Cycas micronesica Trees Alter Local Soil Traits
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Sampling
2.2. Analyses
2.3. Statistics
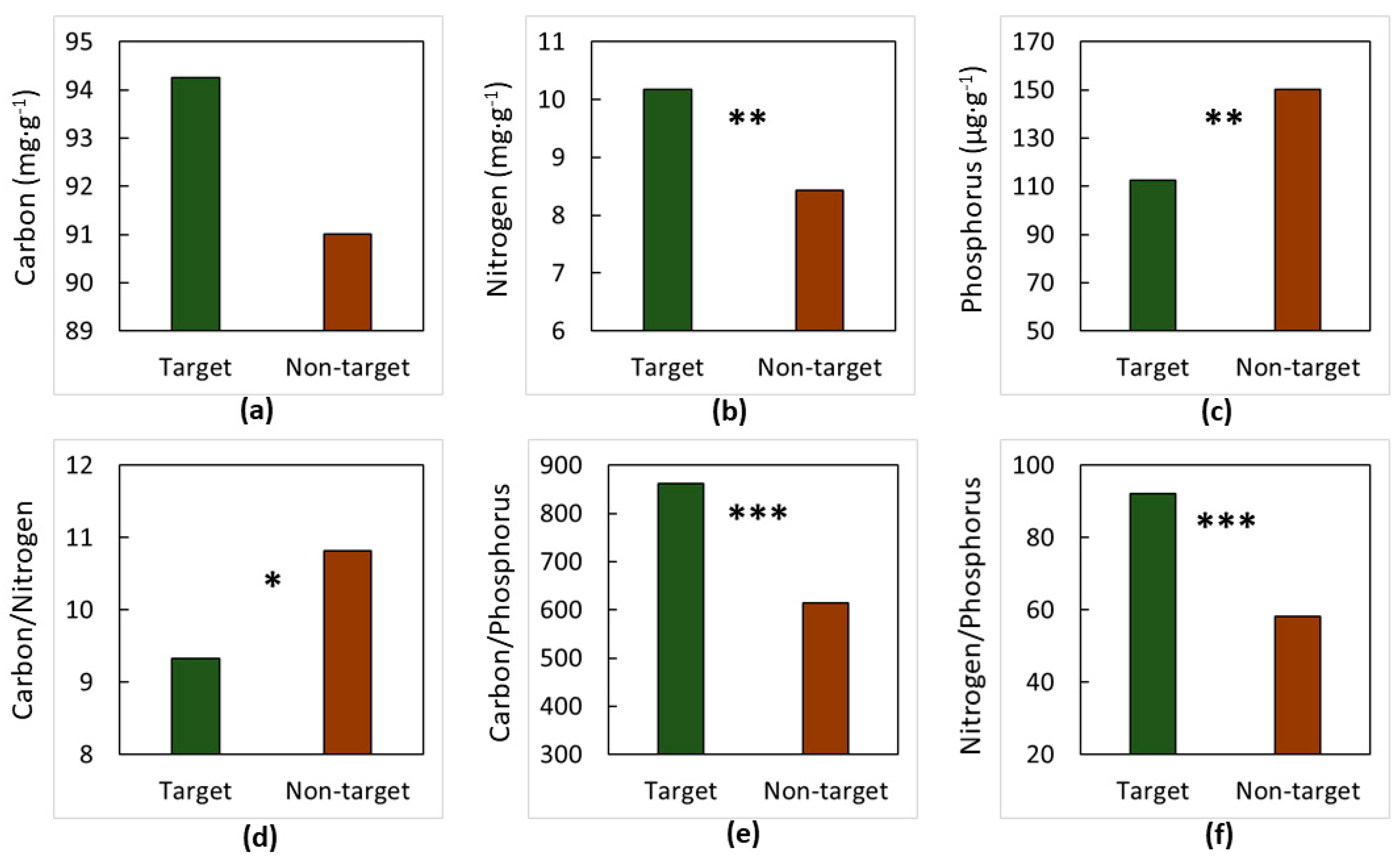
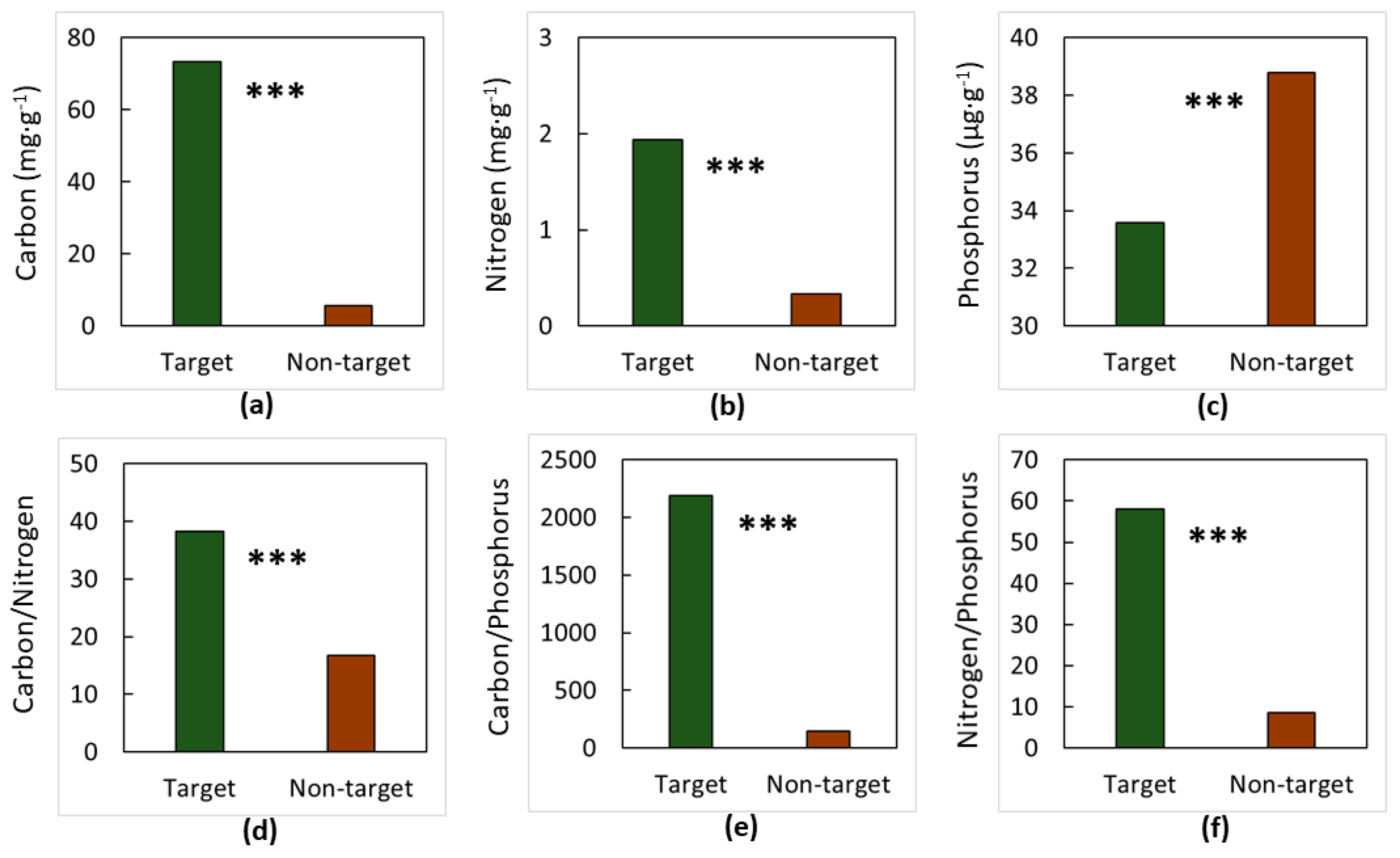
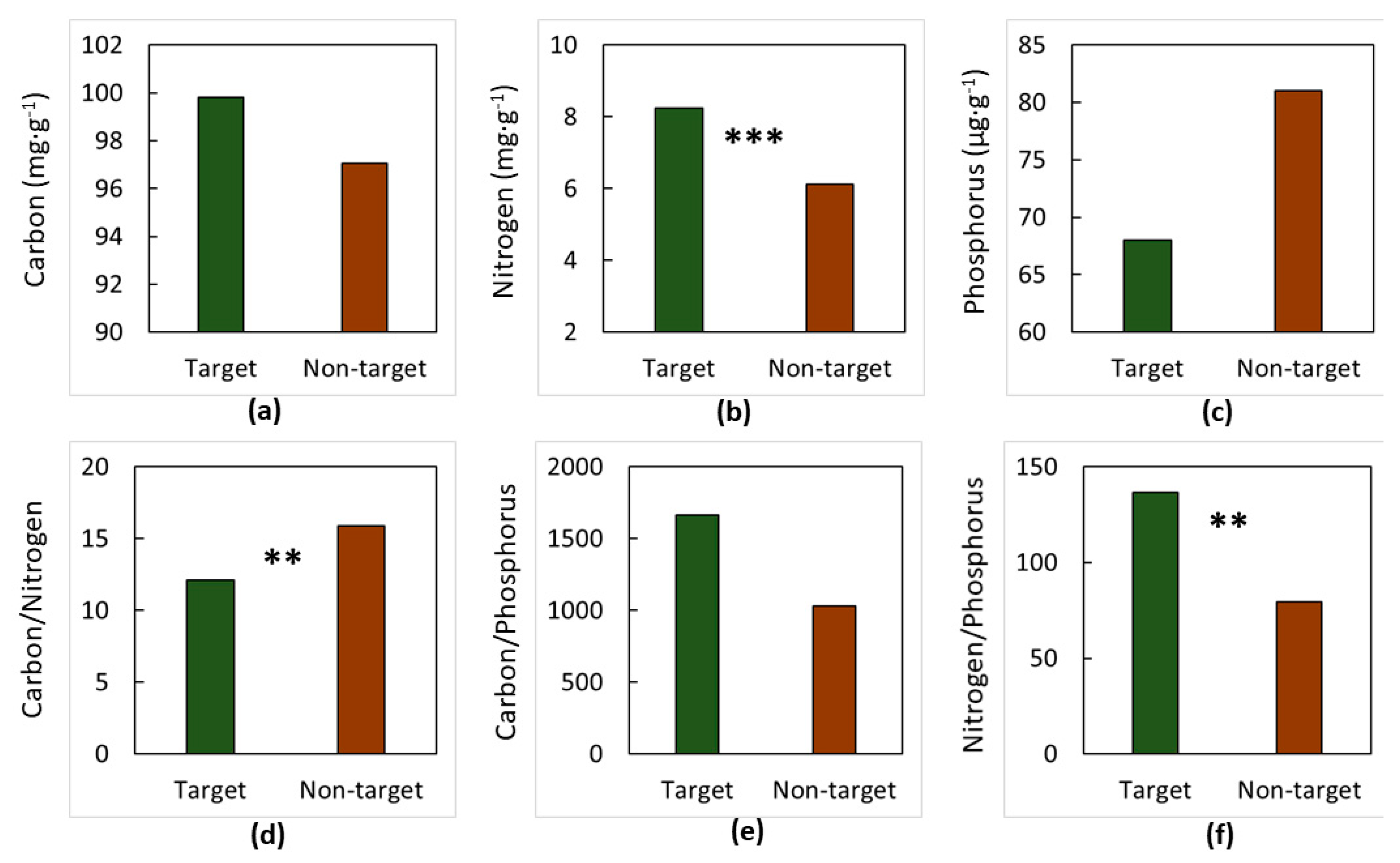
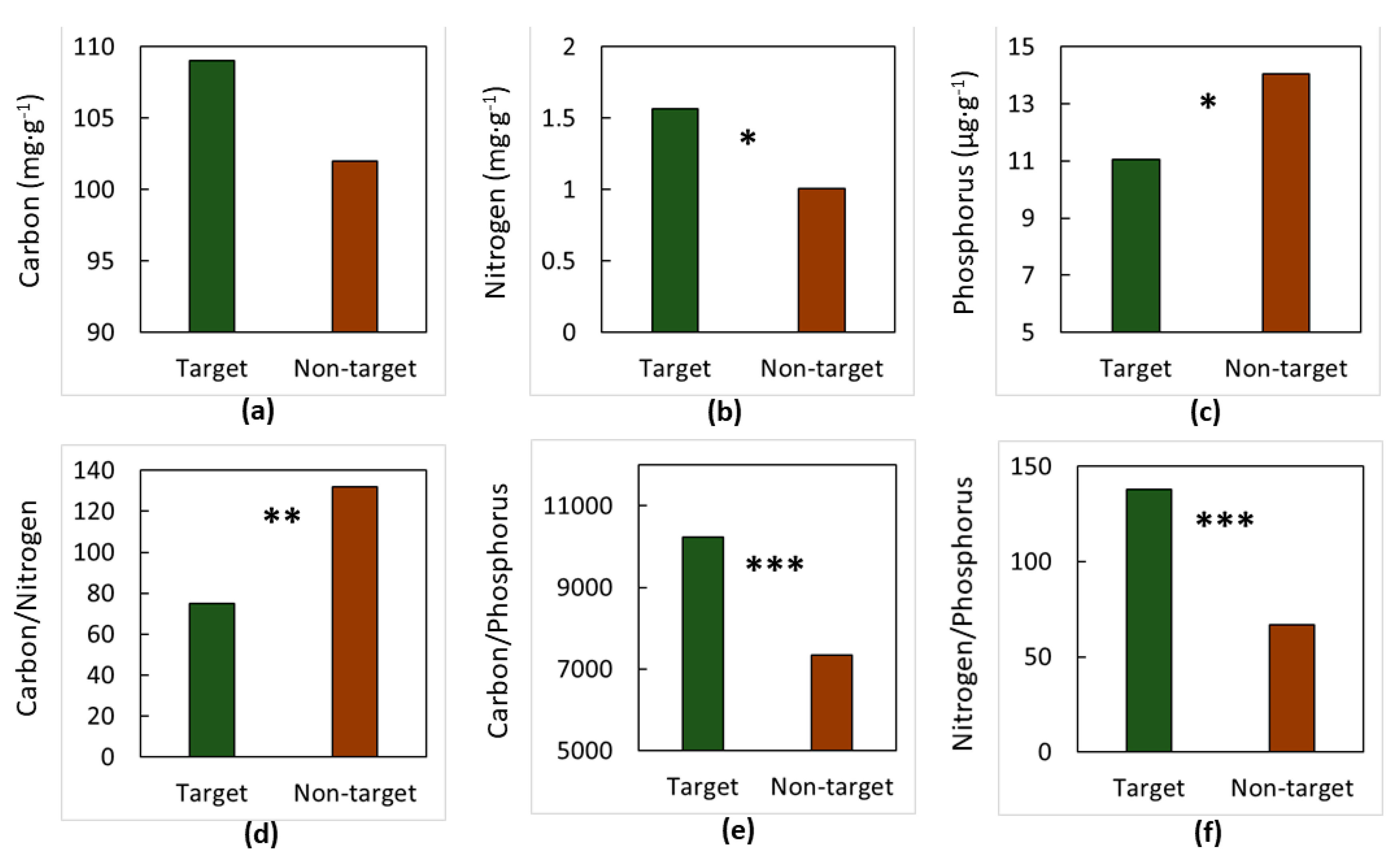
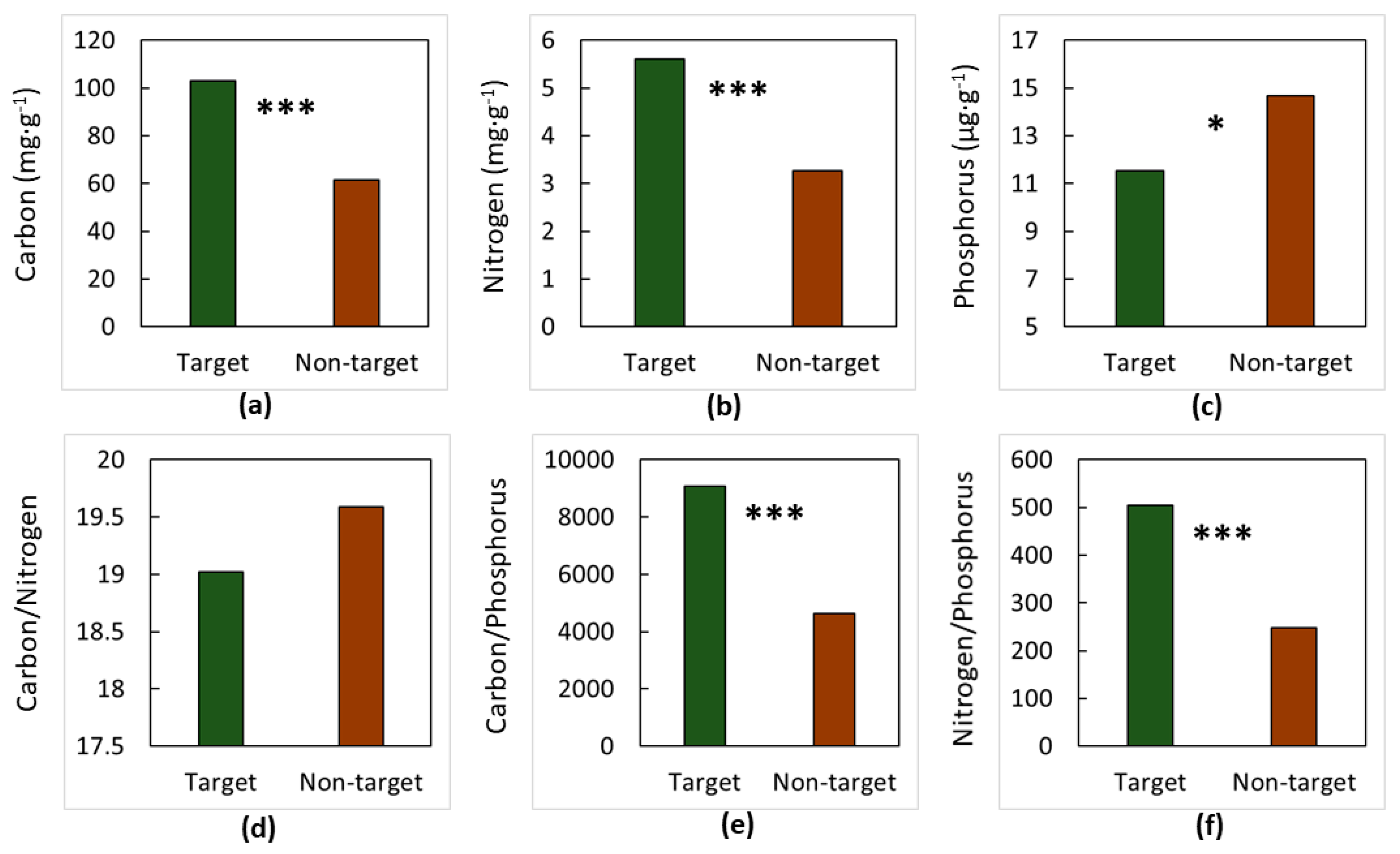
3. Results
4. Discussion
4.1. The Soils
4.2. The Elements
4.3. Informative Sub-Disciplines Literature
4.3.1. Fertile Islands
4.3.2. Plant-Soil Feedback
4.3.3. Home-Field Advantage
4.4. Synthesis
4.5. Conservation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hill, K.D. The Cycas rumphii complex (Cycadaceae) in New Guinea and the Western Pacific. Aust. Syst. Bot. 1994, 7, 543–567. [Google Scholar] [CrossRef]
- Marler, T.E. Cycad aulacaspis scale invades the Mariana Islands. Mem. N. Y. Bot. Gard. 2012, 106, 20–35. [Google Scholar]
- Marler, T.E.; Lawrence, J.H. Demography of Cycas micronesica on Guam following introduction of the armoured scale Aulacaspis yasumatsui. J. Trop. Ecol. 2012, 28, 233–242. [Google Scholar] [CrossRef]
- Marler, T.E.; Lawrence, J.H. Canopy and knowledge gaps when invasive alien insects remove foundation species. Commun. Integr. Biol. 2013, 6, e22331. [Google Scholar] [CrossRef] [PubMed]
- Donnegan, J.A.; Butler, S.L.; Grabowiecki, W.; Hiserote, B.A.; Limtiaco, D. Guam’s Forest Resources, 2002; Resource Bulletin PNW-RB-243; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2004; p. 32.
- Norstog, K.J.; Nicholls, T.J. The Biology of the Cycads; Cornell University Press: Ithica, NY, USA, 1997. [Google Scholar]
- Brenner, E.D.; Stevenson, D.W.; Twigg, R.W. Cycads: Evolutionary innovations and the role of plant-derived neurotoxins. Trends Plant Sci. 2003, 8, 446–452. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N. Three invasive insects alter Cycas micronesica leaf chemistry and predict changes in biogeochemical cycling. Commun. Integr. Biol. 2016, 9, e1208324. [Google Scholar] [CrossRef] [PubMed]
- Young, F.J. Soil Survey of Territory of Guam. U.S. Department of Agriculture, Soil Conservation Service: Washington DC, USA, 1988. [Google Scholar]
- Young, F.J. Soil Survey of the Islands of Aguijan, Rota, Saipan, and Tinian, Commonwealth of the Northern Mariana Islands; U.S. Department of Agriculture, Soil Conservation Service: Washington DC, USA, 1989.
- Smith, C.W. Soil Survey of Islands of Yap Federated States of Micronesia; Department of Agriculture, Soil Conservation Service: Washington DC, USA, 1883.
- Donnegan, J.A.; Butler, S.L.; Kuegler, O.; Hiserote, B.A. Commonwealth of the Northern Mariana Islands’ Forest Resources, 2004; Resource Bulleti PNW-RB-261; U.S. Department of Agriculture, Forest Service, Pacific Northwest Research Station: Portland, OR, USA, 2011; p. 40.
- Falanruw, M.C.; Whitesell, C.D.; Cole, T.G.; MacLean, C.D.; Ambacher, A.H. Vegetation Survey of Yap, Federated States of Micronesia; Resourc. Bull. PSW-21; U.S. Department of Agriculture, Forest Service, Pacific Southwest Forest and Range Experiment Station: Berkeley, OR, USA, 1987; p. 9.
- Marler, T.E. Pacific island tropical cyclones are more frequent and globally relevant, yet less studied. Front. Environ. Sci. 2014, 2, 42. [Google Scholar] [CrossRef]
- Berghage, R.D.; Krauskopf, D.M.; Warncke, D.D.; Widders, I. Micronutrient testing of plant growth media extractant, identification and evaluation. Commun. Soil Sci. Plant Anal. 1987, 18, 1089–1109. [Google Scholar] [CrossRef]
- Zheljazkov, V.D.; Warman, P.R. Comparison of three digestion methods for the recovery of 17 plant essential nutrients and trace elements from six composts. Compost Sci. Utiliz. 2002, 10, 197–203. [Google Scholar] [CrossRef]
- Olsen, S.R.; Cole, C.V.; Watanabe, F.S.; Dean, L.A. Estimation of Available Phosphorus in Soils by Extraction with Sodium Bicarbonate; U.S. Department of Agriculture: Washington, DC, USA, 1954; p. 939.
- Hue, N.V.; Ikawa, H.; Huang, X. Predicting soil phosphorus requirements. In Plant Nutrient Management in Hawaii’s Soils, Approaches for Tropical and Subtropical Agriculture; Silva, J.A., Uchida, R., Eds.; College of Tropical Agriculture and Human Resources, University of Hawaii at Manoa: Honolulu, HI, USA, 2000; pp. 95–99. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a test of whether one of two random variables is stochastically larger than the other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Chimphango, S.B.M.; Potgieter, G.; Cramer, M.D. Differentiation of the biogeochemical niches of legumes and non-legumes in the Cape Floristic Region of South Africa. Plant Ecol. 2015, 216, 1583–1595. [Google Scholar] [CrossRef]
- Smil, V. Cycles of Life: Civilization and the Biosphere; W.H. Freeman & Co.: New York, NY, USA, 2000. [Google Scholar]
- García-Moya, E.; Mckell, C.M. Contribution of shrubs to the nitrogen economy of a desert-wash plant community. Ecology 1970, 51, 81–88. [Google Scholar] [CrossRef]
- Garner, W.; Steinberger, Y. A proposed mechanism for the formation of ‘Fertile Islands’ in the desert ecosystem. J. Arid Environ. 1989, 16, 257–262. [Google Scholar]
- Carmargo-Ricalde, S.L.; Dhillion, S.S. Endemic Mimosa sp. serve as mycorrhizal “resource islands” within semiarid communities of the Tehuacán-Cuacatlán Valley, Mexico. Mycorrhiza 2003, 13, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Ehrenfeld, J.G.; Ravit, B.; Elgersma, K. Feedback in the plant soil system. Annu. Rev. Environ. Resour. 2005, 30, 75–115. [Google Scholar] [CrossRef]
- Van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Kardol, P.; Klironomos, J.N.; Kulmatiski, A.; Schweitzer, J.A.; et al. Plant-soil feedbacks: The past, the present and future challenges. J. Ecol. 2013, 101, 265–276. [Google Scholar] [CrossRef]
- Kulmatiski, A.; Beard, K.H.; Stevens, J.R.; Cobbold, S.M. Plant soil feedbacks: A meta-analytical review. Ecol. Lett. 2008, 11, 980–992. [Google Scholar] [CrossRef] [PubMed]
- Nsikani, M.M.; van Wilgen, B.W.; Gaertner, M. Barriers to ecosystem restoration presented by soil legacy effects of invasive alien N2-fixing woody species: Implications for ecological restoration. Restor. Ecol. 2018, 26, 235–244. [Google Scholar] [CrossRef]
- Marler, T.E.; Dongol, N. Do phytotoxic compounds in soils after scale-infested Cycas micronesica litter deposits explain reduced plant growth? HortScience 2013, 48, 1571–1573. [Google Scholar]
- Watson, G.W.; Marler, T.E. Does cycad aulacaspis scale (Aulacaspis yasumatsui, Hemiptera: Diaspididae) play a direct role in causing soil phytotoxicity? Commun. Integr. Biol. 2014, 7, e278811–e278813. [Google Scholar] [CrossRef] [PubMed]
- Veen, G.F.; Freschet, G.T.; Ordonez, A.; Wardle, D.A. Litter quality and environmental controls of home-field advantage effects on litter decomposition. Oikos 2015, 124, 187–195. [Google Scholar] [CrossRef]
- Pelletier, B.; Fyles, J.W.; Dutilleul, P. Tree species control and spatial structure of forest floor properties in a mixed-species stand. Ecoscience 1999, 6, 79–91. [Google Scholar] [CrossRef]
- Tian, J.; He, N.; Hale, L.; Niu, S.; Yu, G.; Liu, Y.; Blagodatskaya, E.; Kuzyakov, Y.; Gao, Q.; Zhou, J. Soil organic matter availability and climate drive latitudinal patterns in bacterial diversity from tropical to cold temperate forests. Funct. Ecol. 2018, 32, 61–70. [Google Scholar] [CrossRef]
- Meunier, C.L.; Gundale, M.J.; Sanchez, I.S.; Liess, A. Impact of nitrogen deposition on forest and lake food webs in nitrogen-limited environments. Glob. Chang. Biol. 2016, 22, 164–179. [Google Scholar] [CrossRef] [PubMed]
- Schmeller, D.S.; Böhm, M.; Arvanitidis, C.; Barber-Meyer, S.; Brummitt, N.; Chandler, M.; Eva, C.; Mark, J.C.; Hui, D.; Jaime, G.-M.; et al. Building capacity in biodiversity monitoring at the global scale. Biodiv. Cons. 2017, 26, 2765–2790. [Google Scholar] [CrossRef]
- Borges, P.A.V.; Cardoso, P.; Kreft, H.; Whittaker, R.J.; Fattorini, S.; Emerson, B.C.; Artur, G.; Rosemary, G.G.; Thomas, J.M.; Santos, A.M.C.; et al. Global island monitoring scheme (GIMS): A proposal for the long-term coordinated survey and monitoring of native island forest biota. Biodiv. Cons. 2018, 27, 2567–2586. [Google Scholar] [CrossRef]
- Marschner, H. Marschner’s Mineral Nutrition of Higher Plants, 3rd ed.; Academic Press: Waltham, MA, USA, 2011. [Google Scholar]
- Neugebauer, K.; Broadley, M.R.; El-Serehy, H.A.; George, T.S.; McNicol, J.W.; Moraes, M.F.; White, P.J. Variation in the angiosperm ionome. Physiol. Plantarum 2018, 163, 306–322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grove, T.S.; O’Connell, A.M.; Malajczuk, N. Effects of fire on the growth, nutrient content and rate of nitrogen fixation of the cycad Macrozamia riedlei. Aust. J. Bot. 1980, 28, 271–281. [Google Scholar] [CrossRef]
- Watanabe, T.; Broadley, M.R.; Jansen, S.; White, P.J.; Takada, J.; Satake, K.; Takamatsu, T.; Tuah, S.J.; Osaki, M. Evolutionary control of leaf element composition in plants. New Phytol. 2007, 174, 516–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krieg, C.; Watkins, J.E.; Chambers, S.; Husby, C.E. Sex-specific differences in functional traits and resource acquisition in five cycad species. AoB PLANTS 2017, 9, plx013. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-J.; Sack, L.; Cao, K.-F.; Wei, X.-W.; Li, N. Speed versus endurance tradeoff in plants: Leaves with higher photosynthetic rates show stronger seasonal declines. Sci. Rep. 2017, 7, 42085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Cao, K.; Sack, L.; Li, N.; Wei, X.; Goldstein, G. Extending the generality of leaf economic design principles in the cycads, an ancient lineage. New Phytol. 2015, 206, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Álvarez-Yépiz, J.C.; Cueva, A.; Dovčiak, M.; Teece, M.; Yepez, E.A. Ontogenetic resource-use strategies in a rare long-lived cycad along environmental gradients. Conserv. Physiol. 2014, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marler, T.E.; Ferreras, U.F. Disruption of Leaf Nutrient Remobilization in Coastal Cycas Trees by Tropical Cyclone Damage. J. Geogr. Nat. Disast. 2015, 5, 142. [Google Scholar] [CrossRef]
- Marler, T.E.; Ferreras, U.F. Current status, threats and conservation needs of the endemic Cycas wadei Merrill. J. Biodivers. Endanger. Species 2017, 5, 193. [Google Scholar] [CrossRef]
- Brummitt, N.A.; Bachman, S.P.; Griffiths-Lee, J.; Lutz, M.; Moat, J.F.; Farjon, A.; Donaldson, J.S.; Hilton-Taylor, C.; Meagher, T.R.; Albuquerque, S.; et al. Green plants in the red: A baseline global assessment for the IUCN sampled Red List Index for plants. PLoS ONE 2015, 10, e0135152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fragniere, Y.; Bétrisey, S.; Cardinaux, L.; Stoffel, M.; Kozlowski, G. Fighting their last stand? A global analysis of the distribution and conservation status of gymnosperms. J. Biogeogr. 2015, 42, 809–820. [Google Scholar] [CrossRef] [Green Version]
- Marler, P.N.; Marler, T.E. An assessment of Red List data for the Cycadales. Trop. Conserv. Sci. 2015, 8, 1114–1125. [Google Scholar] [CrossRef]

| Soil trait | Target | Non-Target | Statistic 1 | Significance |
|---|---|---|---|---|
| pH | 7.4 ± 0.1 | 7.3 ± 0.1 | t = 0.40 | 0.697 |
| Potassium 2 | 342.7 ± 28.8 | 469.6 ± 58.3 | t = 1.95 | 0.064 |
| Calcium 2 | 10,349.5 ± 625.7 | 10,133.3 ± 676.0 | t = 0.24 | 0.817 |
| Magnesium 2 | 881.8 ± 27.2 | 980.8 ± 40.0 | t = 2.28 | 0.033 |
| Manganese 2 | 55.1 ± 4.3 | 60.5 ± 5.9 | t = 0.60 | 0.552 |
| Iron 2 | 36.1 ± 2.5 | 39.4 ± 2.1 | t = 0.99 | 0.330 |
| Cadmium 3 | 6.2 ± 0.3 | 5.6 ± 0.2 | t = 0.95 | 0.353 |
| Cobalt 3 | 20.3 ± 1.2 | 21.0 ± 1.7 | t = 0.35 | 0.728 |
| Chromium 3 | 102.4 ± 9.0 | 96.9 ± 9.3 | t = 0.42 | 0.682 |
| Copper 3 | 77.4 ± 3.2 | 82.6 ± 5.9 | t = 0.77 | 0.448 |
| Nickel 3 | 17.5 ± 1.1 | 16.0 ± 1.2 | t = 0.91 | 0.371 |
| Lead 3 | 3.1 ± 2.1 | 3.0 ± 2.0 | U = 72 | 0.976 |
| Selenium 3 | 0.5 ± 0.2 | 0.3 ± 0.1 | U = 63 | 0.603 |
| Zinc 3 | 105.8 ± 8.7 | 109.4 ± 8.6 | t = 0.29 | 0.774 |
| Soil Trait | Target | Non-Target | Statistic 1 | Significance |
|---|---|---|---|---|
| pH | 6.8 ± 0.0 | 6.3 ± 0.1 | t = 5.54 | <0.001 |
| Potassium 2 | 1778.0 ± 21.1 | 1918.3 ± 36.4 | t = 3.34 | 0.003 |
| Calcium 2 | 7957.7 ± 67.5 | 5846.5 ± 78.4 | t = 20.42 | <0.001 |
| Magnesium 2 | 2945.9 ± 15.3 | 3293.3 ± 115.2 | t = 2.99 | 0.007 |
| Manganese 2 | 31.8 ± 1.8 | 55.1 ± 1.2 | t = 10.87 | <0.001 |
| Iron 2 | 49.9 ± 1.6 | 95.9 ± 2.1 | t = 17.37 | <0.001 |
| Cobalt 3 | 23.5 ± 1.0 | 17.0 ± 1.0 | t = 5.81 | <0.001 |
| Chromium 3 | 10.9 ± 0.8 | 12.0 ± 0.8 | t = 1.05 | 0.304 |
| Copper 3 | 78.3 ± 1.8 | 80.0 ± 3.8 | t = 0.41 | 0.344 |
| Nickel 3 | 9.3 ± 0.7 | 7.3 ± 0.9 | t = 1.74 | 0.096 |
| Selenium 3 | 0.2 ± 0.0 | 0.1 ± 0.0 | U = 29 | 0.014 |
| Zinc 3 | 60.6 ± 0.9 | 98.2 ± 2.4 | t = 14.48 | <0.001 |
| Soil Trait | Target | Non-Target | Statistic 1 | Significance |
|---|---|---|---|---|
| pH | 7.5 ± 0.1 | 7.4 ± 0.1 | t = 0.32 | 0.752 |
| Potassium 2 | 298.3 ± 19.8 | 3551 ± 28.1 | t = 1.64 | 0.114 |
| Calcium 2 | 8983.2 ± 666.3 | 8632.8 ± 638.3 | t = 0.38 | 0.708 |
| Magnesium 2 | 758.7 ± 31.0 | 827.2 ± 43.3 | t = 1.28 | 0.212 |
| Manganese 2 | 61.5 ± 6.0 | 79.7 ± 9.4 | t = 1.63 | 0.118 |
| Iron 2 | 46.2 ± 3.1 | 54.0 ± 5.1 | t = 1.33 | 0.197 |
| Cadmium 3 | 5.8 ± 0.4 | 5.7 ± 0.4 | t = 0.23 | 0.821 |
| Cobalt 3 | 20.6 ± 1.5 | 21.4 ± 1.5 | t = 0.42 | 0.676 |
| Chromium 3 | 105.0 ± 11.6 | 105.2 ± 10.7 | t = 0.01 | 0.992 |
| Copper 3 | 7500 ± 2.4 | 81.2 ± 4.00 | t = 1.33 | 0.196 |
| Nickel 3 | 19.3 ± 2.0 | 18.3 ± 1.5 | t = 0.41 | 0.684 |
| Lead 3 | 3.4 ± 3.4 | 9.6 ± 7.6 | U = 72 | 0.999 |
| Selenium 3 | 0.4 ± 0.2 | 0.3 ± 0.2 | U = 72 | 0.976 |
| Zinc 3 | 88.9 ± 6.8 | 90.7 ± 5.9 | t = 0.19 | 0.850 |
| Soil Trait | Target | Non-Target | Statistic 1 | Significance |
|---|---|---|---|---|
| pH | 7.9 ± 0.0 | 8.0 ± 0.0 | t = 2.43 | 0.024 |
| Potassium 2 | 21.1 ± 3.1 | 38.9 ± 6.0 | t = 2.64 | 0.015 |
| Calcium 2 | 9525.5 ± 222.8 | 9044.4 ± 235.6 | t = 1.18 | 0.253 |
| Magnesium 2 | 470.2 ± 30.9 | 404.8 ± 19.1 | t = 1.80 | 0.086 |
| Manganese 2 | 18.5 ± 0.8 | 17.7 ± 1.3 | t = 1.54 | 0.138 |
| Iron 2 | 5.7 ± 0.4 | 6.9 ± 0.3 | t = 2.22 | 0.037 |
| Arsenic 3 | 0.3 ± 0.0 | 0.2 ± 0.1 | t = 1.09 | 0.287 |
| Cadmium 3 | 0.1 ± 0.0.0 | 0.1 ± 0.0 | t = 4.01 | 0.001 |
| Cobalt 3 | 0.2 ± 0.0 | 0.1 ± 0.0 | t = 5.60 | <0.001 |
| Copper 3 | 0.4 ± 0.0 | 0.3 ± 0.0 | t = 2.41 | 0.025 |
| Chromium 3 | 3.9 ± 0.3 | 2.4 ± 0.1 | t = 4.73 | <0.001 |
| Lead 3 | 0.1 ± 0.0 | 0.4 ± 0.1 | t = 3.72 | 0.001 |
| Selenium 3 | 3.5 ± 0.0 | 3.0 ± 0.0 | t = 13.82 | <0.001 |
| Zinc 3 | 10.6 ± 1.5 | 7.5 ± 1.1 | t = 1.73 | 0.097 |
| Soil Trait | Target | Non-Target | Statistic 1 | Significance |
|---|---|---|---|---|
| pH | 5.9 ± 0.2 | 5.7 ± 0.1 | t = 2.03 | 0.055 |
| Potassium 2 | 91.3 ± 10.0 | 120.5 ± 22.4 | t = 2.91 | 0.043 |
| Calcium 2 | 2012.5 ± 417.5 | 1212.2 ± 174.2 | t = 4.33 | <0.001 |
| Magnesium 2 | 1031.0 ± 214.1 | 1367.1 ± 203.1 | t = 2.28 | 0.033 |
| Manganese 2 | 11.7 ± 2.3 | 14.2 ± 2.8 | t = 2.79 | 0.011 |
| Iron 2 | 329.2 ± 72.4 | 359.7 ± 41.8 | t = 0.89 | 0.381 |
| Cobalt 3 | 31.5 ± 3.0 | 26.2 ± 2.2 | t = 3.41 | 0.003 |
| Chromium 3 | 521.6 ± 28.3 | 545.8 ± 75.5 | t = 0.74 | 0.469 |
| Copper 3 | 38.2 ± 4.5 | 49.8 ± 6.9 | t = 3.44 | 0.002 |
| Nickel 3 | 231.1 ± 14.6 | 244.5 ± 28.9 | t = 1.02 | 0.321 |
| Selenium 3 | 0.5 ± 0.3 | 0.5 ± 0.5 | U = 60 | 0.509 |
| Zinc 3 | 43.8 ± 12.1 | 41.7 ± 6.2 | t = 0.37 | 0.713 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marler, T.E.; Krishnapillai, M.V. Cycas micronesica Trees Alter Local Soil Traits. Forests 2018, 9, 565. https://doi.org/10.3390/f9090565
Marler TE, Krishnapillai MV. Cycas micronesica Trees Alter Local Soil Traits. Forests. 2018; 9(9):565. https://doi.org/10.3390/f9090565
Chicago/Turabian StyleMarler, Thomas E., and Murukesan V. Krishnapillai. 2018. "Cycas micronesica Trees Alter Local Soil Traits" Forests 9, no. 9: 565. https://doi.org/10.3390/f9090565
APA StyleMarler, T. E., & Krishnapillai, M. V. (2018). Cycas micronesica Trees Alter Local Soil Traits. Forests, 9(9), 565. https://doi.org/10.3390/f9090565





