Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation
Abstract
1. Introduction
2. Material and Method
2.1. Study Area
2.2. Experimental Design
2.3. Soil Respiration Measurement and Soil Sampling
2.4. Analysis of Soil Properties and Enzymes Activities
2.5. Plant Species Identification and Species Diversity Index
2.6. Statistical Analyses
3. Results
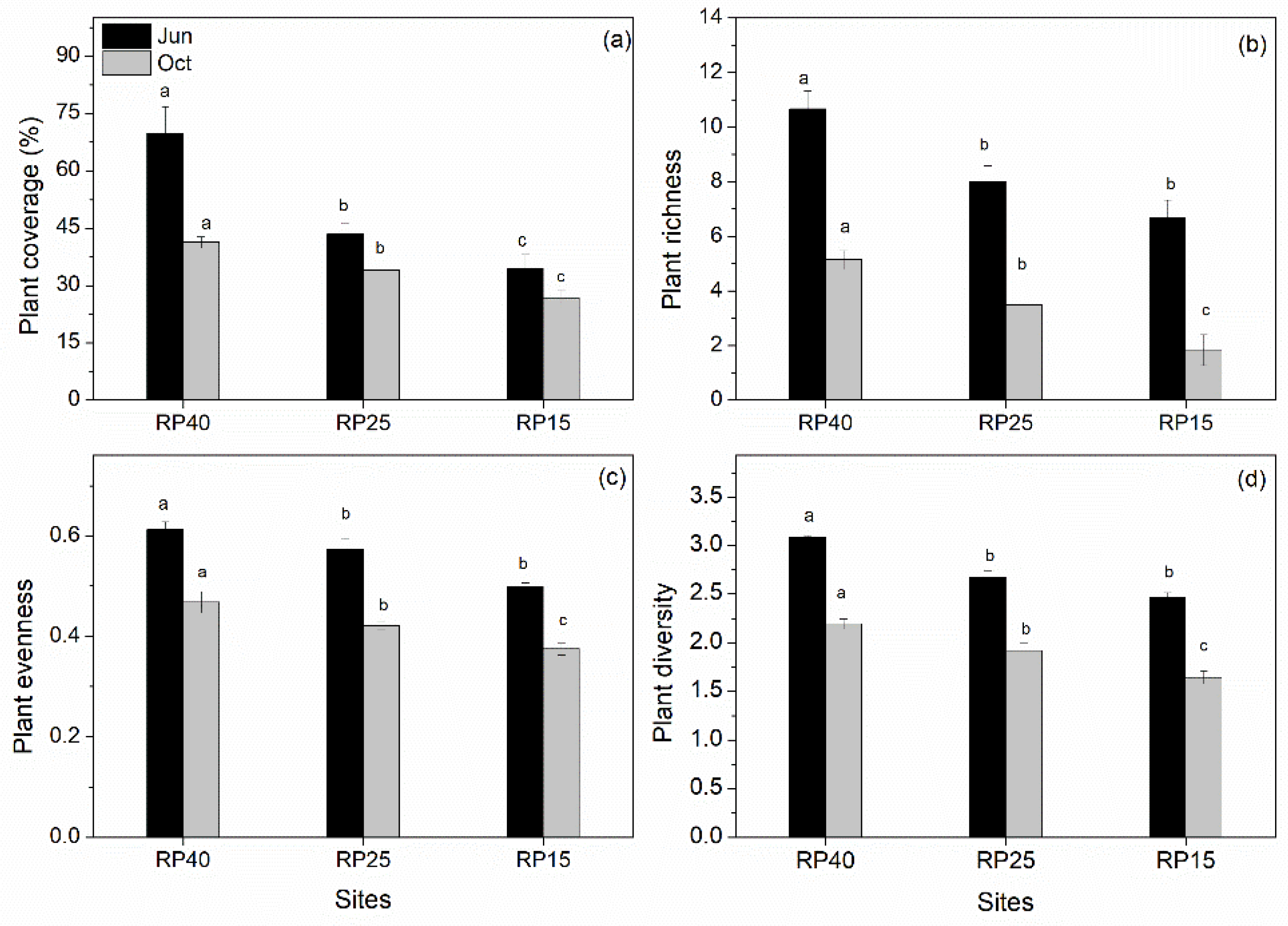
3.1. Changes in Soil Properties and Plant Community Traits after Afforestation
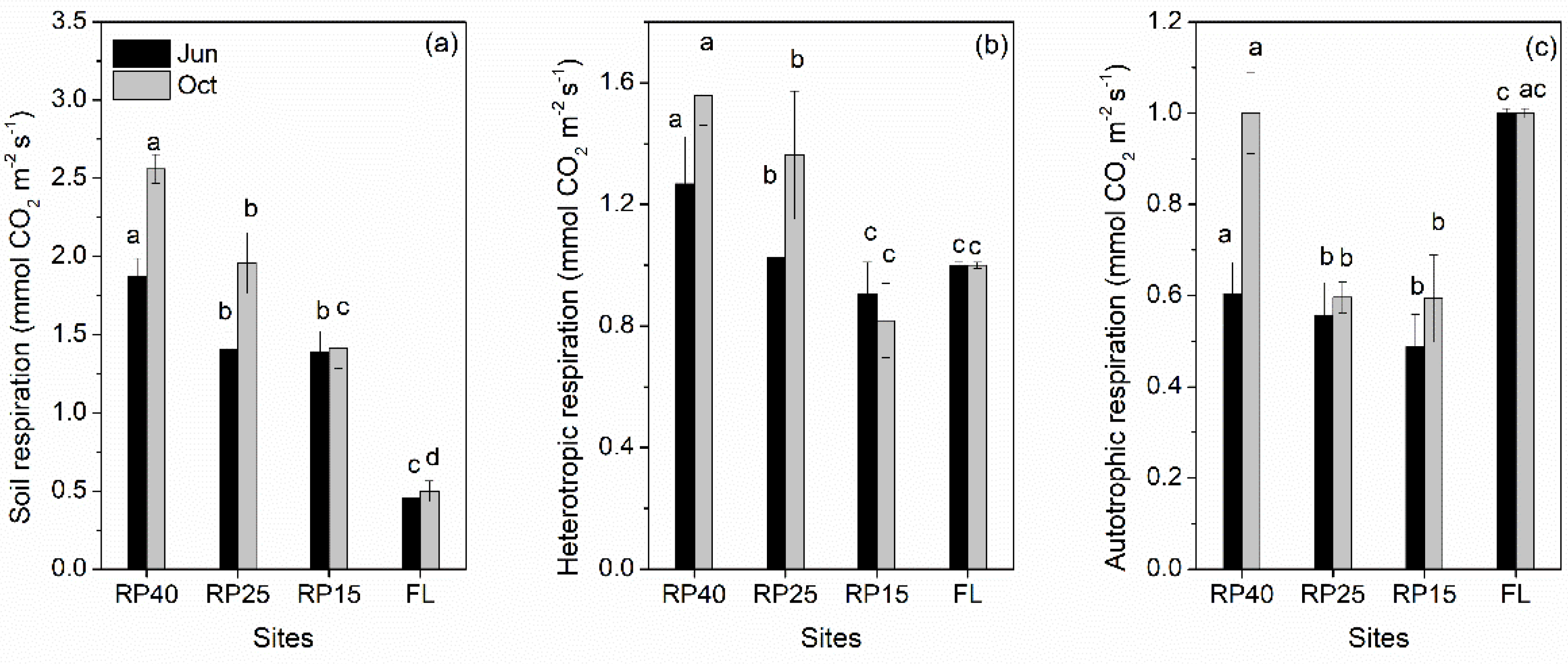
3.2. Changes in Soil Respiration and Its Components after Afforestation
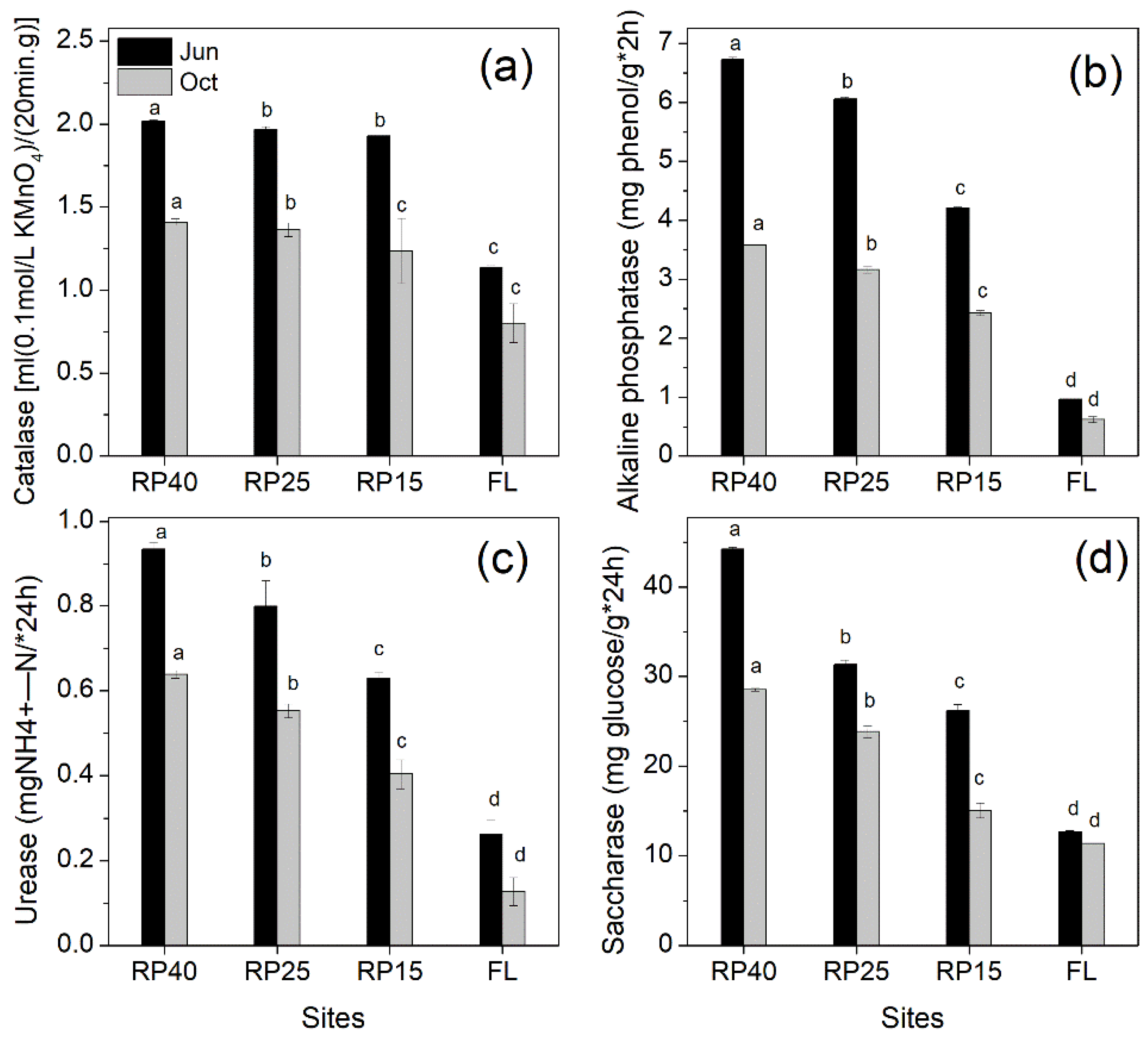
3.3. Changes in Soil Enzyme Activities after Afforestation
3.4. Changes in Soil Microbial Biomass C, N, and P Contents and Its Stoichiometry after Afforestation
3.5. Relationships between Plant and SR Linked to Microbial Biomass C, N, and P Contents and Its Stoichiometry and Soil Enzyme Activities after Afforestation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fornara, D.A.; Tilman, D. Plant functional composition influences rates of soil carbon and nitrogen accumulation. J. Ecol. 2008, 96, 314–322. [Google Scholar] [CrossRef]
- Metcalfe, D.B.; Fisher, R.A.; Wardle, D.A. Plant communities as drivers of soil respiration: Pathways, mechanisms, and significance for global change. Biogeosciences 2011, 8, 2047–2061. [Google Scholar] [CrossRef]
- Schlesinger, W.H.; Andrews, J.A. Soil respiration and the global carbon cycle. Biogeochemistry 2000, 48, 7–20. [Google Scholar] [CrossRef]
- Cong, W.F.; Ruijven, J.V.; Werf, W.V.D.; Deyn, G.B.D.; Mommer, L.; Berendse, F.; Hoffland, E. Plant species richness leaves a legacy of enhanced root litter-induced decomposition in soil. Soil. Biol. Biochem. 2015, 80, 341–348. [Google Scholar] [CrossRef]
- Fang, H.; Cheng, S.; Wang, Y.; Yu, G.; Xu, M.; Dang, X.; Li, L.; Wang, L. Changes in soil heterotrophic respiration, carbon availability, and microbial function in seven forests along a climate gradient. Ecol. Res. 2014, 29, 1077–1086. [Google Scholar] [CrossRef]
- Jewell, M.D.; Shipley, B.; Low-Décarie, E.; Tobner, C.M.; Paquette, A.; Messier, C.; Reich, P.B. Partitioning the effect of composition and diversity of tree communities on leaf litter decomposition and soil respiration. Oikos 2017, 126, 959–971. [Google Scholar] [CrossRef]
- Janssens, I.A.; Lankreijer, H.; Matteucci, G.; Kowalski, A.S.; Buchmann, N.; Epron, D.; Pilegaard, K.; Kutsch, W.; Longdoz, B.; Grünwald, T. Productivity overshadows temperature in determining soil and ecosystem respiration across European forests. Glob. Chang. Biol. 2001, 7, 269–278. [Google Scholar] [CrossRef]
- Esch, E.H.; Lipson, D.; Cleland, E.E. Direct and indirect effects of shifting rainfall on soil microbial respiration and enzyme activity in a semi-arid system. Plant Soil 2016, 411, 333–346. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Shi, Z.; Chen, J.; Han, X.; Yang, G.; Feng, Y.; Ren, G. Differential responses of soil microbial biomass and carbon-degrading enzyme activities to altered precipitation. Soil Biol. Biochem. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Xiao, H.; Li, Z.; Dong, Y.; Chang, X.; Deng, L.; Huang, J.; Nie, X.; Liu, C.; Liu, L.; Wang, D.; et al. Changes in microbial communities and respiration following the revegetation of eroded soil. Agric. Ecosyst. Environ. 2017, 246, 30–37. [Google Scholar] [CrossRef]
- Craine, J.M.; Wedin, D.A.; Reich, P.B. The response of soil CO2 flux to changes in atmospheric CO2, nitrogen supply and plant diversity. Glob. Chang. Biol. 2001, 7, 947–953. [Google Scholar] [CrossRef]
- Johnson, D.; Phoenix, G.K.; Grime, J.P. Plant community composition, not diversity, regulates soil respiration in grasslands. Biol. Lett. 2008, 4, 345–348. [Google Scholar] [CrossRef] [PubMed]
- Dias, A.T.C.; Ruijven, J.V.; Berendse, F. Plant species richness regulates soil respiration through changes in productivity. Oecologia 2010, 163, 805–813. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Xia, H.; Fu, S.; Eisenhauer, N. Tree diversity regulates soil respiration through accelerated tree growth in a mesocosm experiment. Pedobiologia 2017, 65, 24–28. [Google Scholar] [CrossRef]
- Chen, J.; Luo, Y.; Li, J.; Zhou, X.; Cao, J.; Wang, R.W.; Wang, Y.; Shelton, S.; Jin, Z.; Walker, L.M.; et al. Costimulation of soil glycosidase activity and soil respiration by nitrogen addition. Glob. Chang. Biol. 2017, 23, 1328–1337. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, X.; Hruska, T.; Cao, J.; Zhang, B.; Liu, C.; Liu, M.; Shelton, S.; Guo, L.; Wei, Y.; et al. Asymmetric Diurnal and Monthly Responses of Ecosystem Carbon Fluxes to Experimental Warming. CLEAN-Soil Air Water 2017, 45, 1600557. [Google Scholar] [CrossRef]
- Waldrop, M.P.; Zak, D.R.; Sinsabaugh, R.L. Microbial community response to nitrogen deposition in northern forest ecosystems. Soil Biol. Biochem. 2004, 36, 1443–1451. [Google Scholar] [CrossRef]
- Shahzad, T.; Chenu, C.; Genet, P.; Barot, S.; Perveen, N.; Mougin, C.; Fontaine, S. Contribution of exudates, arbuscular mycorrhizal fungi and litter depositions to the rhizosphere priming effect induced by grassland species. Soil Biol. Biochem. 2015, 80, 146–155. [Google Scholar] [CrossRef]
- Liang, C.; Schimel, J.P.; Jastrow, J.D. The importance of anabolism in microbial control over soil carbon storage. Nat. Microbiol. 2017, 2, 17105. [Google Scholar] [CrossRef] [PubMed]
- Devaraju, N.; Bala, G.; Caldeira, K.; Nemani, R. A model based investigation of the relative importance of CO2-fertilization, climate warming, nitrogen deposition and land use change on the global terrestrial carbon uptake in the historical period. Clim. Dynam. 2015, 8, 1–18. [Google Scholar] [CrossRef]
- Zhou, L.; Zhou, X.; Zhang, B.; Lu, M.; Luo, Y.; Liu, L.; Li, B. Different responses of soil respiration and its components to nitrogen addition among biomes: A meta-analysis. Glob. Chang. Biol. 2014, 20, 2332–2343. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Wang, T.; Xu, Y.; Deng, J.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Differential soil microbial community responses to the linkage of soil organic carbon fractions with respiration across land-use changes. For. Ecol. Manag. 2018, 409, 170–178. [Google Scholar] [CrossRef]
- Raich, J.W.; Tufekciogul, A. Vegetation and soil respiration: Correlations and controls. Biogeochemistry 2000, 48, 71–90. [Google Scholar] [CrossRef]
- Igor, N.; Aliaksra, R.; Alyona, S.; Evgeniy, L.; Vadim, Z.; Vladimir, P.; Rakovich, Y.P.; Donegan, J.F.; Rubin, A.B.; Govorov, A.O. Consequences of biodiversity loss for litter decomposition across biomes. Nature 2014, 509, 218–221. [Google Scholar]
- Eisenhauer, N.; Bessler, H.; Engels, C.; Gleixner, G.; Habekost, M.; Milcu, A.; Partsch, S.; Sabais, A.C.; Scherber, C.; Steinbeiss, S. Plant diversity effects on soil microorganisms support the singular hypothesis. Ecology 2010, 91, 485–496. [Google Scholar] [CrossRef] [PubMed]
- Aponte, C.; Marañón, T.; García, L.V. Microbial C, N and P in soils of Mediterranean oak forests: influence of season, canopy cover and soil depth. Biogeochemistry 2010, 101, 77–92. [Google Scholar] [CrossRef]
- Heuck, C.; Weig, A.; Spohn, M. Soil microbial biomass C:N:P stoichiometry and microbial use of organic phosphorus. Soil Biol. Biochem. 2015, 85, 119–129. [Google Scholar] [CrossRef]
- Cleveland, C.C.; Liptzin, D. C:N:P stoichiometry in soil: is there a “Redfield ratio” for the microbial biomass? Biogeochemistry 2007, 85, 235–252. [Google Scholar] [CrossRef]
- Achbergerová, L.; Nahálka, J. Polyphosphate-an ancient energy source and active metabolic regulator. Microb. Cell Fact. 2011, 10, 63. [Google Scholar] [CrossRef] [PubMed]
- Elser, J.J.; Sterner, R.W.; Gorokhova, E.; Fagan, W.F.; Markow, T.A.; Cotner, J.B.; Harrison, J.F.; Hobbie, S.E.; Odell, G.M.; Weider, L.W. Biological stoichiometry from genes to ecosystems. Ecol. Lett. 2010, 3, 540–550. [Google Scholar] [CrossRef]
- Bai, Z.; Dent, D. Recent land degradation and improvement in China. Ambio 2009, 38, 150–156. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Stratification of carbon fractions and carbon management index in deep soil affected by the Grain-to-Green program in China. PLoS ONE 2014, 9, e99657. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Xue, S.; Liu, G.B.; Song, Z.L. A comparison of soil qualities of different revegetation types in the Loess Plateau, China. Plant Soil 2011, 347, 163–178. [Google Scholar] [CrossRef]
- Zhang, C.; Liu, G.; Xue, S.; Wang, G. Soil bacterial community dynamics reflect changes in plant community and soil properties during the secondary succession of abandoned farmland in the Loess Plateau. Soil Biol. Biochem. 2016, 97, 40–49. [Google Scholar] [CrossRef]
- Tian, Q.; Taniguchi, T.; Shi, W.Y.; Li, G.; Yamanaka, N.; Du, S. Land-use types and soil chemical properties influence soil microbial communities in the semiarid Loess Plateau region in China. Sci. Rep. 2017, 7, 45289. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Sun, P.; Kang, D.; Zhao, F.; Feng, Y.; Ren, G.; Han, X.; Yang, G. Responsiveness of soil nitrogen fractions and bacterial communities to afforestation in the Loess Hilly Region (LHR) of China. Sci. Rep. 2016, 6, 28469. [Google Scholar] [CrossRef] [PubMed]
- Du, S.; Wang, Y.L.; Kume, T.; Zhang, J.G.; Otsuki, K.; Yamanaka, N.; Liu, G.B. Sapflow characteristics and climatic responses in three forest species in the semiarid Loess Plateau region of China. Agric. For. Meteorol. 2011, 151, 1–10. [Google Scholar] [CrossRef]
- Zhao, F.Z.; Zhang, L.; Ren, C.J.; Sun, J.; Han, X.H.; Yang, G.H.; Wang, J. Effect of microbial carbon, nitrogen, and phosphorus stoichiometry on soil carbon fractions under a black locust Forest within the central loess plateau of China. Soil Sci. Soc. Am. J. 2016, 80, 1520–1530. [Google Scholar] [CrossRef]
- Ren, C.; Zhao, F.; Kang, D.; Yang, G.; Han, X.; Tong, X.; Feng, Y.; Ren, G. Linkages of C:N:P stoichiometry and bacterial community in soil following afforestation of former farmland. For. Ecol. Manag. 2016, 376, 59–66. [Google Scholar] [CrossRef]
- Ren, C.; Kang, D.; Wu, J.P.; Zhao, F.; Yang, G.; Han, X.; Feng, Y.; Ren, G. Temporal variation in soil enzyme activities after afforestation in the Loess Plateau, China. Geoderma 2016, 282, 103–111. [Google Scholar] [CrossRef]
- Zhao, F.; Kang, D.; Han, X.; Yang, G.; Yang, G.; Feng, Y.; Ren, G. Soil stoichiometry and carbon storage in long-term afforestation soil affected by understory vegetation diversity. Ecol. Eng. 2015, 74, 415–422. [Google Scholar] [CrossRef]
- Hooper, D.U.; Chapin, F.S.; Ewel, J.J.; Hector, A.; Inchausti, P.; Lavorel, S.; Lawton, J.H.; Lodge, D.M.; Loreau, M.; Naeem, S. Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 2005, 75, 3–35. [Google Scholar] [CrossRef]
- Davidson, E.A.; Janssens, I.A. Temperature sensitivity of soil carbon decomposition and feedbacks to climate change. Nature 2006, 440, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Luo, Y.; Xia, J.; Shi, Z.; Jiang, L.; Niu, S.; Zhou, X.; Cao, J. Differential responses of ecosystem respiration components to experimental warming in a meadow grassland on the Tibetan Plateau. Agric. For. Meteorol. 2016, 220, 21–29. [Google Scholar] [CrossRef]
- Breshears, D.D.; Rich, P.M.; Barnes, F.J.; Campbell, K. Overstory-imposed heterogeneity in solar radiation and soil moisture in a semiarid woodland. Ecol. Appl. 1997, 7, 1201–1215. [Google Scholar] [CrossRef]
- Sinsabaugh, R.L.; Hill, B.H.; Follstad Shah, J.J. Ecoenzymatic stoichiometry of microbial organic nutrient acquisition in soil and sediment. Nature 2009, 462, 795–798. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.D.; Wallenstein, M.D.; Bradford, M.A. Soil-carbon response to warming dependent on microbial physiology. Nat. Geosci. 2010, 3, 336–340. [Google Scholar] [CrossRef]
- Ali, R.S.; Ingwersen, J.; Demyan, M.S.; Funkuin, Y.N.; Wizemann, H.D.; Kandeler, E.; Poll, C. Modelling in situ activities of enzymes as a tool to explain seasonal variation of soil respiration from agro-ecosystems. Soil. Biol. Biochem. 2015, 81, 291–303. [Google Scholar] [CrossRef]
- Sistla, S.A.; Schimel, J.P. Seasonal patterns of microbial extracellular enzyme activities in an arctic tundra soil: Identifying direct and indirect effects of long-term summer warming. Soil Biol. Biochem. 2013, 66, 119–129. [Google Scholar] [CrossRef]
- Stone, M.M.; Deforest, J.L.; Plante, A.F. Changes in extracellular enzyme activity and microbial community structure with soil depth at the Luquillo Critical Zone Observatory. Soil Biol. Biochem. 2014, 75, 237–247. [Google Scholar] [CrossRef]
- Spehn, E.M.; Joshi, J.; Schmid, B.; Diemer, M.; Korner, C. Above-ground resource use increases with plant species richness in experimental grassland ecosystems. Funct. Ecol. 2000, 14, 326–337. [Google Scholar]
- Song, B.; Niu, S.; Zhang, Z.; Yang, H.; Li, L.; Wan, S. Light and heavy fractions of soil organic matter in response to climate warming and increased precipitation in a temperate steppe. PLoS ONE 2012, 7, e33217. [Google Scholar] [CrossRef] [PubMed]
- Tucker, C.L.; Bell, J.; Pendall, E.; Ogle, K. Does declining carbon-use efficiency explain thermal acclimation of soil respiration with warming? Glob. Chang. Biol. 2013, 19, 252–263. [Google Scholar] [CrossRef] [PubMed]
- Lloret, F.; Mattana, S.; Curiel Yuste, J. Climate-induced die-off affects plant-soil-microbe ecological relationship and functioning. FEMS Microbiol. Ecol. 2015, 91, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Spohn, M.; Chodak, M. Microbial respiration per unit biomass increases with carbon-to-nutrient ratios in forest soils. Soil Biol. Biochem. 2015, 81, 128–133. [Google Scholar] [CrossRef]
- Schimel, J. Microbial ecology: Linking omics to biogeochemistry. Nat. Microbiol. 2016, 1, 15028. [Google Scholar] [CrossRef] [PubMed]
- Shoemaker, W.R.; Locey, K.J.; Lennon, J.T. A macroecological theory of microbial biodiversity. Nat. Ecol. Evol. 2017, 1, 0107. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Zhang, D.; Wang, P.; Liu, X.; Cheng, K.; Li, L.; Zheng, J.; Zhang, X.; Zheng, J.; Crowley, D.; et al. Changes in microbial biomass and the metabolic quotient with biochar addition to agricultural soils: A Meta-analysis. Agric. Ecosyst. Environ. 2017, 239, 80–89. [Google Scholar] [CrossRef]
- Zhou, Z.; Wang, C.; Jiang, L.; Luo, Y. Trends in soil microbial communities during secondary succession. Soil Biol. Biochem. 2017, 115, 92–99. [Google Scholar] [CrossRef]
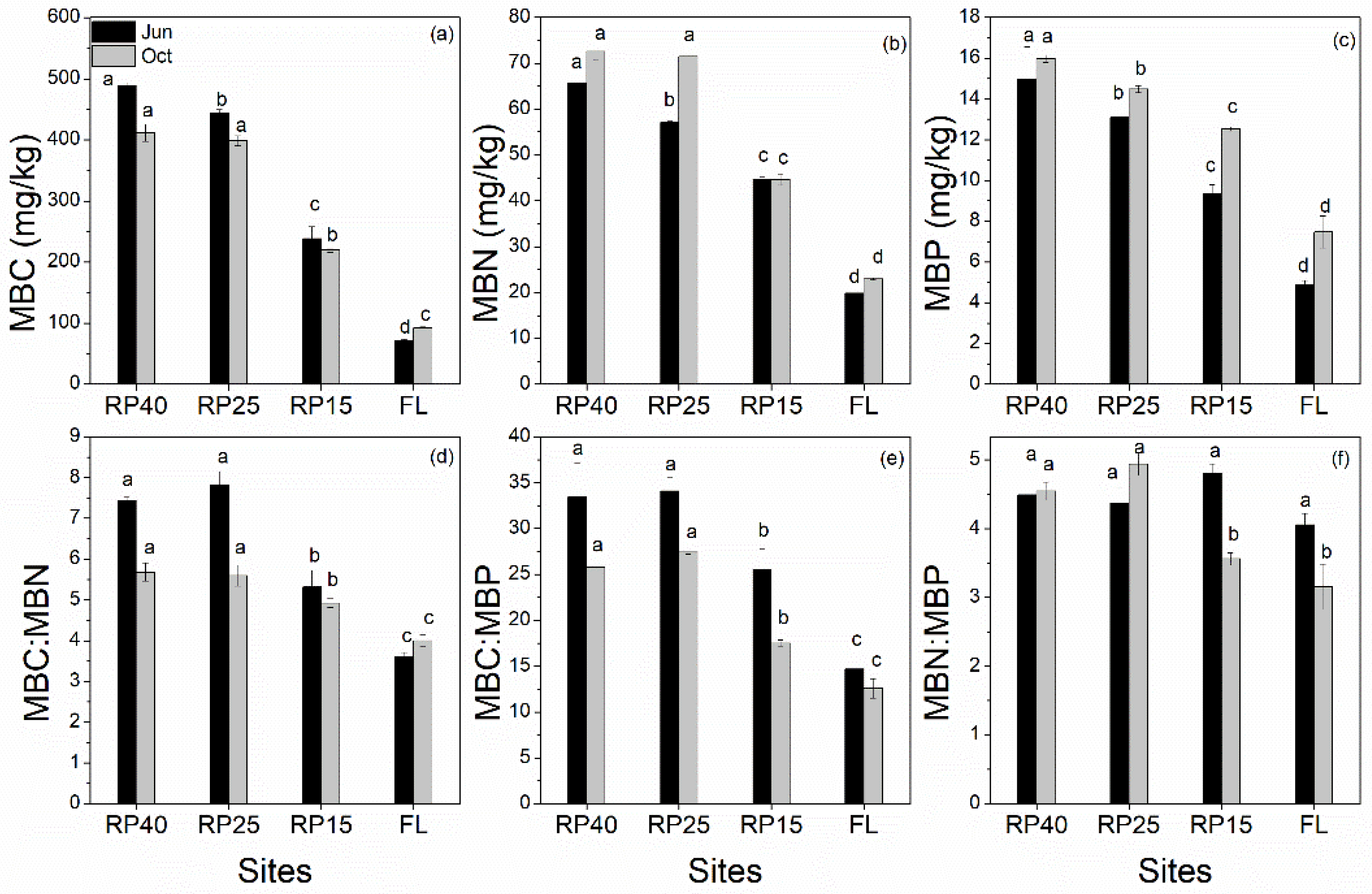
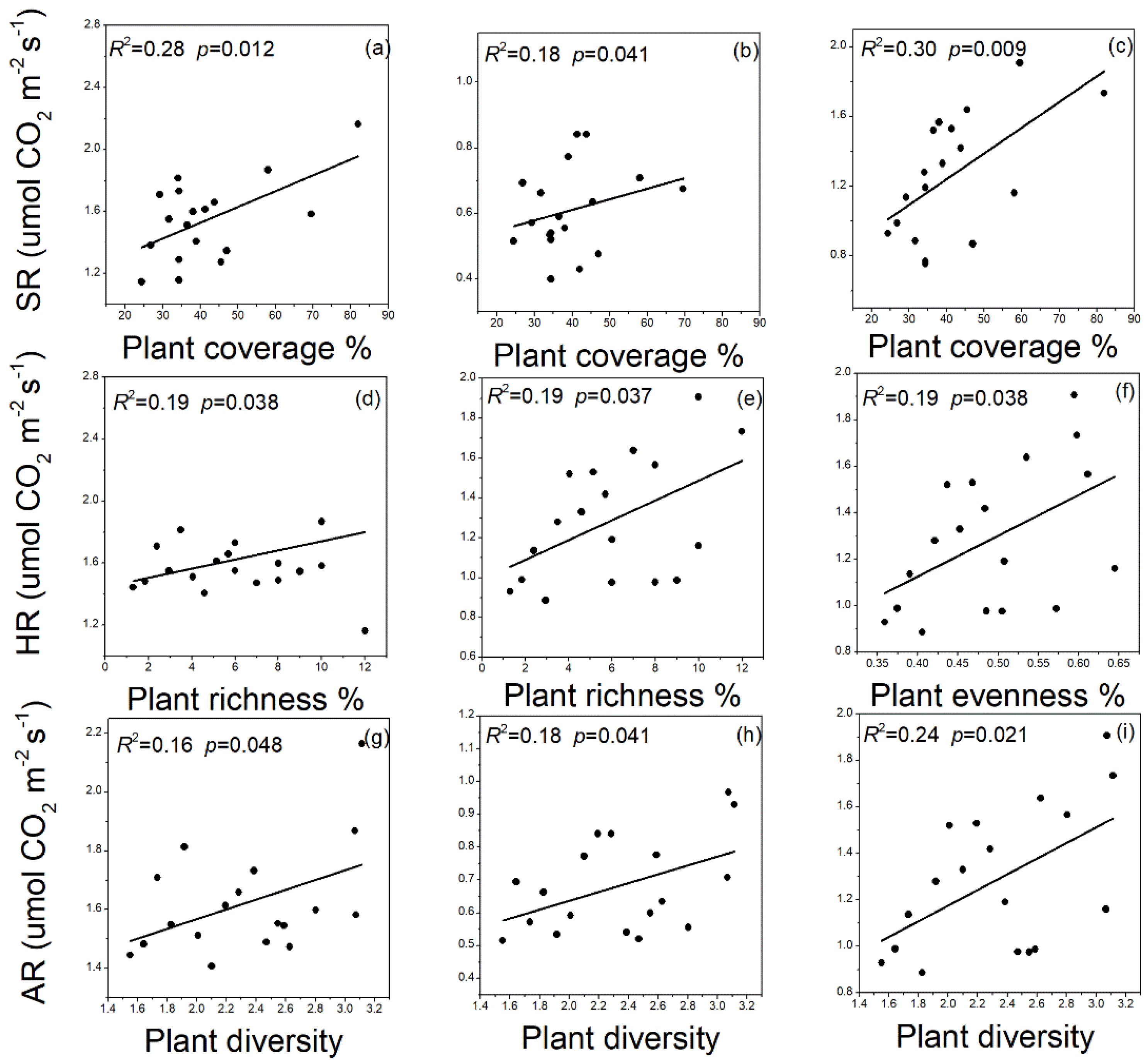
| Sites | Farmland | Robinia pseudoacacia Linn. (RP15 year) | Robinia pseudoacacia Linn. (RP25 year) | Robinia pseudoacacia Linn. (RP40 year) |
|---|---|---|---|---|
| Elevation (m) | 1205 | 1303 | 1298 | 1293 |
| Clay (%) | 8.12 ± 0.21 A | 8.55 ± 0.14 A | 9.54 ± 0.13 A | 10.11 ± 0.12 A |
| Silt (%) | 60.45 ± 0.22 A | 62.53 ± 0.19 A | 60.36 ± 0.19 A | 64.35 ± 0.21 A |
| Sand (%) | 31.43 ± 0.10 A | 28.92 ± 0.09 A | 30.10 ± 0.18 A | 25.54 ± 0.11 A |
| Dominate species | Artemisia capillaries Thunb., Heteropappus altaicus (Willd.) Novopokr., Stipa bungeana Trin., Oxytropis bicolor Bunge., Cleistogenes squarrosa (Trin.) Keng | Artemisia capillaries Thunb., Heteropappus altaicus (Willd.) Novopokr., Stipa bungeana Trin., Salsola collina Pall., Oxytropis bicolor Bunge., Cleistogenes squarrosa (Trin.) Keng | Artemisia capillaries Thunb., Heteropappus altaicus (Willd.) Novopokr., Artemisia sacrorum Ledeb., Stipa bungeana Trin., Oxytropis bicolor Bunge., Cleistogenes squarrosa (Trin.) Keng |
| Sites | SBD (g·cm−3) a | pH | WHC (%) b |
|---|---|---|---|
| Farmland | 1.14 ± 0.02 A | 9.38 ± 0.01 A | 10.27 ± 0.74 C |
| R. pseudoacacia (RP15 year) | 1.17 ± 0.01 A | 8.67 ± 0.11 A | 15.75 ± 0.98 B |
| R. pseudoacacia (RP25 year) | 1.20 ± 0.01 A | 8.65 ± 0.01 A | 16.92 ± 0.79 B |
| R. pseudoacacia (RP40 year) | 1.24 ± 0.01 A | 8.48 ± 0.02 A | 24.33 ± 1.21 A |
| Soil Enzyme Actives | ||||||
|---|---|---|---|---|---|---|
| catalase | Alkaline phosphatase | urease | saccharase | |||
| SR | −0.653 ** | −0.649 ** | −0.677 ** | −0.757 ** | ||
| HR | −0.520 * | −0.518 * | −0.410 | −0.558 * | ||
| AR | −0.595 ** | −0.649 ** | −0.774 ** | −0.717 ** | ||
| MBC | MBN | MBP | MBC:MBN | MBC:MBP | MBN:MBP | |
| SR | 0.42 | 0.582 * | 0.676 ** | 0.613 ** | −0.695 ** | 0.447 |
| HR | 0.553 * | 0.364 | 0.517 * | 0.658 ** | −0.699 ** | 0.481 * |
| AR | 0.24 | 0.586 * | 0.568 * | 0.620 ** | −0.717 ** | 0.478 * |
| Number Variables | R | Predictor Variables ** |
|---|---|---|
| 1 | 0.142 | Coverage |
| 2 | 0.116 | Coverage, Evenness |
| 3 | 0.093 | Coverage, Evenness, Diversity |
| 4 | 0.037 | Coverage, Evenness, Diversity, Richness |
| Number Variables | Soil Enzyme Actives | Soil Microbial C, N, P | Soil Microbial Stoichiometry | |||
|---|---|---|---|---|---|---|
| R | Predictor Variables | R | Predictor Variables | R | Predictor Variables | |
| 1 | 0.131 | urease | 0.112 | MBP | 0.136 | MBC:MBP |
| 2 | 0.126 | alkaline phosphatase, urease | 0.158 | MBP | 0.193 | MBC:MBP, MBN:MBP |
| 3 | 0.118 | catalase, alkaline phos-phatase, urease | 0.138 | MBP, MBN | 0.132 | MBC:MBP, MBN:MBP |
| 4 | 0.036 | catalase, alkaline phosphatase, urease, saccharase | ||||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, F.; Wang, J.; Zhang, L.; Ren, C.; Han, X.; Yang, G.; Doughty, R.; Deng, J. Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation. Forests 2018, 9, 436. https://doi.org/10.3390/f9070436
Zhao F, Wang J, Zhang L, Ren C, Han X, Yang G, Doughty R, Deng J. Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation. Forests. 2018; 9(7):436. https://doi.org/10.3390/f9070436
Chicago/Turabian StyleZhao, Fazhu, Jieying Wang, Lu Zhang, Chengjie Ren, Xinhui Han, Gaihe Yang, Russell Doughty, and Jian Deng. 2018. "Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation" Forests 9, no. 7: 436. https://doi.org/10.3390/f9070436
APA StyleZhao, F., Wang, J., Zhang, L., Ren, C., Han, X., Yang, G., Doughty, R., & Deng, J. (2018). Understory Plants Regulate Soil Respiration through Changes in Soil Enzyme Activity and Microbial C, N, and P Stoichiometry Following Afforestation. Forests, 9(7), 436. https://doi.org/10.3390/f9070436








