wang, H.; Wang, X.; Eizema, K.; Pauschinger, M.; Schoemaker, R.; Veghel, R.; Houtsmuller, A.; Schultheiss, H.P.; et al. Expression of coxsackie adenovirus receptor and αv-integrin does not correlate with adenovector targeting in vivo indicating anatomical vector barriers. Gene Ther. 1999, 6, 1520–1535. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.A.; Idamakanti, N.; Marshall-Neff, J.; Rollence, M.L.; Wright, P.; Kaloss, M.; King, L.; Mech, C.; Dinges, L.; Iverson, W.O.; et al. Receptor interactions involved in adenoviral-mediated gene delivery after systemic administration in non-human primates. Hum. Gene Ther. 2003, 14, 1595–1604. [Google Scholar] [CrossRef] [PubMed]
- Shayakhmetov, D.M.; Gaggar, A.; Ni, S.; Li, Z.Y.; Lieber, A. Adenovirus binding to blood factors results in liver cell infection and hepatotoxicity. J. Virol. 2005, 79, 7478–7491. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Mestre-Frances, N.; Verdier, J.M.; Henaff, D.; Baker, A.H. Coagulation factor X mediates adenovirus type 5 liver gene transfer in non-human primates (Microcebus murinus). Gene Ther. 2012, 19, 109–113. [Google Scholar] [CrossRef] [PubMed]
- Parker, A.L.; Waddington, S.N.; Nicol, C.G.; Shayakhmetov, D.M.; Buckley, S.M.; Denby, L.; Kemball-Cook, G.; Ni, S.; Lieber, A.; McVey, J.H.; et al. Multiple vitamin K-dependent coagulation zymogens promote adenovirus-mediated gene delivery to hepatocytes. Blood 2006, 108, 2554–2561. [Google Scholar] [CrossRef] [PubMed]
- Alba, R.; Bradshaw, A.C.; Parker, A.L.; Bhella, D.; Waddington, S.N.; Nicklin, S.A.; van Rooijen, N.; Custers, J.; Goudsmit, J.; Barouch, D.H.; et al. Identification of coagulation factor (F)X binding sites on the adenovirus serotype 5 hexon: Effect of mutagenesis on FX interactions and gene transfer. Blood 2009, 114, 965–971. [Google Scholar] [CrossRef] [PubMed]
- Kalyuzhniy, O.; Di Paolo, N.C.; Silvestry, M.; Hofherr, S.E.; Barry, M.A.; Stewart, P.L.; Shayakhmetov, D.M. Adenovirus serotype 5 hexon is critical for virus infection of hepatocytes in vivo. Proc. Natl. Acad. Sci. USA 2008, 105, 5483–5488. [Google Scholar] [CrossRef] [PubMed]
- Waddington, S.N.; McVey, J.H.; Bhella, D.; Parker, A.L.; Barker, K.; Atoda, H.; Pink, R.; Buckley, S.M.; Greig, J.A.; Denby, L.; et al. Adenovirus serotype 5 hexon mediates liver gene transfer. Cell 2008, 132, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, A.C.; Parker, A.L.; Duffy, M.R.; Coughlan, L.; van Rooijen, N.; Kähäri, V.M.; Nicklin, S.A.; Baker, A.H. Requirements for receptor engagement during infection by adenovirus complexed with blood coagulation factor X. PLoS Pathog. 2010, 6, e1001142. [Google Scholar] [CrossRef] [PubMed]
- Duffy, M.R.; Bradshaw, A.C.; Parker, A.L.; McVey, J.H.; Baker, A.H. A cluster of basic amino acids in the factor X serine protease mediates surface attachment of adenovirus/FX complexes. J. Virol. 2011, 85, 10914–10919. [Google Scholar] [CrossRef] [PubMed]
- Zaiss, A.K.; Foley, E.M.; Lawrence, R.; Schneider, L.S; Hoveida, H.; Secrest, P.; Catapang, A.B.; Yamaguchi, Y.; Alemany, R.; Shayakhmetov, D.M.; et al. Hepatocyte Heparan Sulfate Is Required for Adeno-Associated Virus 2 but Dispensable for Adenovirus 5 Liver Transduction In Vivo. J. Virol. 2015, 90, 412–420. [Google Scholar] [CrossRef] [PubMed]
- Greig, J.A.; Buckley, S.M.; Waddington, S.N.; Parker, A.L.; Bhella, D.; Pink, R.; Rahim, A.A.; Morita, T.; Nicklin, S.A.; McVey, J.H.; et al. Influence of coagulation factor X on in vitro and in vivo gene delivery by adenovirus (Ad) 5, Ad35, and chimeric Ad5/Ad35 vectors. Mol. Ther. 2009, 17, 1683–1691. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Ahonen, M.; Hämäläinen, H.; Bergelson, J.M.; Kähäri, V.M.; Lahesmaa, R. High-efficiency gene transfer to primary T lymphocytes by recombinant adenovirus vectors. J. Immunol. Methods 2002, 260, 79–89. [Google Scholar] [CrossRef]
- Schroers, R.; Hildebrandt, Y.; Hasenkamp, J.; Glass, B.; Lieber, A.; Wulf, G.; Piesche, M. Gene transfer into human T lymphocytes and natural killer cells by Ad5/F35 chimeric adenoviral vectors. Exp. Hematol. 2004, 32, 536–546. [Google Scholar] [CrossRef] [PubMed]
- Jung, D.; Neron, S.; Drouin, M.; Jacques, A. Efficient gene transfer into normal human B lymphocytes with the chimeric adenoviral vector Ad5/F35. J. Immunol. Methods 2005, 304, 78–87. [Google Scholar] [CrossRef] [PubMed]
- Drouin, M.; Cayer, M.P.; Jung, D. Adenovirus 5 and chimeric adenovirus 5/F35 employ distinct B-lymphocyte intracellular trafficking routes that are independent of their cognate cell surface receptor. Virology 2010, 401, 305–313. [Google Scholar] [CrossRef] [PubMed]
- Pahl, J.H.; Verhoeven, D.H.; Kwappenberg, K.M.; Vellinga, J.; Lankester, A.C.; van Tol, M.J.; Schilham, M.W. Adenovirus type 35, but not type 5, stimulates NK cell activation via plasmacytoid dendritic cells and TLR9 signaling. Mol. Immunol. 2012, 51, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.F.; Shao, H.W.; Wu, F.L.; Xie, X.; Li, Z.M.; Bo, H.B.; Shen, H.; Wang, T.; Huang, S.L. Influence of cell physiological state on gene delivery to T lymphocytes by chimeric adenovirus Ad5F35. Sci. Rep. 2016, 6, 22688. [Google Scholar] [CrossRef] [PubMed]
- Roberts, M.L.; Virdee, K.; Sampson, C.P.; Gordon, I.; Parone, P.; Tolkovsky, A.M. The combination of bcl-2 expression and NGF-deprivation facilitates the selective destruction of BAD protein in living sympathetic neurons. Mol. Cell. Neurosci. 2000, 16, 97–110. [Google Scholar] [CrossRef] [PubMed]
- Yu, L.; Shimozato, O.; Li, Q.; Kawamura, K.; Ma, G.; Namba, M.; Ogawa, T.; Kaiho, I.; Tagawa, M. Adenovirus type 5 substituted with type 11 or 35 fiber structure increases its infectivity to human cells enabling dual gene transfer in CD46-dependent and -independent manners. Anticancer Res. 2007, 27, 2311–2316. [Google Scholar] [PubMed]
- Hamdan, S.; Verbeke, C.S.; Fox, N.; Booth, J.; Bottley, G.; Pandha, H.S.; Blair, G.E. The roles of cell surface attachment molecules and coagulation Factor X in adenovirus 5-mediated gene transfer in pancreatic cancer cells. Cancer Gene Ther. 2011, 18, 478–488. [Google Scholar] [CrossRef] [PubMed]
- Mittereder, N.; March, K.L.; Trapnell, B.C. Evaluation of the concentration and bioactivity of adenovirus vectors for gene therapy. J. Virol. 1996, 70, 7498–7509. [Google Scholar] [PubMed]
- Esko, J.D.; Stewart, T.E.; Taylor, W.H. Animal cell mutants defective in glycosaminoglycan biosynthesis. Proc. Natl. Acad. Sci. USA 1985, 82, 3197–3201. [Google Scholar] [CrossRef] [PubMed]
- David, G.; Bai, X.M.; van den Schueren, B.; van den Berghe, H. Developmental changes in heparan sulphate expression: In situ detection with mAbs. J. Cell Biol. 1992, 119, 961–975. [Google Scholar] [CrossRef] [PubMed]
- Fadnes, B.; Husebekk, A.; Svineng, G.; Rekdal, Ø.; Yanagishita, M.; Kolet, S.O.; Uhlin-Hansen, L. The proteoglycan repertoire of lymphoid cells. Glycoconj. J. 2012, 29, 513–523. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Qiu, Q.; Tian, J.; Smith, J.S.; Conenello, G.M.; Morita, T.; Byrnes, A.P. Coagulation factor X shields adenovirus type 5 from attack by natural antibodies and complement. Nat. Med. 2013, 19, 452–457. [Google Scholar] [CrossRef] [PubMed]
- Garnett, C.T.; Talekar, G.; Mahr, J.A.; Huang, W.; Zhang, Y.; Ornelles, D.A.; Gooding, L.R. Latent species C adenoviruses in human tonsil tissues. J. Virol. 2009, 83, 2417–2428. [Google Scholar] [CrossRef] [PubMed]
- Corjon, S.; Gonzalez, G.; Henning, P.; Grichine, A.; Lindholm, L.; Boulanger, P.; Fender, P.; Hong, S.S. Cell entry and trafficking of human adenovirus bound to blood factor X is determined by the fiber serotype and not hexon:heparan sulphate interaction. PLoS ONE 2011, 6, e18205. [Google Scholar] [CrossRef] [PubMed]
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Findlay, J.S.; Cook, G.P.; Blair, G.E. Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes. Viruses 2018, 10, 20. https://doi.org/10.3390/v10010020
Findlay JS, Cook GP, Blair GE. Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes. Viruses. 2018; 10(1):20. https://doi.org/10.3390/v10010020
Chicago/Turabian StyleFindlay, James S., Graham P. Cook, and G. Eric Blair. 2018. "Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes" Viruses 10, no. 1: 20. https://doi.org/10.3390/v10010020
APA StyleFindlay, J. S., Cook, G. P., & Blair, G. E. (2018). Blood Coagulation Factor X Exerts Differential Effects on Adenovirus Entry into Human Lymphocytes. Viruses, 10(1), 20. https://doi.org/10.3390/v10010020





