Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks
Abstract
:1. Introduction
2. Materials and Methods
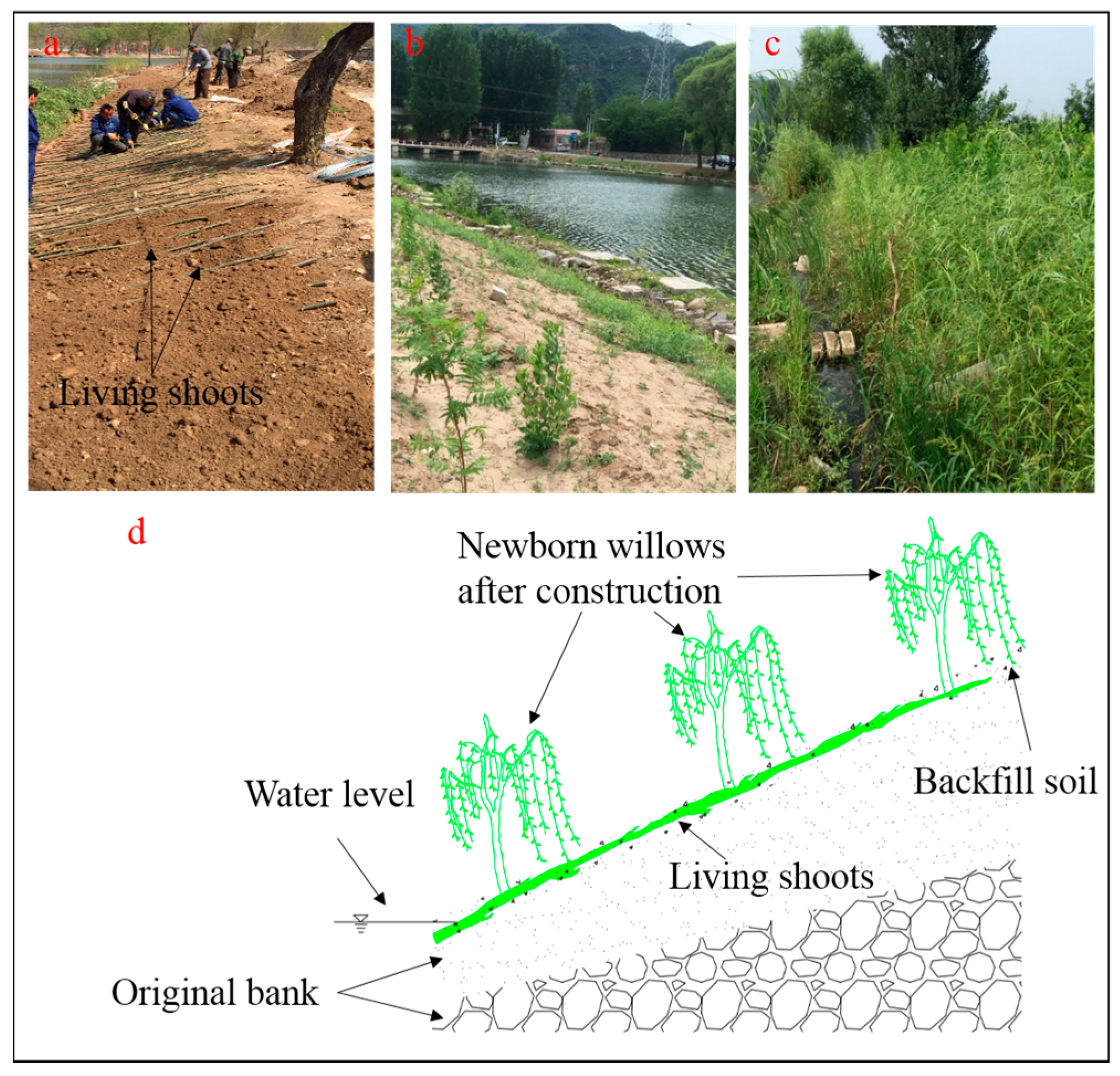
2.1. Construction Methods for Living Brush Mattress and Salix alba L. ‘Tristis’ (LBS)
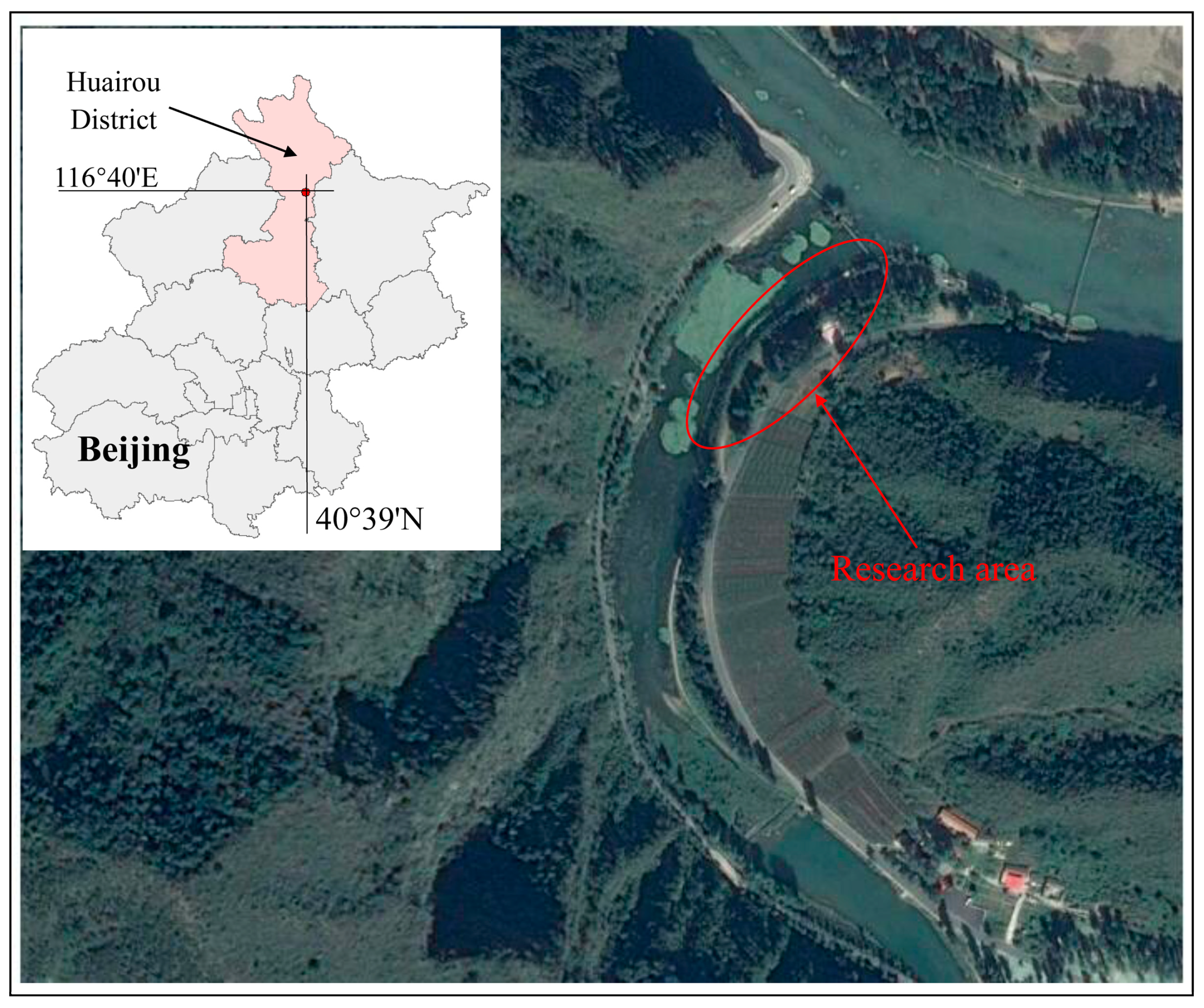
2.2. Site Details
2.3. Tree Selection and Extraction
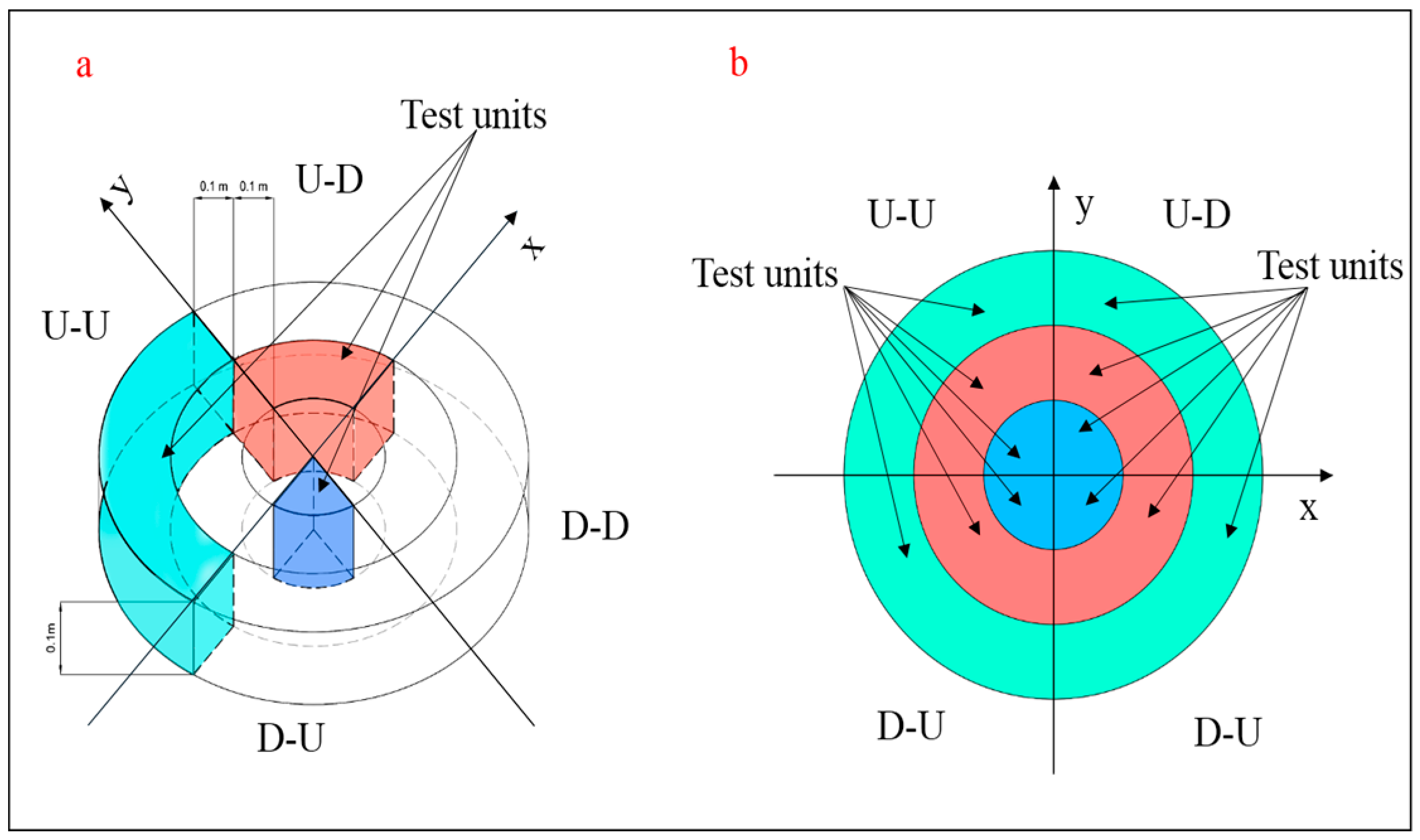
2.4. Root System Parameter Measurements
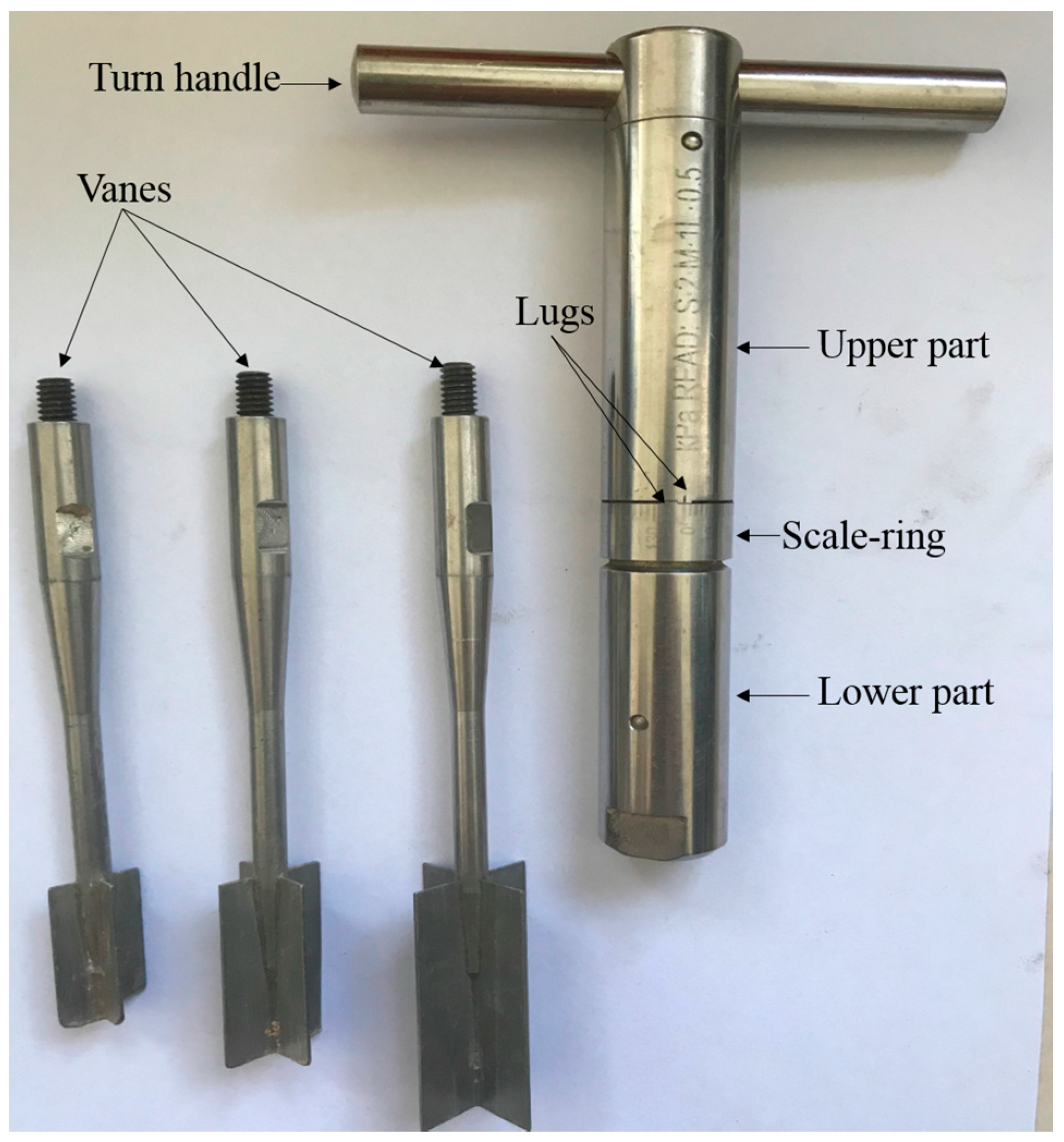
2.5. Shear Strength Determination
2.6. Statistical Analysis
3. Results
3.1. LBS Dimensions
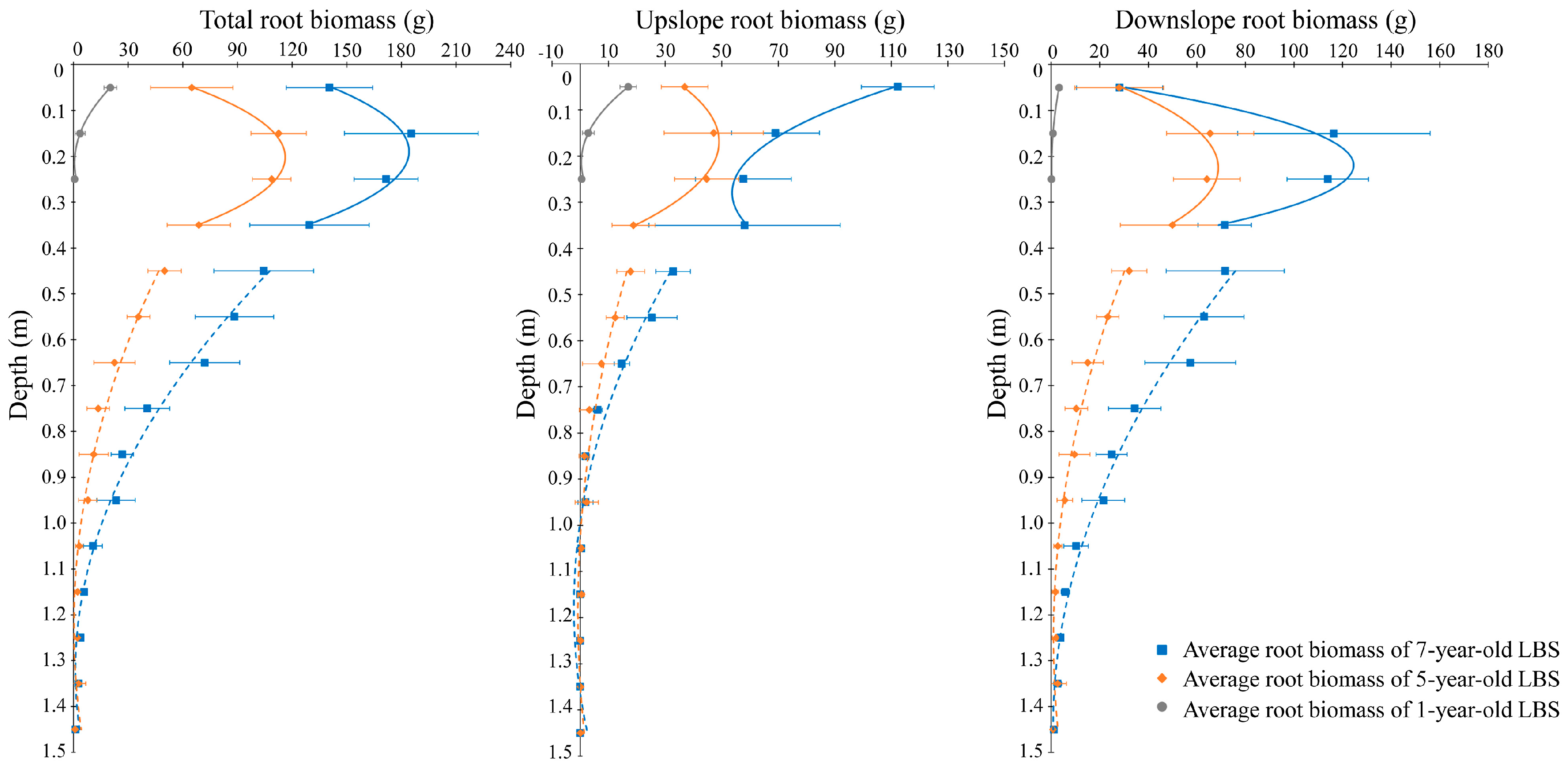
3.2. Spatio-Temporal Root Biomass Distribution with the Depth below the Ground Surface
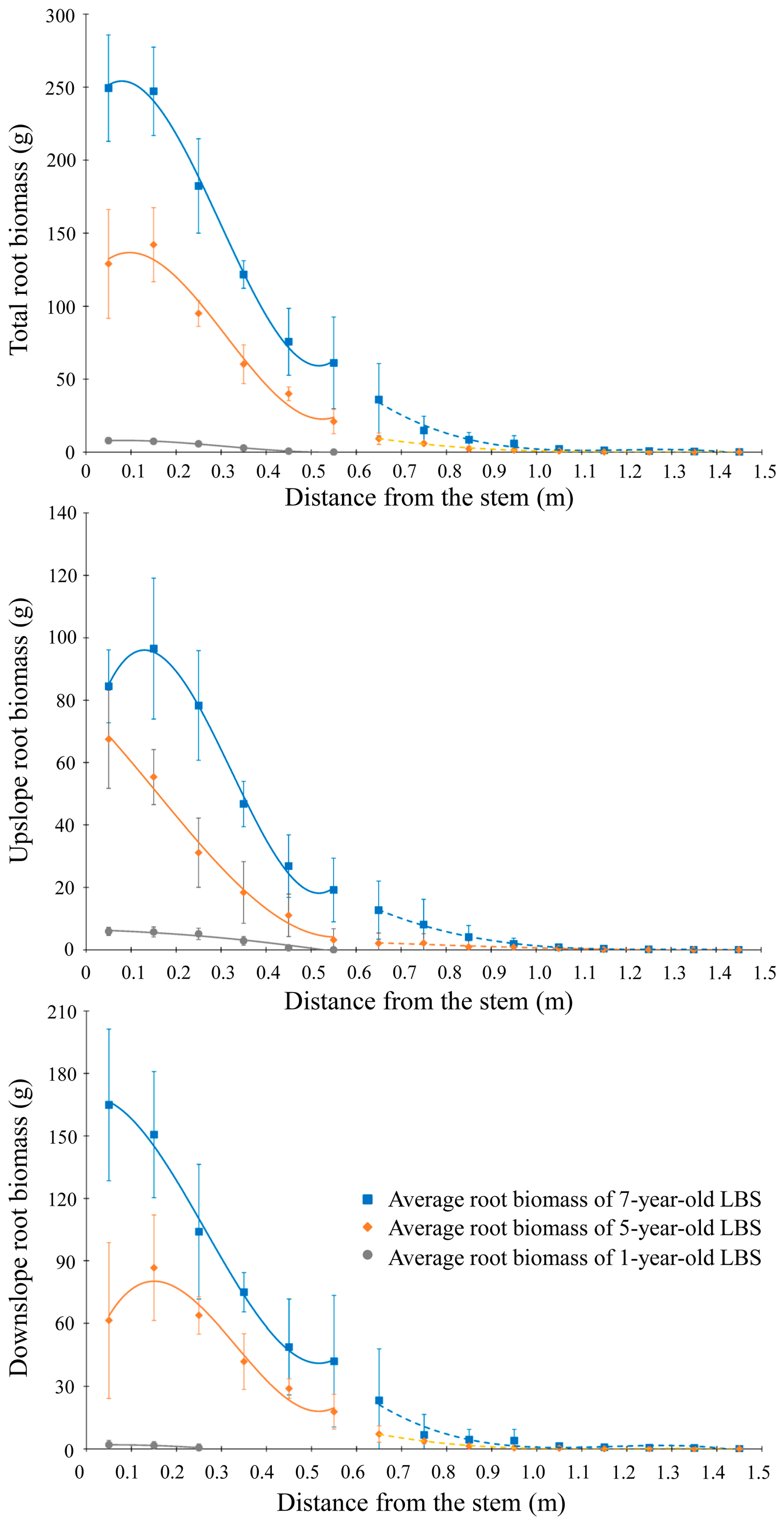
3.3. Spatio-Temporal Root Biomass Distribution with Lateral Distance from the Tree Stem
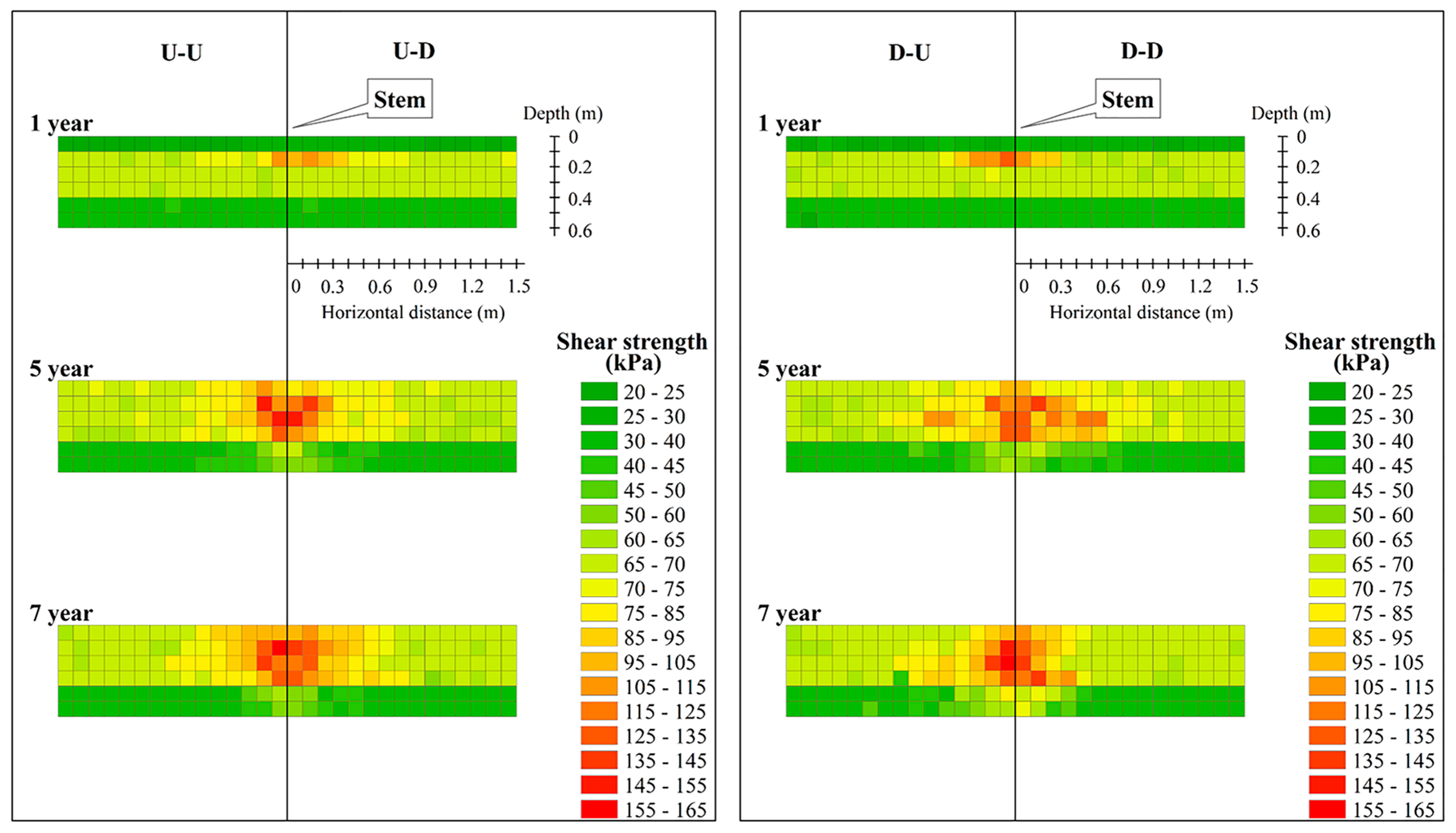
3.4. Spatio-Temporal Distribution of the Soil Shear Strength with LBS Roots
4. Discussion
4.1. Spatio-Temporal Dynamic Distribution Characteristics of the LBS Root System
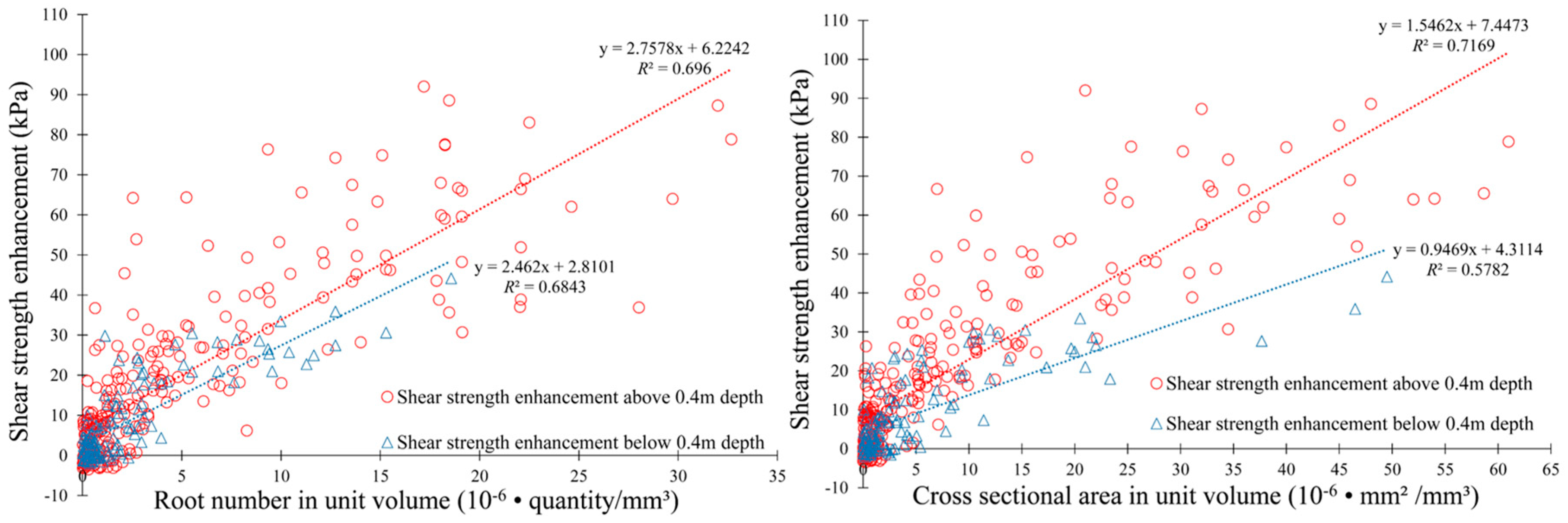
4.2. Soil Shear Strength Enhancement by the LBS Root System
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cheng, X.; Chen, L.; Sun, R.; Kong, P. Land use changes and socio-economic development strongly deteriorate river ecosystem health in one of the largest basins in China. Sci. Total Environ. 2018, 616–617, 376–385. [Google Scholar] [CrossRef] [PubMed]
- Samadi, A.; Amiri-Tokaldany, E.; Darby, S.E. Identifying the effects of parameter uncertainty on the reliability of riverbank stability modelling. Geomorphology 2009, 106, 219–230. [Google Scholar] [CrossRef]
- Darby, S.E.; Thorne, C.R. Prediction of tension crack location and riverbank erosion hazards along destabilized channels. Earth Surf. Process. Landf. 1994, 19, 233–245. [Google Scholar] [CrossRef]
- Yao, S.; Yue, H.; Li, L. Analysis on Current Situation and Development Trend of Ecological Revetment Works in Middle and Lower Reaches of Yangtze River. Procedia Eng. 2012, 28, 307–313. [Google Scholar] [CrossRef]
- Li, X.; Zhang, L.; Zhang, Z. Soil bioengineering and the ecological restoration of riverbanks at the airport Town, Shanghai, China. Ecol. Eng. 2006, 26, 304–314. [Google Scholar] [CrossRef]
- Li, M.H.; Eddleman, K.E. Biotechnical engineering as an alternative to traditional engineering methods: A biotechnical streambank stabilization design approach. Landsc. Urban Plan. 2002, 60, 225–242. [Google Scholar] [CrossRef]
- Wang, P.; Chen, G.Q. Contaminant transport in wetland flows with bulk degradation and bed absorption. J. Hydrol. 2017, 552, 674–683. [Google Scholar] [CrossRef]
- Bischetti, G.B.; Chiaradia, E.A.; D’Agostino, V.; Simonato, T. Quantifying the effect of brush layering on slope stability. Ecol. Eng. 2010, 36, 258–264. [Google Scholar] [CrossRef]
- Dhital, Y.P.; Tang, Q. Soil bioengineering application for flood hazard minimization in the foothills of siwaliks, Nepal. Ecol. Eng. 2015, 74, 458–462. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Guiomar, N. Simulating the stabilization effect of soil bioengineering interventions in Mediterranean environments using limit equilibrium stability models and combinations of plant species. Ecol. Eng. 2016, 88, 122–142. [Google Scholar] [CrossRef]
- Rauch, H.P.; Sutili, F.; Hörbinger, S. Installation of a riparian forest by means of soil bio engineering techniques—Monitoring results from a river restoration work in southern Brazil. Open J. For. 2014, 4, 161–169. [Google Scholar] [CrossRef]
- Capilleri, P.P.; Motta, E.; Raciti, E. Experimental study on native plant root tensile strength for slope stabilization. Procedia Eng. 2016, 158, 116–121. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Ma, C.; Zhang, H.; Wang, Y.; Song, S.; Zhu, J. Influence of the spatial layout of plant roots on slope stability. Ecol. Eng. 2016, 91, 477–486. [Google Scholar] [CrossRef]
- Fan, C.C.; Lai, Y.F. Influence of the spatial layout of vegetation on the stability of slopes. Plant Soil 2014, 377, 83–95. [Google Scholar] [CrossRef]
- Stokes, A.; Atger, C.; Bengough, A.G.; Fourcaud, T.; Sidle, R.C. Desirable plant root traits for protecting natural and engineered slopes against landslides. Plant Soil 2009, 324, 1–30. [Google Scholar] [CrossRef]
- Giadrossich, F.; Cohen, D.; Schwarz, M.; Seddaiu, G.; Contran, N.; Lubino, M.; Valdes-Rodriguez, O.A.; Niedda, M. Modeling bio-engineering traits of Jatropha curcas L. Ecol. Eng. 2016, 89, 40–48. [Google Scholar] [CrossRef]
- Docker, B.B.; Hubble, T.C.T. Modelling the distribution of enhanced soil shear strength beneath riparian trees of south-eastern Australia. Ecol. Eng. 2009, 35, 921–934. [Google Scholar] [CrossRef]
- Fan, C.C.; Tsai, M.H. Spatial distribution of plant root forces in root-permeated soils subject to shear. Soil Tillage Res. 2016, 156, 1–15. [Google Scholar] [CrossRef]
- McIvor, I.R.; Douglas, G.B.; Hurst, S.E.; Hussain, Z.; Foote, A.G. Structural root growth of young Veronese poplars on erodible slopes in the southern north island, New Zealand. Agrofor. Syst. 2008, 72, 75–86. [Google Scholar] [CrossRef]
- Di, I.A.; Lasserre, B.; Scippa, G.S.; Chiatante, D. Root system architecture of Quercus pubescens trees growing on different sloping conditions. Ann. Bot. 2005, 95, 351–361. [Google Scholar] [CrossRef]
- Nicoll, B.C.; Berthier, S.; Achim, A.; Gouskou, K.; Danjon, F.; Beek, L.P.H.V. The architecture of Picea sitchensis, structural root systems on horizontal and sloping terrain. Trees 2006, 20, 701–712. [Google Scholar] [CrossRef]
- Yang, X.; Blagodatsky, S.; Liu, F.; Beckschäfer, P.; Xu, J.; Cadisch, G. Rubber tree allometry, biomass partitioning and carbon stocks in mountainous landscapes of sub-tropical China. For. Ecol. Manag. 2017, 404, 84–99. [Google Scholar] [CrossRef]
- Luo, T.; Luo, J.; Pan, Y. Leaf traits and associated ecosystem characteristics across subtropical and timberline forests in the Gongga Mountains, Eastern Tibetan Plateau. Oecologia 2005, 142, 261–273. [Google Scholar] [CrossRef] [PubMed]
- Czernin, A.; Phillips, C. Below-ground morphology of Cordyline australis (New Zealand cabbage tree) and its suitability for river bank stabilization. N. Z. J. Bot. 2005, 43, 851–864. [Google Scholar] [CrossRef]
- Stokes, A.; Douglas, G.B.; Fourcaud, T.; Giadrossich, F.; Gillies, C.; Hubble, T.; Walker, L.R. Ecological mitigation of hillslope instability: Ten key issues facing researchers and practitioners. Plant Soil 2014, 377, 1–23. [Google Scholar] [CrossRef] [Green Version]
- Schwarz, M.; Preti, F.; Giadrossich, F.; Lehmann, P.; Or, D. Quantifying the role of vegetation in slope stability: A casestudy in Tuscany (Italy). Ecol. Eng. 2010, 36, 285–291. [Google Scholar] [CrossRef]
- Schwarz, M.; Lehmann, P.; Or, D. Quantifying lateral root reinforcement in steep slopes-From a bundle of roots to tree stand. Earth Surf. Process. Landf. 2010, 35, 354–367. [Google Scholar] [CrossRef]
- Cohen, D.; Schwarz, M.; Or, D. An analytical fiber bundle model for pullout mechanics of root bundles. J. Geophys. Res. Earth Surf. 2011, 116, F03010. [Google Scholar] [CrossRef]
- Wu, T.H.; Iii, M.K.; Swanston, D.N. Strength of tree roots and landslides on Prince of Wales Island, Alaska. Can. Geotech. J. 1979, 16, 19–33. [Google Scholar] [CrossRef]
- Wang, P.; Chen, G.Q. Solute dispersion in open channel flow with bed absorption. J. Hydrol. 2016, 543, 208–217. [Google Scholar] [CrossRef]
- Zhang, C.B.; Chen, L.H.; Liu, Y.P.; Ji, X.D.; Liu, X.P. Triaxial compression test of soil-root composites to evaluate influence of roots on soil shear strength. Ecol. Eng. 2010, 36, 19–26. [Google Scholar] [CrossRef]
- Giadrossich, F.; Schwarz, M.; Cohen, D.; Preti, F.; Or, D. Mechanical interactions between neighbouring roots during pullout tests. Plant Soil 2013, 367, 391–406. [Google Scholar] [CrossRef]
- Wu, T.H. Investigation of landslides on Prince of Wales Island, Alska; Geotechnical Engineering Report 5; Ohio State University: Columbus, OH, USA, 1976. [Google Scholar]
- Waldron, L.J. The shear resistance of root-permeated homogeneous and stratified soil. Soil Sci. Soc. Am. J. 1977, 41, 843–849. [Google Scholar] [CrossRef]
- Pollen, N.; Simon, A. Estimating the mechanical effects of riparian vegetation on stream bank stability using a fiber bundle model. Water Resour. Res. 2005, 41, 226–244. [Google Scholar] [CrossRef]
- Schwarz, M.; Cohen, D.; Or, D. Pullout tests of root analogs and natural root bundles in soil: Experiments and modeling. J. Geophys. Res. Earth Surf. 2011, 116, F02007(1-14). [Google Scholar] [CrossRef]
- Liu, Y.; Rauch, H.P.; Zhang, J.; Yang, X.; Gao, J.R. Development and soil reinforcement characteristics of five native species planted as cuttings in local area of Beijing. Ecol. Eng. 2014, 71, 190–196. [Google Scholar] [CrossRef]
- Liu, Y. Effects of Soil bioengineering Techniques Applied in Riverbank Ecological Restoration. Ph.D. Thesis, Beijing Forestry University, Beijing, China, 2011. [Google Scholar]
- Qian, B.T. Research on Plant Materials Selection and Construction Methods of Soil Bio-Engineering. Master’s Thesis, Beijing Forestry University, Beijing, China, 2013. [Google Scholar]
- Studer, R.; Zeh, H.; Givoanni, D.C. SOIL BIOENGINEERING—Construction Type Manual, 5th ed.; vdf Hochschulverlag AG der ETH Zürich: Zürich, Switzerland, 2014; pp. 218–250. ISBN 978-3-7281-3642-8. [Google Scholar]
- Böhm, W. Methods of Studying Root Systems, 12th ed.; Springer: Berlin/Heidelberg, Germany, 1979; pp. 125–138. ISBN 978-3-642-67284-2. [Google Scholar]
- Fan, C.C.; Chen, Y.W. The effect of root architecture on the shearing resistance of root-permeated soils. Ecol. Eng. 2010, 36, 813–826. [Google Scholar] [CrossRef]
- Ziemer, R.R. Roots and the stability of forested slopes. In Erosion and Sediment Transport in Pacific Rim Steeplands, 3rd ed.; Davies, T.R.H., Pearce, A.J., Eds.; International Association of Hydrological Sciences: Christchurch, New Zealand, 1981; Publication 132; pp. 343–361. [Google Scholar]
- Abernethy, B.; Rutherfurd, I.D. The distribution and strength of riparian tree roots in relation to riverbank reinforcement. Hydrol. Process. 2001, 15, 63–79. [Google Scholar] [CrossRef]
- Kajimoto, T.; Matsuura, Y.; Osawa, A.; Abaimov, A.P.; Zyryanova, O.A.; Isaev, A.P.; Yefremov, D.P.; Mori, S.; Koike, T. Size-mass allometry and biomass allocation of two larch species growing on the continuous permafrost region in Siberia. For. Ecol. Manag. 2006, 222, 314–325. [Google Scholar] [CrossRef]
- Greenway, D.R. Vegetation and slope stability. In Slope Stability Geotechnical Engineering and Geomorphology, 3rd ed.; Anderson, M.G., Richards, K.S., Eds.; John Wiley & Sons: Chichester, UK, 1987; pp. 187–230. [Google Scholar]
- Wu, T.H. Slope stabilization. In Slope Stabilization and Erosion Control: A Bioengineering Approach, 7th ed.; Morgan, R.P.C., Rickson, R.J., Eds.; E & FN Spon: London, UK, 1995; pp. 221–248. ISBN 0-419-15630-5. [Google Scholar]
- Reubens, B.; Achten, W.M.J.; Maes, W.H.; Danjon, F.; Aerts, R.; Poesen, J.; Muys, B. More than biofuel? Jatropha curcas root system symmetry and potential for soil erosion control. J. Arid Environ. 2011, 75, 201–205. [Google Scholar] [CrossRef] [Green Version]
- Amichev, B.Y.; Bailey, B.E.; Rees, K.C.J.V. White spruce (Picea glauca) structural root system development and symmetry influenced by disc trenching site preparation. For. Ecol. Manag. 2014, 326, 1–8. [Google Scholar] [CrossRef]
- Pasquale, N.; Perona, P.; Francis, R.; Burlando, P. Effects of streamflow variability on the vertical root density distribution of willow cutting experiments. Ecol. Eng. 2012, 40, 167–172. [Google Scholar] [CrossRef]
- Shields, F.D.; Gray, D.H. Effects of woody vegetation on sandy levee integrity. J. Am. Water Resour. Assoc. 1992, 28, 917–931. [Google Scholar] [CrossRef]
- Waldron, L.J.; Dakessian, S. Effect of grass, legume, and tree roots on soil shearing resistance. Soil Sci. Soc. Am. J. 1982, 46, 894–899. [Google Scholar] [CrossRef]
- Guo, K.L. Salix× aureo-pendula Root System Distribution and Tensile Mechanical Properties in Soil Bioengineering Revetment. Master’s Thesis, Beijing Forestry University, Beijing, China, 2016. [Google Scholar]
- Vergani, C.; Chiaradia, E.A.; Bischetti, G.B. Variability in the tensile resistance of roots in alpine forest tree species. Ecol. Eng. 2012, 46, 43–56. [Google Scholar] [CrossRef]


| Age | 1 | 5 | 7 | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Plot Area (m2) | 3 m × 2 m | 3 m × 4 m | 3 m × 4 m | ||||||
| Plot Number | 1 | 2 | 3 | 1 | 2 | 3 | 1 | 2 | 3 |
| Plot Slope | 15° | 15° | 15° | 15° | 15° | 15° | 15° | 15° | 15° |
| Number of Trees | 8 | 8 | 8 | 13 | 9 | 11 | 12 | 7 | 8 |
| Mean Basal Diameter (mm) | 10.7 | 10.2 | 10.6 | 75.4 | 82.7 | 86.8 | 115.6 | 130.4 | 113.2 |
| Age (Year) | N | H (m) | D (mm) | RB (g) | MLD (m) | MVD (m) |
|---|---|---|---|---|---|---|
| 1 | 5 | 2.01 ± 0.35 a | 9.16 ± 1.36 a | 24.5 ± 7.02 a | 0.45 ± 0.06 a | 0.20 ± 0.03 a |
| 5 | 5 | 9.79 ± 1.00 b | 79.92 ± 11.46 b | 507.28 ± 49.00 b | 1.00 ± 0.20 b | 1.46 ± 0.11 b |
| 7 | 5 | 14.11 ± 1.22 c | 122.08 ± 11.66 c | 1006.25 ± 157.85 c | 1.27 ± 0.11 c | 1.73 ± 0.07 c |
| Age (Year) | MLD/H | MVD/H |
|---|---|---|
| 1 | 0.22 | 0.1 |
| 5 | 0.1 | 0.15 |
| 7 | 0.09 | 0.12 |
| 1-Year-Old | 5-Year-Old | 7-Year-Old | ||
|---|---|---|---|---|
| Active Layer | Total | B = 727.9d2 − 311.9d + 34.03 R2 = 0.9148 | B = −2193.2d2 + 885.03d + 26.90 R2 = 0.6731 | B = −2174.9d2 + 823.17d + 106.31 R2 = 0.4272 |
| Upslope | B = 623.2d2 − 265.68d + 28.68 R2 = 0.921 | B = −902.55d2 + 304.34d + 23.41 R2 = 0.5164 | B = 1091.8d2 − 610.72d + 139.09 R2 = 0.5717 | |
| Downslope | B = 104.7d2 − 46.22d + 5.35 R2 = 0.711 | B = −1290.6d2 + 580.69d + 3.50 R2 = 0.4486 | B = −3266.7d2 + 1433.9d − 32.78 R2 = 0.7217 | |
| Stable Layer | Total | B = 77.72d2 − 190.25d + 116.66 R2 = 0.8665 | B = 140.34d2 − 371.09d + 246.37 R2 = 0.8891 | |
| Upslope | B = 32.01d2 − 75.92d + 44.20 R2 = 0.7618 | B = 64.561d2 − 152.06d + 87.29 R2 = 0.8966 | ||
| Downslope | B = 47.71d2 − 114.33d + 72.47 R2 = 0.8418 | B = 75.78d2 − 219.03d + 159.08 R2 = 0.8368 |
| Vertical Depth (m) | Growth Rate | |
|---|---|---|
| 1−5 Years | 5−7 Years | |
| 0.0–0.1 | 11.16 | 37.79 |
| 0.1–0.2 | 27.44 | 36.42 |
| 0.2–0.3 | 27.05 | 31.38 |
| 0.3–0.4 | 17.19 | 30.38 |
| 0.4–0.5 | 12.50 | 27.23 |
| 0.5–0.6 | 8.92 | 26.31 |
| 0.6–0.7 | 5.64 | 24.75 |
| 0.7–0.8 | 3.40 | 13.48 |
| 0.8–0.9 | 2.77 | 7.86 |
| 0.9–1.0 | 1.98 | 7.74 |
| 1.0–1.1 | 0.80 | 3.74 |
| 1.1–1.2 | 0.57 | 1.80 |
| 1.2–1.3 | 0.56 | 0.85 |
| 1.3–1.4 | 0.71 | −0.08 |
| 1.4–1.5 | 0.22 | 0.13 |
| p-Value | |||||
|---|---|---|---|---|---|
| Soil Depth (m) | Paired t-Test | ANOVA | |||
| Slope Position | Age | ||||
| 1-Year-Old | 5-Year-Old | 7-Year-Old | Upslope | Downslope | |
| 0.0–0.1 | <0.01 | 0.308 | <0.01 | <0.01 | 0.030 |
| 0.1–0.2 | 0.038 | 0.271 | 0.090 | <0.01 | <0.01 |
| 0.2–0.3 | 0.398 | 0.130 | 0.012 | <0.01 | <0.01 |
| 0.3–0.4 | - | 0.062 | 0.477 | <0.01 | <0.01 |
| 0.4–0.5 | - | 0.019 | 0.018 | <0.01 | <0.01 |
| 0.5–0.6 | - | <0.01 | <0.01 | <0.01 | <0.01 |
| 0.6–0.7 | - | 0.060 | <0.01 | <0.01 | <0.01 |
| 0.7–0.8 | - | 0.045 | <0.01 | <0.01 | <0.01 |
| 0.8–0.9 | - | 0.020 | <0.01 | 0.061 | <0.01 |
| 0.9–1.0 | - | 0.227 | <0.01 | 0.428 | <0.01 |
| 1.0–1.1 | - | 0.037 | 0.011 | 0.471 | <0.01 |
| 1.1–1.2 | - | 0.122 | <0.01 | 0.397 | <0.01 |
| 1.2–1.3 | - | 0.035 | <0.01 | 0.397 | <0.01 |
| 1.3–1.4 | - | 0.160 | 0.021 | 0.397 | 0.142 |
| 1.4–1.5 | - | 0.174 | 0.012 | 0.397 | 0.078 |
| 1-Year-Old | 5-Year-Old | 7-Year-Old | ||
|---|---|---|---|---|
| Active layer | Total | B = −5.2l2 − 14.70l + 9.11 R2 = 0.8109 | B = 2909.4l3 − 2713.2l2 + 443.9l + 116.4 R2 = 0.8426 | B = 4651.7l3 − 4162l2 + 573.2l + 232.5 R2 = 0.895 |
| Upslope | B = −15.80l2 − 3.94l +6.44 R2 = 0.7758 | B = 368.7l3 − 173.1l2 − 147.8l + 76.4 R2 = 0.8612 | B = 2666.1l3 − 2587.3l2 + 535.9l + 64.2 R2 = 0.8406 | |
| Downslope | B = -35.7l2 + 3.72l + 1.89 R2 = 0.3868 | B = 2540.6l3 − 2540.1l2 + 591.7l + 40 R2 = 0.6702 | B = 1985.6l3 − 1574.7l2 +37.2l + 168.3 R2 = 0.8358 | |
| Stable layer | Total | B = −38.6l3 + 147.2l2 − 187.1l + 79.3 R2 = 0.8225 | B = −218.8l3 + 783.6l2 − 930.7l + 368 R2 = 0.6232 | |
| Upslope | B = 3.9l3 − 7.93l2 + 0.4l + 4.3 R2 = 0.2126 | B = −48.1l3 + 186.3l2 − 240.7l + 103.9 R2 = 0.5382 | ||
| Downslope | B = −42.5l3 + 155.2l2 − 187.5l + 75.1 R2 = 0.5966 | B = −170.7l3 + 597.3l2 − 689.9l + 264.2 R2 = 0.6001 |
| Distance from the Stem (m) | Growth Rate | |
|---|---|---|
| 1−5 Years | 5−7 Years | |
| 0.0–0.1 | 30.26 | 60.19 |
| 0.1–0.2 | 33.67 | 52.53 |
| 0.2–0.3 | 22.32 | 43.65 |
| 0.3–0.4 | 14.33 | 30.71 |
| 0.4–0.5 | 9.83 | 17.81 |
| 0.5–0.6 | 5.26 | 20.05 |
| 0.6–0.7 | 2.30 | 13.36 |
| 0.7–0.8 | 1.48 | 4.43 |
| 0.8–0.9 | 0.58 | 3.11 |
| 0.9–1.0 | 0.36 | 2.22 |
| 1.0–1.1 | 0.21 | 0.69 |
| 1.1–1.2 | 0.03 | 0.56 |
| 1.2–1.3 | 0.01 | 0.37 |
| 1.3–1.4 | 0.01 | 0.21 |
| Distance from the Stem (m) | Paired t-Test | ANOVA | |||
|---|---|---|---|---|---|
| Slope Position | Age | ||||
| 1-Year-Old | 5-Year-Old | 7-Year-Old | Upslope | Downslope | |
| 0.0–0.1 | <0.01 | 0.376 | 0.012 | <0.01 | <0.01 |
| 0.1–0.2 | <0.01 | 0.132 | <0.01 | <0.01 | <0.01 |
| 0.2–0.3 | <0.01 | 0.018 | 0.043 | <0.01 | <0.01 |
| 0.3–0.4 | 0.010 | 0.016 | 0.038 | <0.01 | <0.01 |
| 0.4–0.5 | 0.052 | 0.017 | 0.092 | <0.01 | <0.01 |
| 0.5–0.6 | 0.374 | 0.065 | 0.051 | <0.01 | <0.01 |
| 0.6–0.7 | - | 0.219 | 0.054 | <0.01 | <0.01 |
| 0.7–0.8 | - | 0.584 | 0.671 | 0.062 | <0.01 |
| 0.8–0.9 | - | 0.755 | 0.857 | 0.049 | <0.01 |
| 0.9–1.0 | - | 0.693 | 0.331 | 0.108 | 0.070 |
| 1.0–1.1 | - | 0.898 | 0.689 | 0.231 | 0.230 |
| 1.1–1.2 | - | 0.374 | 0.565 | 0.292 | 0.111 |
| 1.2–1.3 | - | 0.374 | 0.404 | 0.175 | 0.117 |
| 1.3–1.4 | - | 0.374 | 0.293 | 0.619 | 0.257 |
| 1.4–1.5 | - | - | - | - | - |
| Depth (m) | SSP (kPa) | MSSC (kPa) | ESS (kPa) |
|---|---|---|---|
| 0–0.4 | 67.61 ± 4.41 | 159.61 | 24.05 ± 22.27 |
| 0.4–0.6 | 36.78 ± 2.33 | 80.94 | 11.74 ± 10.53 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Cheng, J.; Liu, Y.; Zhang, H.; Ma, L.; Mei, X.; Sun, Y. Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks. Forests 2018, 9, 493. https://doi.org/10.3390/f9080493
Zhang D, Cheng J, Liu Y, Zhang H, Ma L, Mei X, Sun Y. Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks. Forests. 2018; 9(8):493. https://doi.org/10.3390/f9080493
Chicago/Turabian StyleZhang, Dong, Jinhua Cheng, Ying Liu, Hongjiang Zhang, Lan Ma, Xuemei Mei, and Yihui Sun. 2018. "Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks" Forests 9, no. 8: 493. https://doi.org/10.3390/f9080493
APA StyleZhang, D., Cheng, J., Liu, Y., Zhang, H., Ma, L., Mei, X., & Sun, Y. (2018). Spatio-Temporal Dynamic Architecture of Living Brush Mattress: Root System and Soil Shear Strength in Riverbanks. Forests, 9(8), 493. https://doi.org/10.3390/f9080493




