Subwatershed-Level Lodgepole Pine Attributes Associated with a Mountain Pine Beetle Outbreak
Abstract
:1. Introduction
2. Materials and Methods
3. Results
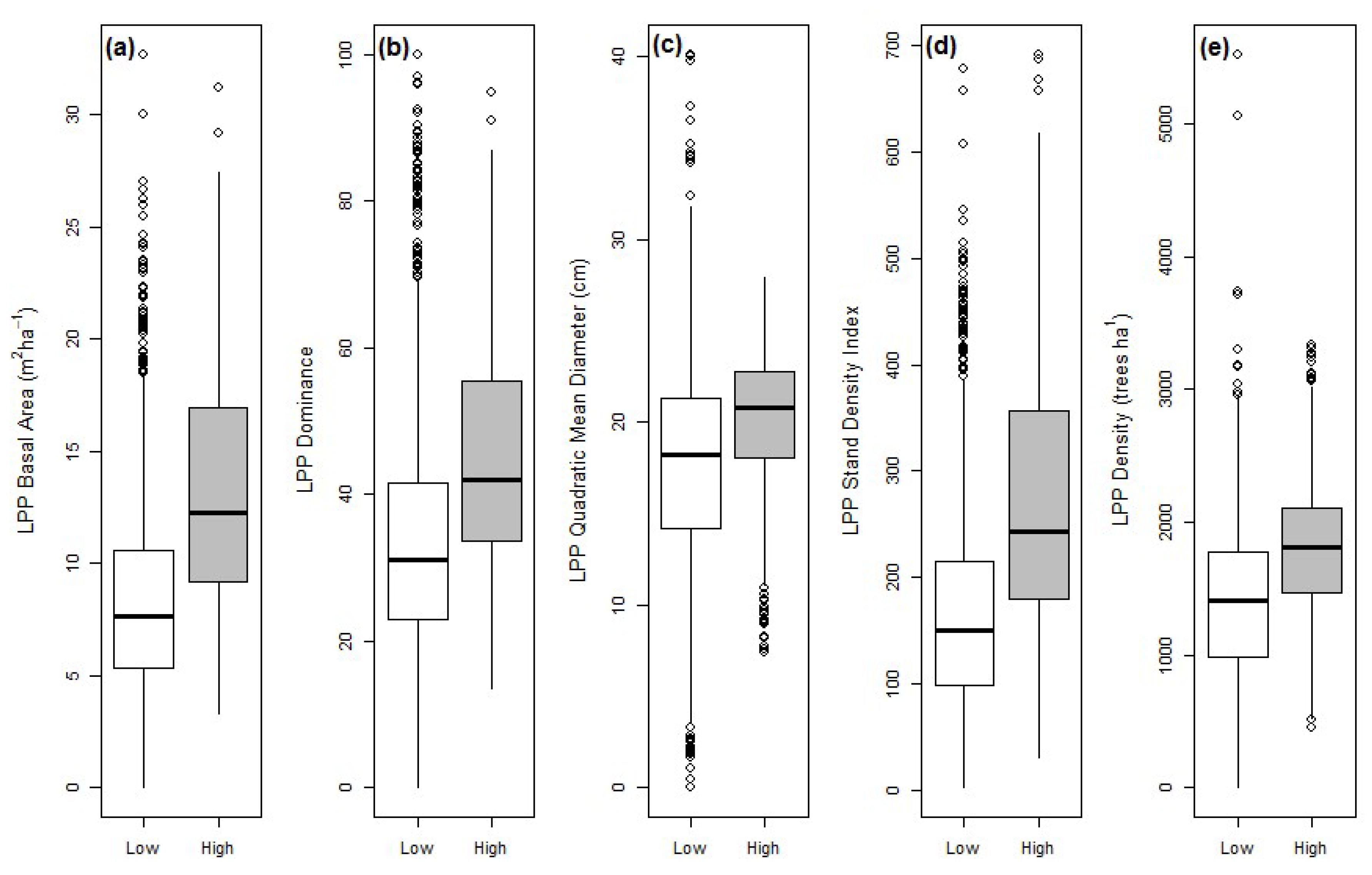
3.1. LPP Forest Attributes during the 2000s Outbreak
3.2. Subwatershed MPB Attack Severity during the 2000s Outbreak
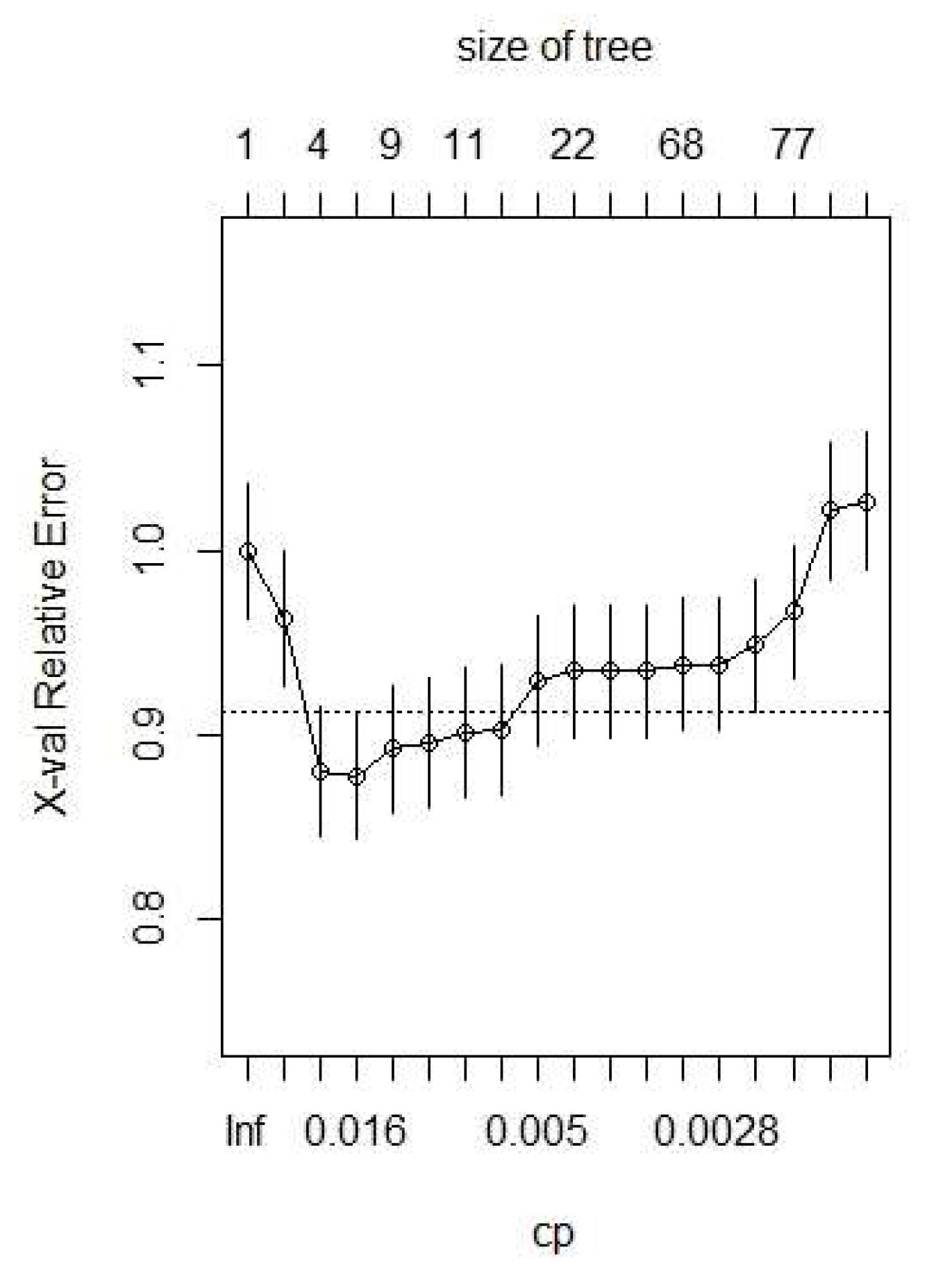
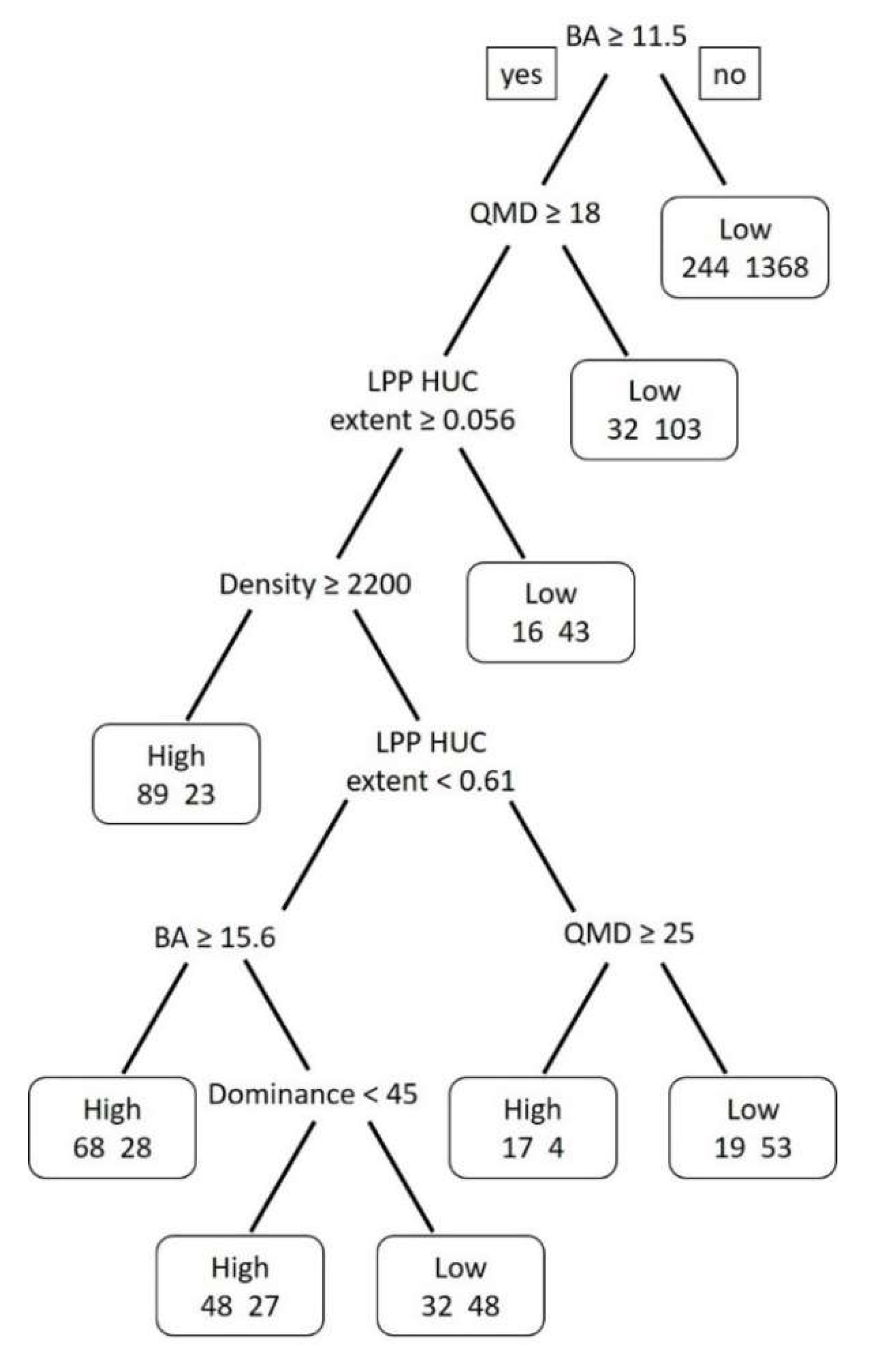
3.3. Modeling Results
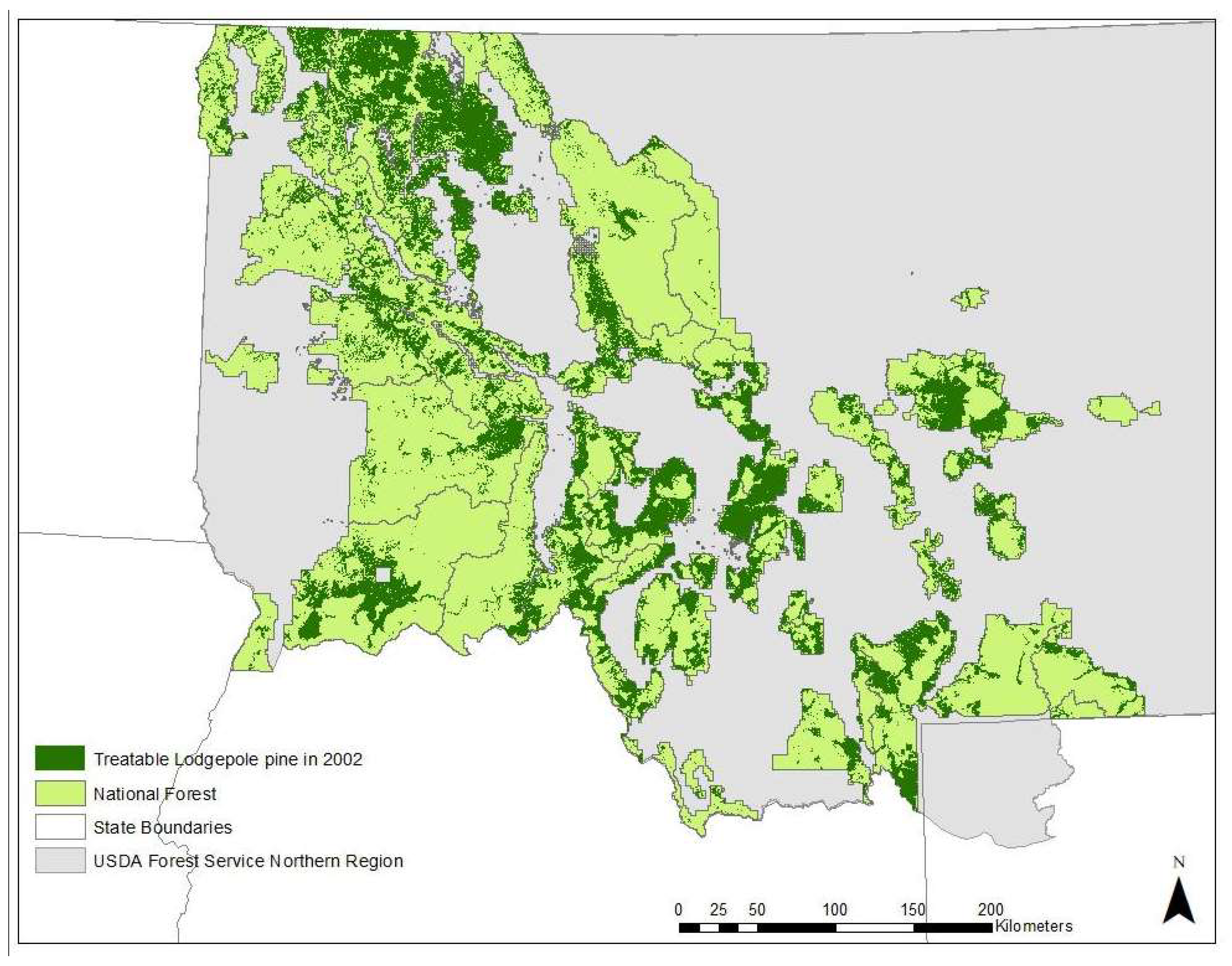
3.4. Potential for Treatment
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Appendix A
| Classification Tree Model | Data Splits | Cross Validation Error | Cost Complexity | Misclassification Rate |
|---|---|---|---|---|
| 1 a | 118 | 1.03 | 0.001 | 0.08 |
| 2 b | 8 | 0.85 | 0.01 | 0.18 |
| 3 | 4 | 0.87 | 0.014 | 0.18 |
| 4 | 3 | 0.88 | 0.018 | 0.19 |
| 5 c | 2 | 0.96 | 0.048 | 0.22 |
| National Forest | Total LPP | Restricted LPP | Treatable LPP |
|---|---|---|---|
| Beaverhead | 274,115 | 187,146 (68%) | 86,969 (32%) |
| Bitterroot | 90,592 | 65,545 (72%) | 25,047 (28%) |
| Clearwater | 45,986 | 39,976 (87%) | 6010 (13%) |
| Coeur d’Alene | 4220 | 1332 (32%) | 2888 (68%) |
| Custer | 20,113 | 12,908 (64%) | 7205 (36%) |
| Deerlodge | 232,002 | 80,540 (35%) | 151,462 (65%) |
| Flathead | 75,886 | 45,335 (60%) | 30,551 (40%) |
| Gallatin | 122,973 | 69,296 (56%) | 53,677 (44%) |
| Helena | 109,411 | 65,180 (60%) | 44,231 (40%) |
| Kaniksu | 24,022 | 11,651 (49%) | 12,371 (51%) |
| Kootenai | 72,584 | 17,437 (24%) | 55,147 (76%) |
| Lewis & Clark | 161,003 | 101,299 (63%) | 59,704 (37%) |
| Lolo | 102,969 | 57,124 (55%) | 45,845 (45%) |
| Nez Perce | 96,497 | 69,224 (72%) | 27,273 (28%) |
| St. Joe | 14,863 | 9577 (64%) | 5286 (36%) |
| Northern Region Total | 1,447,236 | 833,570 (58%) | 613,666 (42%) |
References
- Raffa, K.F.; Aukema, B.H.; Bentz, B.J.; Carroll, A.L.; Hicke, J.A.; Turner, M.G.; Romme, W.H. Cross-scale drivers of natural disturbances prone to anthropogenic amplification: The dynamics of bark beetle eruptions. Bioscience 2008, 58, 501–517. [Google Scholar] [CrossRef]
- Berryman, A.A.; Raffa, K.R.; Millstein, J.A.; Stenseth, N.C. Interaction dynamics of bark beetle aggregation and conifer defense rates. Oikos 1989, 56, 256–263. [Google Scholar] [CrossRef]
- Graham, R.T.; Asherin, L.A.; Battaglia, M.A.; Jain, T.; Mata, S.A. Mountain Pine Beetles: A Century of Knowledge, Control Attempts, and Impacts Central to the Black Hills; General Technical Report. RMRS-GTR-353; US Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2016; p. 193. [Google Scholar]
- Creeden, E.P.; Hicke, J.A.; Buotte, P.C. Climate, weather, and recent mountain pine beetle outbreaks in the western United States. For. Ecol. Manag. 2014, 312, 239–251. [Google Scholar] [CrossRef]
- Aukema, B.H.; Carroll, A.L.; Zheng, Y.; Zhu, J.; Raffa, K.F.; Dan Moore, R.; Stahl, K.; Taylor, S.W. Movement of outbreak populations of mountain pine beetle: Influences of spatiotemporal patterns and climate. Ecography 2008, 31, 348–358. [Google Scholar] [CrossRef]
- Preisler, H.K.; Hicke, J.A.; Ager, A.A.; Hayes, J.L. Climate and weather influences on spatial temporal patterns of mountain pine beetle populations in Washington and Oregon. Ecology 2012, 93, 2421–2434. [Google Scholar] [CrossRef] [PubMed]
- Sambaraju, K.R.; Carroll, A.L.; Zhu, J.; Stahl, K.; Moore, R.D.; Aukema, B.H. Climate change could alter the distribution of mountain pine beetle outbreaks in western Canada. Ecography 2012, 35, 211–223. [Google Scholar] [CrossRef]
- Nelson, K.; Rocca, M.; Diskin, M.; Aoki, C.; Romme, W. Predictors of bark beetle activity and scale-dependent spatial heterogeneity change during the course of an outbreak in a subalpine forest. Landsc. Ecol. 2014, 29, 97–109. [Google Scholar] [CrossRef]
- Kashian, D.M.; Jackson, R.M.; Lyons, H.D. Forest structure altered by mountain pine beetle outbreaks affects subsequent attack in a Wyoming lodgepole pine forest, USA. Can. J. For. Res. 2011, 41, 2403–2412. [Google Scholar] [CrossRef]
- Simard, M.; Powell, E.N.; Raffa, K.F.; Turner, M.G. What explains landscape patterns of tree mortality caused by bark beetle outbreaks in greater Yellowstone? Glob. Ecol. Biogeogr. 2012, 21, 556–567. [Google Scholar] [CrossRef]
- Klutsch, J.G.; Negron, J.F.; Costello, S.L.; Rhoades, C.C.; West, D.R.; Popp, J.; Caissie, R. Stand characteristics and downed woody debris accumulations associated with a mountain pine beetle (Dendroctonus ponderosae Hopkins) outbreak in Colorado. For. Ecol. Manag. 2009, 258, 641–649. [Google Scholar] [CrossRef]
- Taylor, S.W.; Carroll, A.L.; Alfaro, R.I.; Safranyik, L. Forest, climate and mountain pine beetle outbreak dynamics in western Canada. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Canadian Forest Service: Victoria, BC, Canada, 2006; pp. 67–94. [Google Scholar]
- Meddens, A.J.H.; Hicke, J.A.; Ferguson, C.A. Spatiotemporal patterns of observed bark beetle-caused tree mortality in British Columbia and the western United States. Ecol. Appl. 2012, 22, 1876–1891. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Barclay, H.J.; Hawkes, B.C.; Taylor, S. Lodgepole pine forest age class dynamics and susceptibility to mountain pine beetle attack. Ecol. Complex. 2005, 2, 232–239. [Google Scholar] [CrossRef]
- Safranyik, L. Effects of climate and weather on mountain pine beetle populations. In Theory and Practice of Mountain Pine Beetle Management in Lodgepole Pine Forests; Symposium Proceedings, Berryman, A.A., Amman, G.D., Stark, R.W., Eds.; University of Idaho: Moscow, ID, USA, 1978; pp. 74–84. [Google Scholar]
- Mitchell, R.G.; Preisler, H.K. Analysis of spatial patterns of lodgepole pine attacked by outbreak populations of the mountain pine beetle. For. Sci. 1991, 37, 1390–1408. [Google Scholar]
- Kolb, T.E.; Fettig, C.J.; Ayres, M.P.; Bentz, B.J.; Hicke, J.A.; Mathiasen, R.; Stewart, J.E.; Weed, A.S. Observed and anticipated impacts of drought on forest insects and diseases in the United States. For. Ecol. Manag. 2016, 380, 321–334. [Google Scholar] [CrossRef]
- Chapman, T.B.; Veblen, T.T.; Schoennagel, T. Spatiotemporal patterns of mountain pine beetle activity in the southern Rocky Mountains. Ecology 2012, 93, 2175–2185. [Google Scholar] [CrossRef] [PubMed]
- Safranyik, L.; Carroll, A.L. The biology and epidemiology of the mountain pine beetle in lodgepole pine forests. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2006; pp. 3–66. [Google Scholar]
- Jakubauskas, M.E.; Price, K.P. Empirical relationships between structural and spectral factors of Yellowstone lodgepole pine forests. Photogramm. Eng. Remote Sens. 1997, 63, 1375–1380. [Google Scholar]
- Roe, A.L.; Amman, G.D. Mountain pine beetle in lodgepole pine forests RP-INT-71; US Department of Agriculture, Forest Service, Intermountain Forest and Range Experiment Station: Ogden, UT, USA, 1970; p. 23. [Google Scholar]
- Cole, W.E.; Amman, G.D. Mountain Pine Beetle Infestations in Relation to Lodgepole Pine Diameters; Research Note INT-95; US Department of Agriculture, Forest Service, Intermountain Forest & Range Experiment Station: Ogden, UT, USA, 1969; p. 7. [Google Scholar]
- Mitchell, R.; Waring, R.H.; Pitman, G. Thinning lodgepole pine increases tree vigor and resistance to mountain pine beetle. For. Sci. 1983, 29, 204–211. [Google Scholar]
- Whitehead, R.J.; Russo, G.L. “Beetle-Proofed” Lodgepole Pine Stands in Interior British Columbia have Less Damage from Mountain Pine Beetle; Information Report BC-X-402; Natural Resources Canada. Canadian Forest Service: Victoria, BC, Canada, 2005. [Google Scholar]
- Negrón, J.F.; Allen, K.K.; Ambourn, A.; Cook, B.; Marchand, K. Large-scale thinnings, ponderosa pine, and mountain pine beetle in the Black Hills, USA. For. Sci. 2017, 63, 529–536. [Google Scholar] [CrossRef]
- Taylor, S.; Carroll, A. Disturbance, forest age, and mountain pine beetle outbreak dynamics. In Mountain Pine Beetle Symposium: Challenges and Solutions; Shore, T.L., Brooks, J.E., Stone, J.E., Eds.; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2004; pp. 41–51. [Google Scholar]
- Hicke, J.A.; Jenkins, J.C. Mapping lodgepole pine stand structure susceptibility to mountain pine beetle attack across the western United States. For. Ecol. Manag. 2008, 255, 1536–1547. [Google Scholar] [CrossRef]
- Krist, F.J.; Ellenwood, J.R.; Woods, M.E.; McMahan, A.; Cowardin, J.; Ryerson, D.; Saplo, F.J.; Zwelfler, M.O.; Romero, S. 2013–2027 National Insect and Disease Forest Risk Assessment; US Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Fort Collins, CO, USA, 2014; p. 209. [Google Scholar]
- McConnell, T.J.; Johnson, E.W.; Burns, B. A Guide to Conducting Aerial Sketchmapping Surveys; US Department of Agriculture, Forest Service, Forest Health Technology Enterprise Team: Fort Collins, CO, USA, 2000; p. 88. [Google Scholar]
- Williams, H.L.; Hood, S.M.; Keyes, C.R.; Egan, J.M. Mountain Pine Beetle Attack Severity Data in Lodgepole Pine in the Northern Rockies from 1999–2014; Forest Service Research Data Archive: Fort Collins, CO, USA, 2018. [CrossRef]
- National Oceanic and Atmospheric Administration, Narrative Summaries, Tables and Maps for Each State with Overview of State Climatologist Programs, Third Edition; Volume 1: Alabama-New Mexico; Volume 2: New York-Wyoming. 1985. Available online: https://wrcc.dri.edu/Climate/narratives.php (accessed on 28 August 2018).
- Montana Natural Resources Conservation Service State Office. Watershed Boundary Data; Montana Natural Resources Conservation Service State Office: Bozeman, MT, USA, 2006.
- Reineke, L.H. Perfecting a stand-density index for even-aged forests. J. Agric. Res. 1933, 46, 627–638. [Google Scholar]
- Egan, J.M.; Jenne, J.; Stasey, A. Techniques to Enhance Assessment and Reporting of Pest Damages Estimated with Aerial Detection Surveys; US Department of Agriculture, Forest Service, Northern Region, Forest Health Protection: Missoula, MT, USA, 2018; p. 35. [Google Scholar]
- Breiman, L.; Friedman, J.; Stone, C.J.; Olshen, R.A. Classification and Regression Trees; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 1984. [Google Scholar]
- De’Ath, G.; Fabricius, K.E. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology 2000, 81, 3178–3192. [Google Scholar] [CrossRef]
- Evans, J.S.; Cushman, S.A. Gradient modeling of conifer species using random forests. Landsc. Ecol. 2009, 24, 673–683. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing, 3.2.3 ed.; R Foundation for Statistical Computing: Vienna, Austria, 2015. [Google Scholar]
- Therneau, T.; Atkinson, B.; Ripley, B. Rpart: Recursive partitioning and regression trees, R package version 4.1–10. 2015. Available online: https://cran.r-project.org/web/packages/rpart/index.html (accessed on 28 August 2018).
- Fettig, C.J.; Klepzig, K.D.; Billings, R.F.; Munson, A.S.; Nebeker, T.E.; Negrón, J.F.; Nowak, J.T. The effectiveness of vegetation management practices for prevention and control of bark beetle infestations in coniferous forests of the western and southern United States. For. Ecol. Manag. 2007, 238, 24–53. [Google Scholar] [CrossRef]
- Björklund, N.; Lindgren, B.S. Diameter of lodgepole pine and mortality caused by the mountain pine beetle: Factors that influence their relationship and applicability for susceptibility rating. Can. J. For. Res. 2009, 39, 908–916. [Google Scholar] [CrossRef]
- Robertson, C.; Wulder, M.; Nelson, T.; White, J. Risk rating for mountain pine beetle infestation of lodgepole pine forests over large areas with ordinal regression modelling. For. Ecol. Manag. 2008, 256, 900–912. [Google Scholar] [CrossRef]
- Shore, T.L.; Safranyik, L. Susceptibility and Risk Rating Systems for the Mountain Pine Beetle in Lodgepole Pine Stands; Information Report BC-X-336; Forestry Canada, Pacific Forestry Centre: Victoria, BC, Canada, 1992; p. 12. [Google Scholar]
- Nelson, W.A.; Potapov, A.; Lewis, M.A.; Hundsdörfer, A.E.; He, F. Balancing ecological complexity in predictive models: A reassessment of risk models in the mountain pine beetle system. J. Appl. Ecol. 2008, 45, 248–257. [Google Scholar] [CrossRef]
- Shore, T.L.; Riel, W.; Safranyik, L.; Fal, A. Decision support systems. In The Mountain Pine Beetle: A Synthesis of Biology, Management, and Impacts on Lodgepole Pine; Safranyik, L., Wilson, B., Eds.; Natural Resources Canada, Canadian Forest Service, Pacific Forestry Centre: Victoria, BC, Canada, 2006; pp. 193–230. [Google Scholar]
- Bentz, B.J.; Amman, G.D.; Logan, J.A. A critical assessment of risk classification systems for the mountain pine beetle. For. Ecol. Manag. 1993, 61, 349–366. [Google Scholar] [CrossRef]
- Dymond, C.C.; Wulder, M.A.; Shore, T.L.; Nelson, T.; Boots, B.; Riel, B.G. Evaluation of risk assessment of mountain pine beetle infestations. West. J. Appl. For. 2006, 21, 5–13. [Google Scholar]
- Dixon, G.E. Essential FVS: A User’s Guide to the Forest Vegetation Simulator; Internal Report; US Department of Agriculture, Forest Service, Forest Management Service Center: Fort Collins, CO, USA, 2018; p. 226. [Google Scholar]
- Bentz, B.J.; Regniere, J.; Fettig, C.J.; Hansen, E.M.; Hayes, J.L.; Hicke, J.A.; Kelsey, R.G.; Negron, J.F.; Seybold, S.J. Climate change and bark beetles of the western United States and Canada: Direct and indirect effects. Bioscience 2010, 60, 602–613. [Google Scholar] [CrossRef]
- Buotte, P.C.; Hicke, J.A.; Preisler, H.K.; Abatzoglou, J.T.; Raffa, K.F.; Logan, J.A. Climate influences on whitebark pine mortality from mountain pine beetle in the greater Yellowstone ecosystem. Ecol. Appl. 2016, 26, 2507–2524. [Google Scholar] [CrossRef] [PubMed]
- Liang, L.; Hawbaker, T.J.; Chen, Y.; Zhu, Z.; Gong, P. Characterizing recent and projecting future potential patterns of mountain pine beetle outbreaks in the southern Rocky Mountains. Appl. Geogr. 2014, 55, 165–175. [Google Scholar] [CrossRef]
- Axelson, J.N.; Alfaro, R.I.; Hawkes, B.C. Influence of fire and mountain pine beetle on the dynamics of lodgepole pine stands in British Columbia, Canada. For. Ecol. Manag. 2009, 257, 1874–1882. [Google Scholar] [CrossRef]
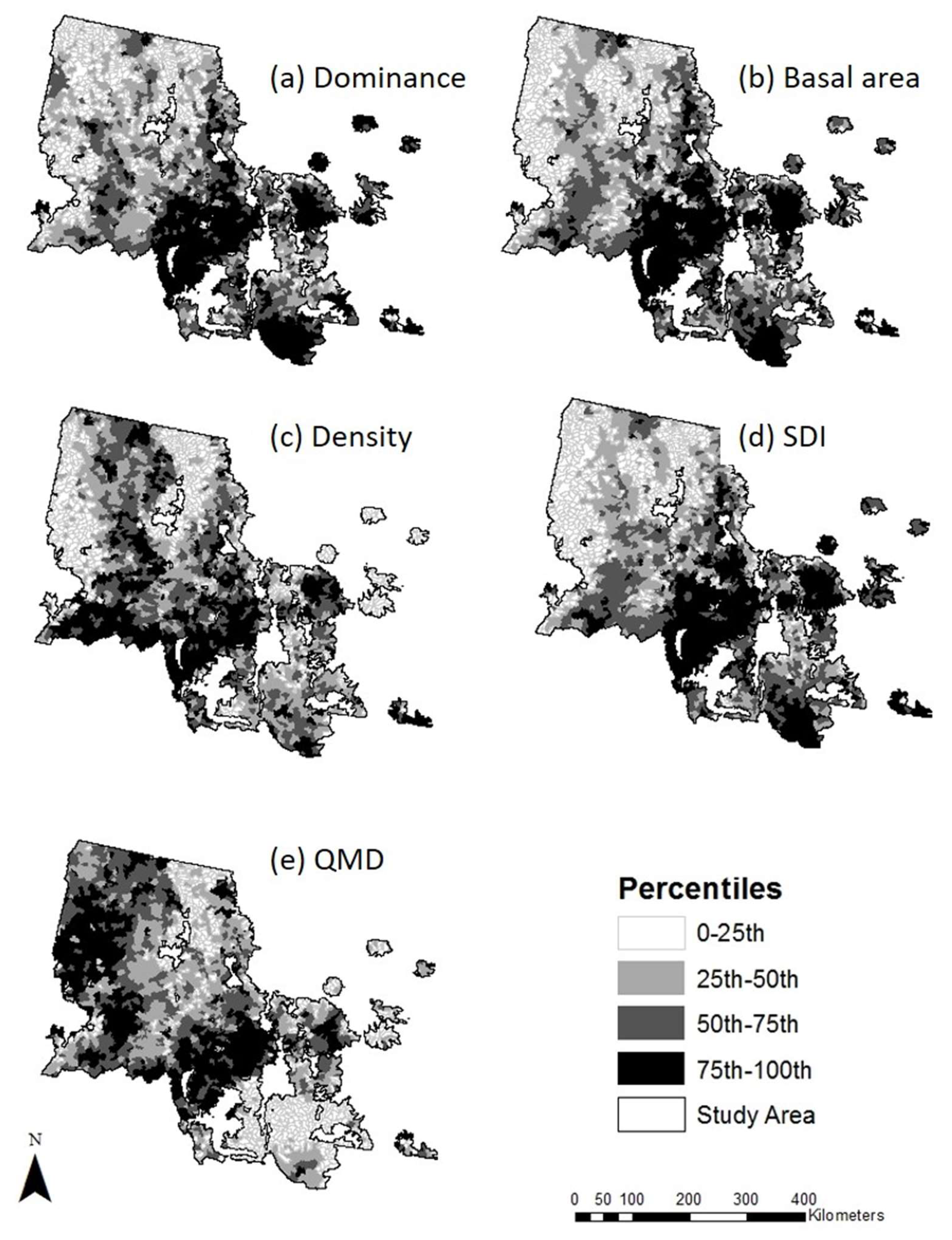
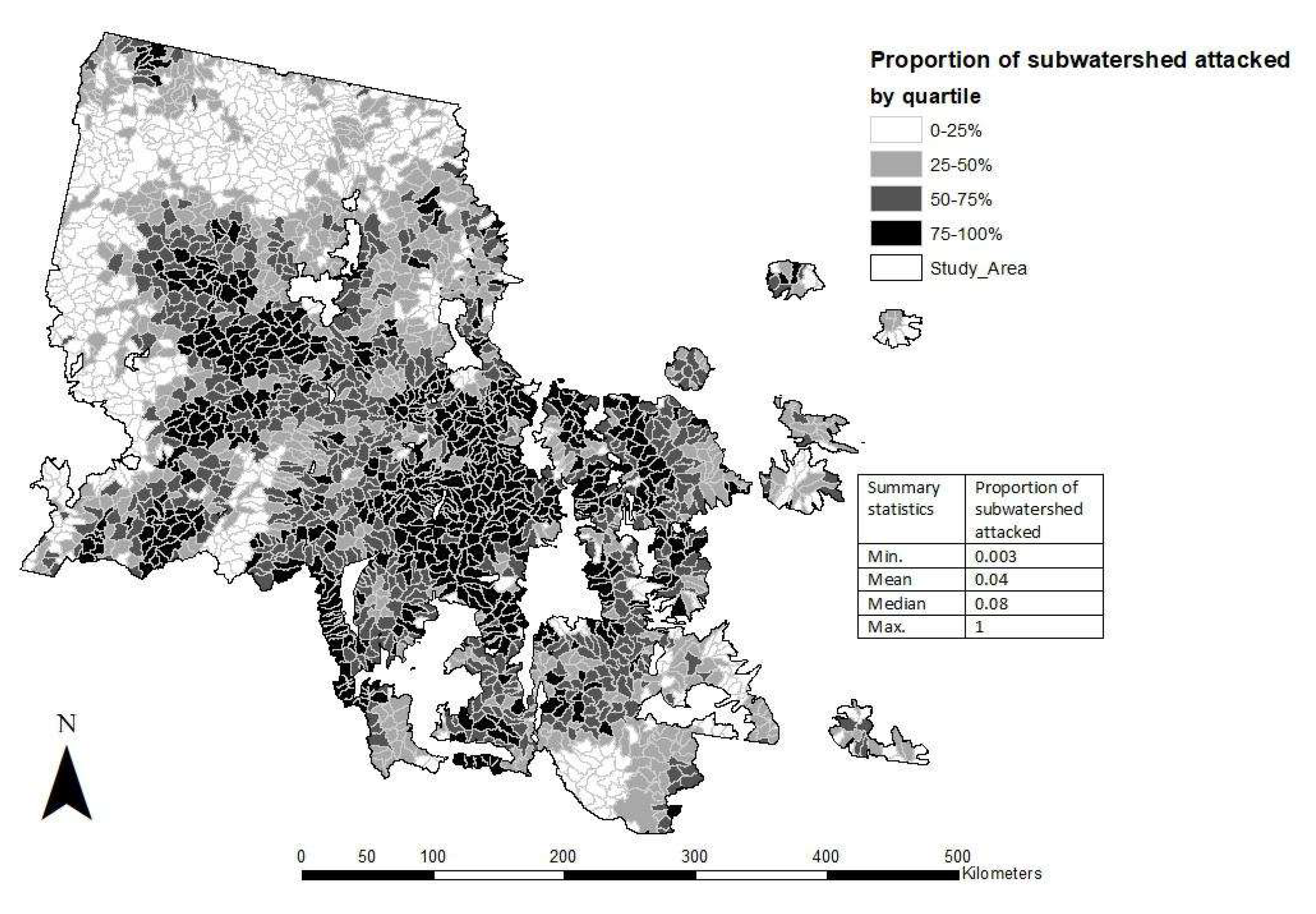
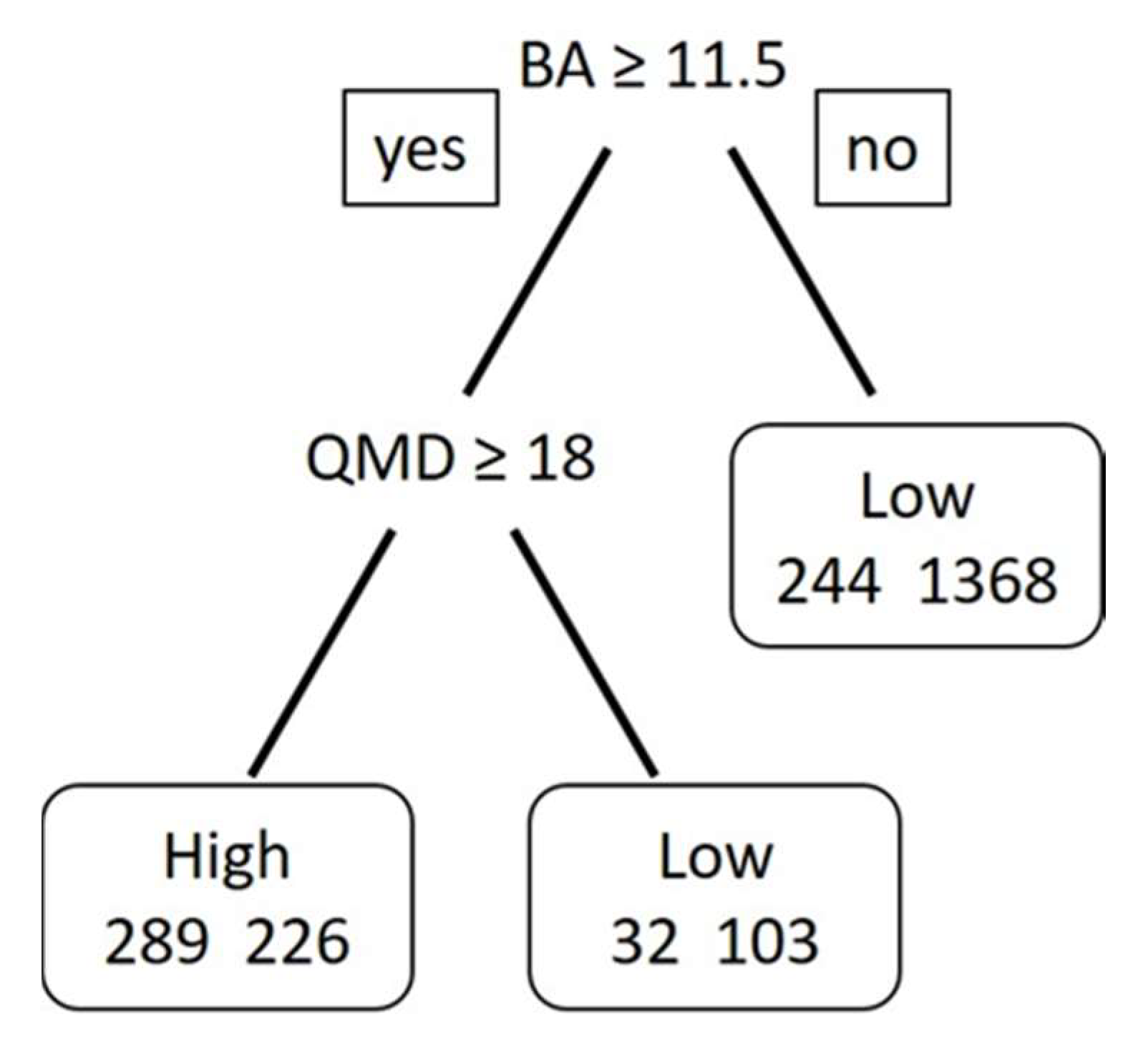
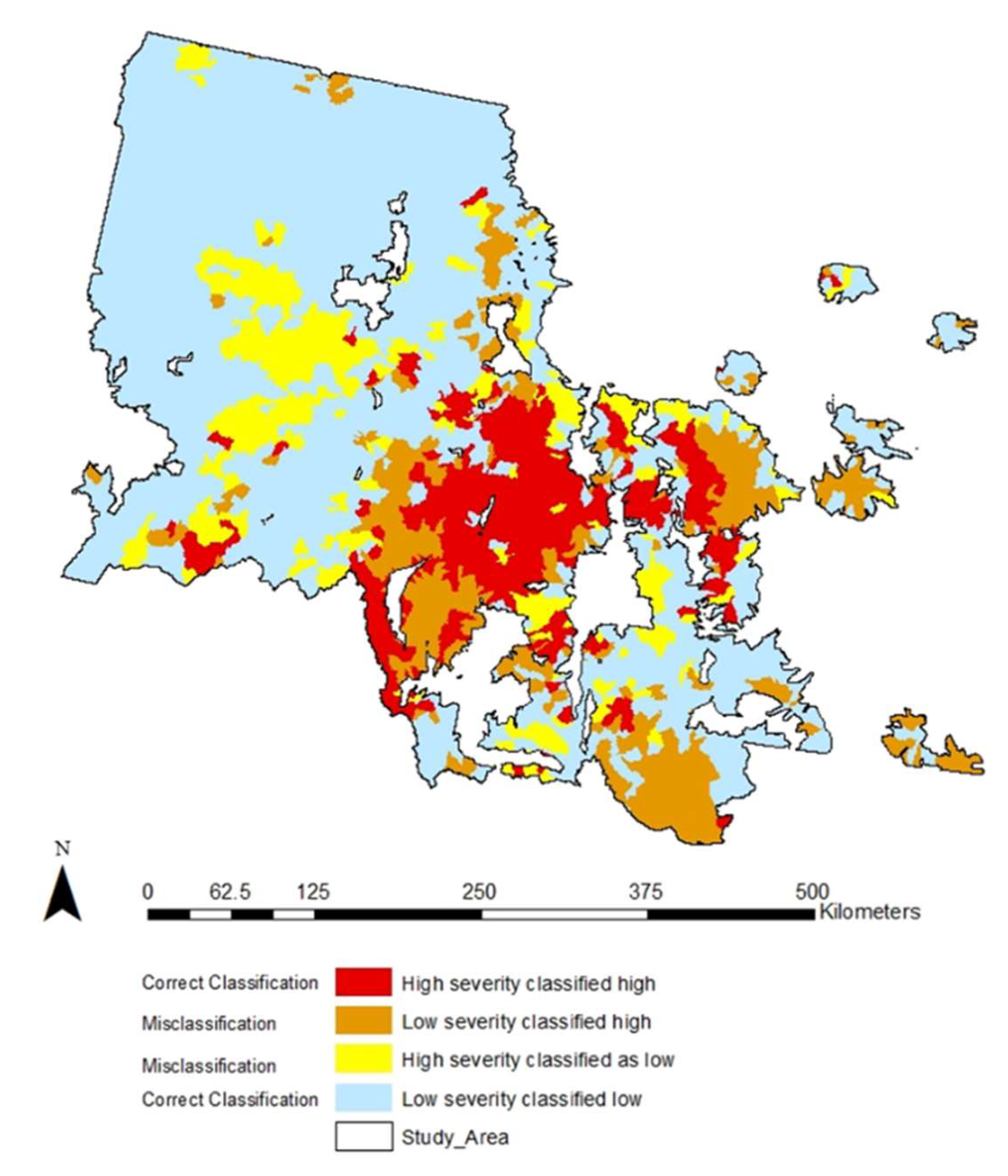
| Variable | Description | Units | Range | Median | Mean | Std. Error |
|---|---|---|---|---|---|---|
| Stand-Level Variables | ||||||
| Dominance | Mean LPP proportion of total basal area | Proportion | 0–1 | 0 | 0.37 | 0.004 |
| BA | Mean LPP basal area | m2 ha−1 | 0–33 | 9 | 10 | 0.1 |
| Density | Mean LPP trees per ha | Trees ha−1 | 0.8–5522 | 1507 | 1487 | 12.7 |
| SDI | Mean number of 25 cm DBH LPP trees per hectare | Unitless index (metric) | 3–691 | 170 | 194 | 2.4 |
| QMD | LPP quadratic mean diameter | cm | 0–40 | 19 | 18 | 0.11 |
| Subwatershed-Level Variables | ||||||
| LPP area | LPP areal extent | ha | 0.09–29,984 | 1262 | 1957 | 46 |
| LPP HUC extent | Mean proportion of subwatershed occupied by LPP | Proportion | 0–1 | 0.2 | 0.25 | 0.005 |
| High BA | Area with LPP basal area > 18.4 m2 ha−1 | ha | 0.09–17,515 | 168 | 499 | 19 |
| High dominance area | Area with LPP dominance > 50% | ha | 0.09–24,323 | 306 | 790 | 27 |
| Large QMD | Area with LPP QMD > 20.3 cm | ha | 0.09–22,116 | 687 | 1266 | 33 |
| Variable | LPP Unattacked | LPP Attacked | All LPP |
|---|---|---|---|
| BA | 8.1 (0.08) a | 10.8 (0.11) b | 9.7 (0.11) |
| High BA | 209.4 (13.0) a | 334.0 (13.4) a | 499.3 (19.0) |
| LPP area | 1100.4 (35.0) a | 940.8 (30.4) b | 1956.8 (45.8) |
| Dominance | 0.34 (0.003) a | 0.39 (0.004) b | 0.37 (0.004) |
| High Dominance | 377.9 (20.0) a | 466.7 (18.2) a | 789.9 (27.4) |
| QMD | 17.3 (0.1) a | 18.8 (0.1) b | 18.3 (0.1) |
| Large QMD | 671.6 (24.1) a | 659.3 (23.2) a | 1266.1 (33.2) |
| SDI | 161.4 (1.9) a | 217.8 (2.6) b | 193.7 (2.4) |
| Density | 1371.3 (12.3) a | 1590.3 (15.5) b | 1487.2 (12.8) |
| LPP HUC extent | 0.14 (0.004) a | 0.12 (0.004) a | 0.25 (0.005) |
| Variable | Attack Severity | Range | Median | Mean | Std. Error |
|---|---|---|---|---|---|
| BA | LOW | 0.12–33 | 8 | 8 | 0.11 |
| HIGH | 0–31 | 12 | 13 | 0.21 | |
| High BA | LOW | 0–17,510 | 111 | 379 | 21 |
| HIGH | 0–5905 | 441 | 825 | 40 | |
| LPP area | LOW | 0–29,980 | 1002 | 1768 | 53 |
| HIGH | 0–13,400 | 1944 | 2488 | 87 | |
| Dominance | LOW | 0–1 | 0.31 | 0.34 | 0.004 |
| HIGH | 0–0.95 | 0.42 | 0.45 | 0.007 | |
| High dominance area | LOW | 0–24,320 | 216 | 640 | 31 |
| HIGH | 0–7514 | 713 | 1205 | 55 | |
| QMD | LOW | 0–40 | 18 | 18 | 0.1 |
| HIGH | 0–28 | 20 | 20 | 0.17 | |
| Large QMD | LOW | 0–22,120 | 520 | 1097 | 37 |
| HIGH | 0–10,050 | 1222 | 1742 | 68 | |
| SDI | LOW | 3–677 | 149 | 167 | 2 |
| HIGH | 31–691 | 240 | 269 | 5 | |
| Density | LOW | 0–5522 | 1400 | 1379 | 15 |
| HIGH | 5–3739 | 1794 | 1791 | 22 | |
| LPP HUC extent | LOW | 0–0.97 | 0.14 | 0.22 | 0.01 |
| HIGH | 0–0.99 | 0.29 | 0.33 | 0.01 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Williams, H.; Hood, S.M.; Keyes, C.R.; Egan, J.M.; Negrón, J. Subwatershed-Level Lodgepole Pine Attributes Associated with a Mountain Pine Beetle Outbreak. Forests 2018, 9, 552. https://doi.org/10.3390/f9090552
Williams H, Hood SM, Keyes CR, Egan JM, Negrón J. Subwatershed-Level Lodgepole Pine Attributes Associated with a Mountain Pine Beetle Outbreak. Forests. 2018; 9(9):552. https://doi.org/10.3390/f9090552
Chicago/Turabian StyleWilliams, Howard, Sharon M. Hood, Christopher R. Keyes, Joel M. Egan, and José Negrón. 2018. "Subwatershed-Level Lodgepole Pine Attributes Associated with a Mountain Pine Beetle Outbreak" Forests 9, no. 9: 552. https://doi.org/10.3390/f9090552
APA StyleWilliams, H., Hood, S. M., Keyes, C. R., Egan, J. M., & Negrón, J. (2018). Subwatershed-Level Lodgepole Pine Attributes Associated with a Mountain Pine Beetle Outbreak. Forests, 9(9), 552. https://doi.org/10.3390/f9090552





