Effects of Growing-Season Drought on Phenology and Productivity in the West Region of Central Hardwood Forests, USA
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
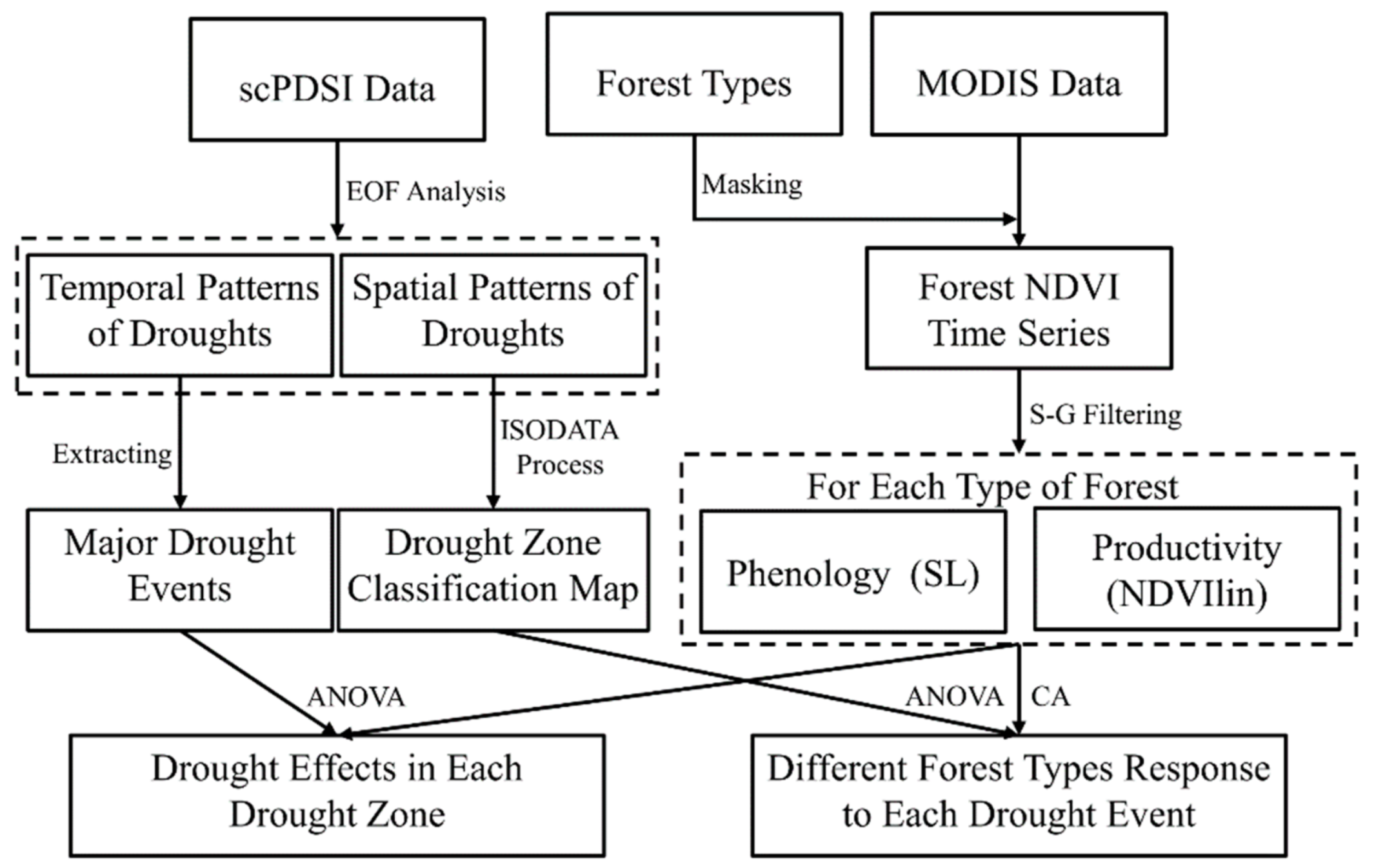
2.2. General Approach
2.3. Drought Dataset
2.4. The Forest Type Data
2.5. NDVI Time Series from MODIS
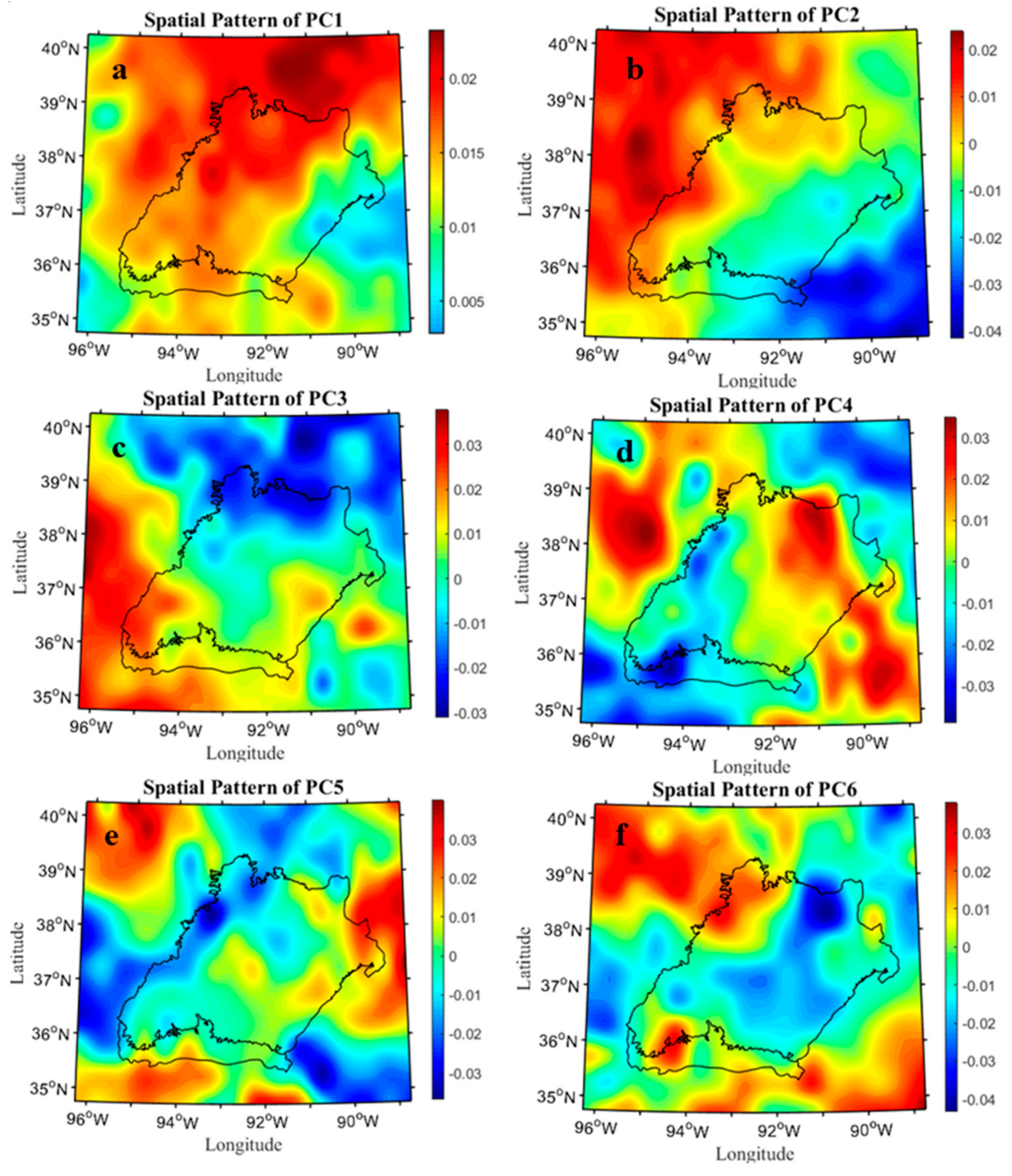
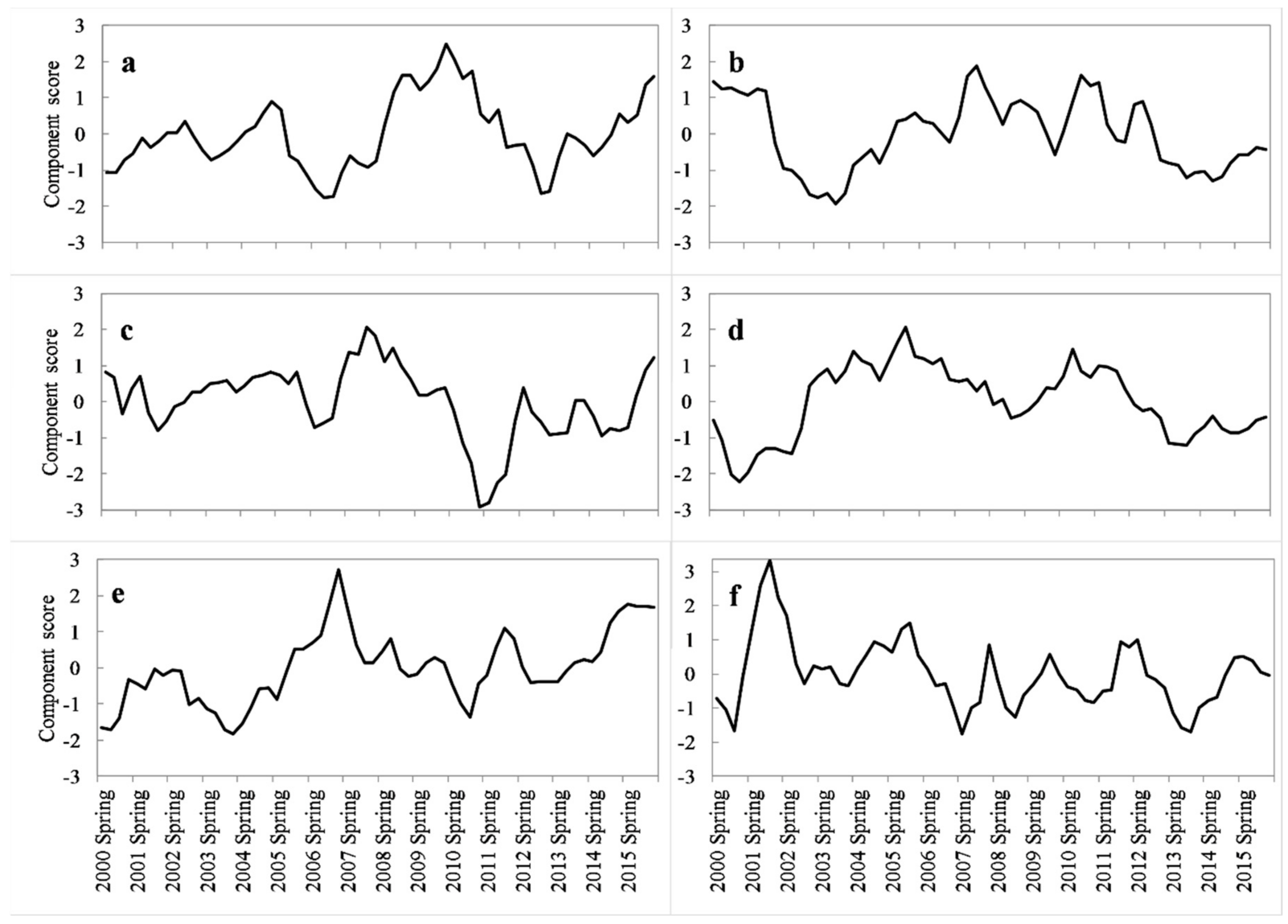
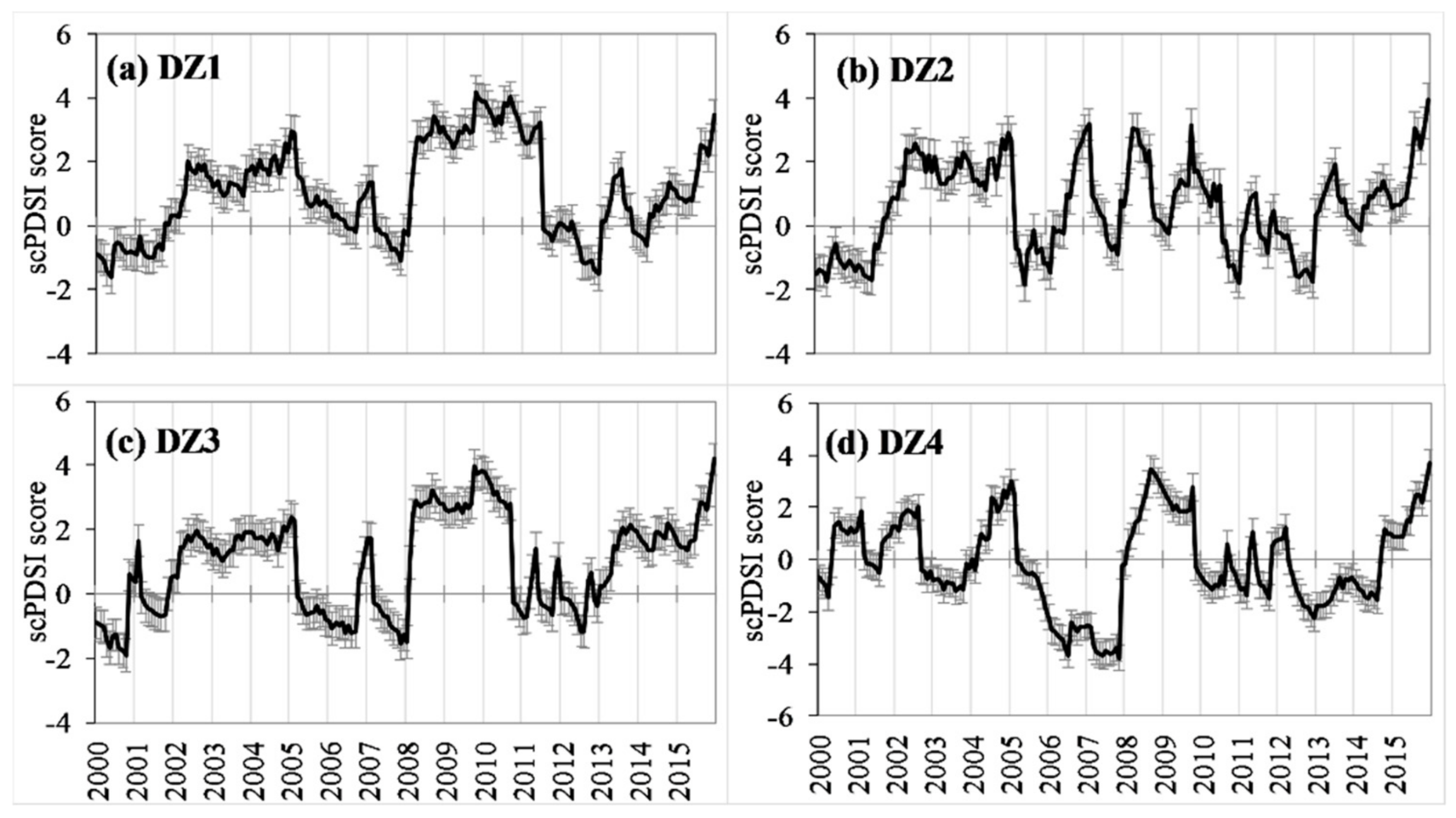
2.6. Spatial-Temporal Pattern Analysis of Drought Variability
2.7. Extraction of Forest Growth Phenology and Productivity
2.8. Drought Effects on Forest Growth Phenology and Productivity
2.9. The Response of Different Forest Types to Drought
3. Results
3.1. Drought Conditions in the West Region of Central Hardwood Forests
3.2. Forest Growth Phenology and Productivity Response to Drought of Different Severities
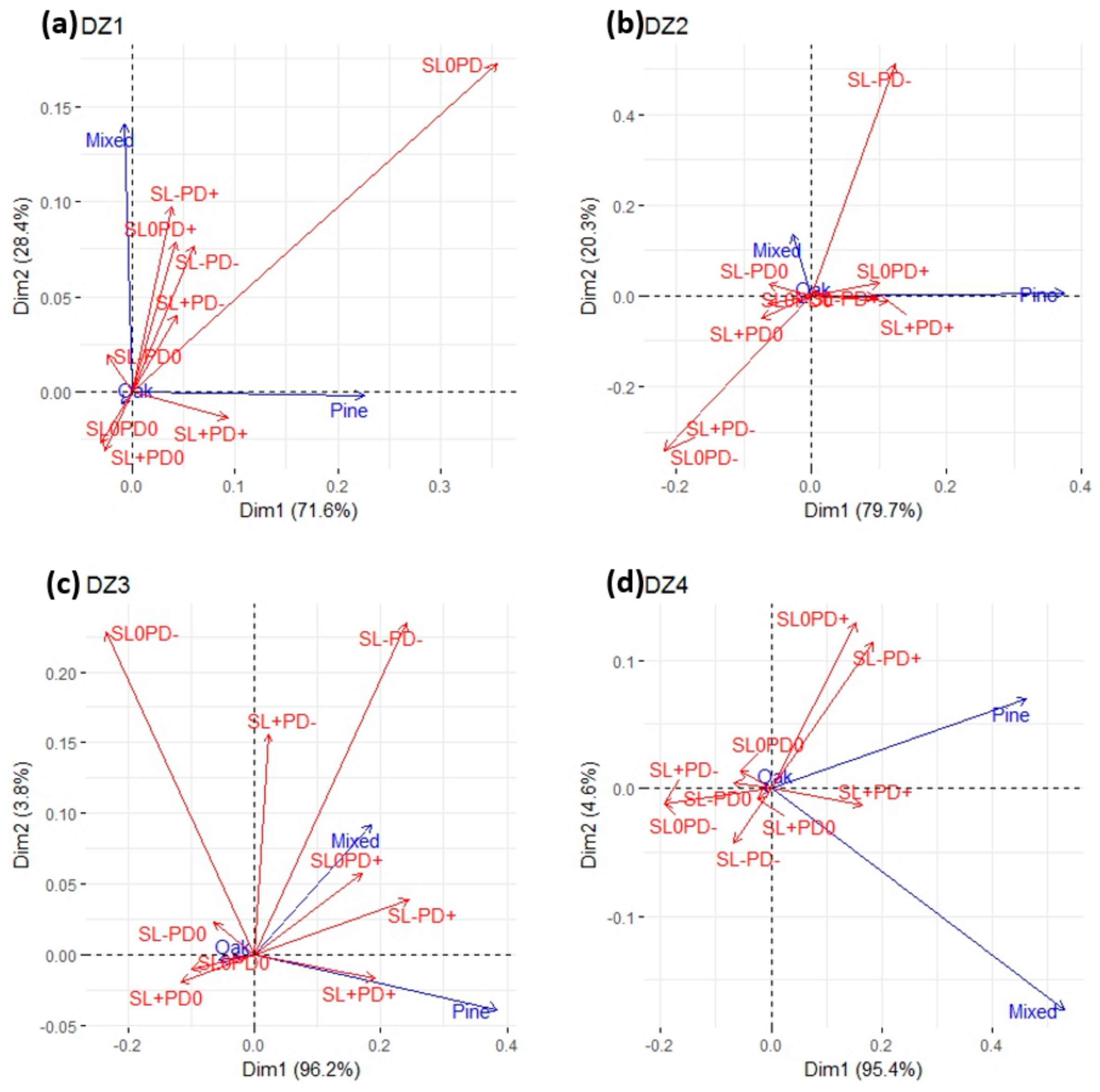
3.3. Forest Type Responses to Drought
4. Discussion
4.1. Drought Variation Estimation
4.2. Phenology and Productivity Response to Drought
4.3. Forest Types Response to Drought
4.4. Advantages and Disadvantages of the Applied Methodology
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Appendix B
| DZ1 for NDVIlin | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 3 | 22,147,183,435 | 7,382,394,478.2 | 84.06 | <0.0001 |
| Error | 2208 | 193,910,331,251 | 87,821,707.994 | ||
| Corrected Total | 2211 | 216,057,514,686 | |||
| DZ1 for SL | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 3 | 121.249634 | 40.416545 | 58.94 | <0.0001 |
| Error | 2208 | 1514.094937 | 0.685731 | ||
| Corrected Total | 2211 | 1635.344571 | |||
| DZ2 for NDVIlin: | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 3 | 15,449,150,256 | 5,149,716,752 | 137.10 | <0.0001 |
| Error | 1828 | 68,661,833,033 | 37,561,178 | ||
| Corrected Total | 1831 | 84,110,983,289 | |||
| DZ2 for SL | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 3 | 62.8476856 | 20.9492285 | 65.52 | <0.0001 |
| Error | 1828 | 584.4726638 | 0.3197334 | ||
| Corrected Total | 1831 | 647.3203493 | |||
| DZ3 for NDVIlin | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 4 | 5,541,997,181.2 | 1,385,499,295.3 | 15.66 | <0.0001 |
| Error | 2220 | 196,472,945,188 | 88,501,326.661 | ||
| Corrected Total | 2224 | 202,014,942,369 | |||
| DZ3 for SL | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 4 | 169.883622 | 42.470906 | 74.01 | <0.0001 |
| Error | 2220 | 1273.999775 | 0.573874 | ||
| Corrected Total | 2224 | 1443.883398 | |||
| DZ4 for NDVIlin | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 4 | 33,791,990,482 | 8,447,997,620.4 | 129.15 | <0.0001 |
| Error | 2600 | 170,076,503,144 | 65,414,039.671 | ||
| Corrected Total | 2604 | 203,868,493,625 | |||
| DZ4 for SL | |||||
| Source | DF | Sum of Squares | Mean Square | F Value | Pr > F |
| Model | 4 | 539.976791 | 134.994198 | 336.67 | <0.0001 |
| Error | 2600 | 1042.503992 | 0.400963 | ||
| Corrected Total | 2604 | 1582.480783 | |||
Appendix C
| Drought Zone | Type of Forest | Phenology Parameters | Normal Year Values |
| Minor drought zone | Oak forest | SL (days) | 200.2 |
| NDVIlin | 117,956.2 | ||
| Pine forest | SL (days) | 203.0 | |
| NDVIlin | 123,084.9 | ||
| Mixed forest | SL (days) | 199.0 | |
| NDVIlin | 116,653.3 | ||
| Cyclic drought zone | Oak forest | SL (days) | 195.8 |
| NDVIlin | 119,606.1 | ||
| Pine forest | SL (days) | 201.4 | |
| NDVIlin | 127,575.2 | ||
| Mixed forest | SL (days) | 208.8 | |
| NDVIlin | 121,881.1 | ||
| Moderate drought zone | Oak forest | SL (days) | 204.0 |
| NDVIlin | 124,277.9 | ||
| Pine forest | SL (days) | 213.6 | |
| NDVIlin | 123,600.5 | ||
| Mixed forest | SL (days) | 209.8 | |
| NDVIlin | 123,106.8 | ||
| Sporadic severe drought zone | Oak forest | SL (days) | 201.4 |
| NDVIlin | 118,559.1 | ||
| Pine forest | SL (days) | 198.4 | |
| NDVIlin | 119,523.7 | ||
| Mixed forest | SL (days) | 204.8 | |
| NDVIlin | 121,145.2 |
References
- Asner, G.P.; Alencar, A. Drought impacts on the amazon forest: The remote sensing perspective. New Phytol. 2010, 187, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Jackson, S.T.; Kutzbach, J.E. Projected distributions of novel and disappearing climates by 2100 AD. Proc. Natl. Acad. Sci. USA 2007, 104, 5738–5742. [Google Scholar] [CrossRef] [PubMed]
- Breshears, D.D.; Cobb, N.S.; Rich, P.M.; Price, K.P.; Allen, C.D.; Balice, R.G.; Romme, W.H.; Kastens, J.H.; Floyd, M.L.; Belnap, J. Regional vegetation die-off in response to global-change-type drought. Proc. Natl. Acad. Sci. USA 2005, 102, 15144–15148. [Google Scholar] [CrossRef] [PubMed]
- Scherrer, D.; Bader, M.K.-F.; Körner, C. Drought-sensitivity ranking of deciduous tree species based on thermal imaging of forest canopies. Agric. For. Meteorol. 2011, 151, 1632–1640. [Google Scholar] [CrossRef]
- Bonan, G.B. Forests and climate change: Forcings, feedbacks, and the climate benefits of forests. Science 2008, 320, 1444–1449. [Google Scholar] [CrossRef] [PubMed]
- Brandt, L.; He, H.; Iverson, L.; Thompson, F.R.; Butler, P.; Handler, S.; Janowiak, M.; Shannon, P.D.; Swanston, C.; Albrecht, M. Central Hardwoods Ecosystem Vulnerability Assessment and Synthesis: A Report from the Central Hardwoods Climate Change Response Framework Project; Gen. Tech. Rep. NRS-124; US Department of Agriculture, Forest Service, Northern Research Station: Newtown Square, PA, USA, 2014; Volume 124, pp. 1–254.
- Xie, Y.; Wang, X.; Silander, J.A. Deciduous forest responses to temperature, precipitation, and drought imply complex climate change impacts. Proc. Natl. Acad. Sci. USA 2015, 112, 13585–13590. [Google Scholar] [CrossRef] [PubMed]
- Camarero, J.J.; Franquesa, M.; Sangüesa-Barreda, G. Timing of drought triggers distinct growth responses in holm oak: Implications to predict warming-induced forest defoliation and growth decline. Forests 2015, 6, 1576–1597. [Google Scholar] [CrossRef]
- Jeong, S.J.; HO, C.H.; GIM, H.J.; Brown, M.E. Phenology shifts at start vs. End of growing season in temperate vegetation over the northern hemisphere for the period 1982–2008. Glob. Chang. Biol. 2011, 17, 2385–2399. [Google Scholar] [CrossRef]
- Dragoni, D.; Rahman, A.F. Trends in fall phenology across the deciduous forests of the eastern USA. Agric. For. Meteorol. 2012, 157, 96–105. [Google Scholar] [CrossRef]
- Dragoni, D.; Schmid, H.P.; Wayson, C.A.; Potter, H.; Grimmond, C.S.B.; Randolph, J.C. Evidence of increased net ecosystem productivity associated with a longer vegetated season in a deciduous forest in south-central indiana, USA. Glob. Chang. Biol. 2011, 17, 886–897. [Google Scholar] [CrossRef]
- Richardson, A.D.; Hollinger, D.Y.; Dail, D.B.; Lee, J.T.; Munger, J.W.; O’keefe, J. Influence of spring phenology on seasonal and annual carbon balance in two contrasting new england forests. Tree Physiol. 2009, 29, 321–331. [Google Scholar] [CrossRef] [PubMed]
- Kljun, N.; Black, T.; Griffis, T.; Barr, A.; Gaumont-Guay, D.; Morgenstern, K.; McCaughey, J.; Nesic, Z. Response of net ecosystem productivity of three boreal forest stands to drought. Ecosystems 2006, 9, 1128–1144. [Google Scholar] [CrossRef]
- Richardson, A.D.; Black, T.A.; Ciais, P.; Delbart, N.; Friedl, M.A.; Gobron, N.; Hollinger, D.Y.; Kutsch, W.L.; Longdoz, B.; Luyssaert, S. Influence of spring and autumn phenological transitions on forest ecosystem productivity. Philos. Trans. R. Soc. B Biol. Sci. 2010, 365, 3227–3246. [Google Scholar] [CrossRef] [PubMed]
- Piao, S.; Ciais, P.; Friedlingstein, P.; Peylin, P.; Reichstein, M.; Luyssaert, S.; Margolis, H.; Fang, J.; Barr, A.; Chen, A. Net carbon dioxide losses of northern ecosystems in response to autumn warming. Nature 2008, 451, 49–52. [Google Scholar] [CrossRef] [PubMed]
- Richardson, A.D.; Bailey, A.S.; Denny, E.G.; Martin, C.W.; O’KEEFE, J. Phenology of a northern hardwood forest canopy. Glob. Chang. Biol. 2006, 12, 1174–1188. [Google Scholar] [CrossRef]
- White, M.A.; Beurs, D.; Kirsten, M.; Didan, K.; Inouye, D.W.; Richardson, A.D.; Jensen, O.P.; O’Keefe, J.; Zhang, G.; Nemani, R.R. Intercomparison, interpretation, and assessment of spring phenology in north america estimated from remote sensing for 1982–2006. Glob. Chang. Biol. 2009, 15, 2335–2359. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I. Phenology feedbacks on climate change. Science 2009, 324, 887–888. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, C.A.; Edwards, N.T.; Walker, A.V.; O’hara, K.H.; Campion, C.M.; Hanson, P.J. Forest phenology and a warmer climate–growing season extension in relation to climatic provenance. Glob. Chang. Biol. 2012, 18, 2008–2025. [Google Scholar] [CrossRef]
- Hamunyela, E.; Verbesselt, J.; Roerink, G.; Herold, M. Trends in spring phenology of western european deciduous forests. Remote Sens. 2013, 5, 6159–6179. [Google Scholar] [CrossRef]
- Anderson, L.O.; Malhi, Y.; Aragão, L.E.; Ladle, R.; Arai, E.; Barbier, N.; Phillips, O. Remote sensing detection of droughts in amazonian forest canopies. New Phytol. 2010, 187, 733–750. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.; Unger, N.; Zhang, X.; Vogel, C. Probing the past 30-year phenology trend of us deciduous forests. Biogeosciences 2015, 12, 4693. [Google Scholar] [CrossRef]
- Ciais, P.; Reichstein, M.; Viovy, N.; Granier, A.; Ogée, J.; Allard, V.; Aubinet, M.; Buchmann, N.; Bernhofer, C.; Carrara, A. Europe-wide reduction in primary productivity caused by the heat and drought in 2003. Nature 2005, 437, 529. [Google Scholar] [CrossRef] [PubMed]
- Marchin, R.; Zeng, H.; Hoffmann, W. Drought-deciduous behavior reduces nutrient losses from temperate deciduous trees under severe drought. Oecologia 2010, 163, 845–854. [Google Scholar] [CrossRef] [PubMed]
- Leuzinger, S.; Zotz, G.; Asshoff, R.; Körner, C. Responses of deciduous forest trees to severe drought in central europe. Tree Physiol. 2005, 25, 641–650. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.D.; Breshears, D.D.; McDowell, N.G. On underestimation of global vulnerability to tree mortality and forest die-off from hotter drought in the anthropocene. Ecosphere 2015, 6, 1–55. [Google Scholar] [CrossRef]
- Dietze, M.C.; Moorcroft, P.R. Tree mortality in the eastern and central united states: Patterns and drivers. Glob. Chang. Biol. 2011, 17, 3312–3326. [Google Scholar] [CrossRef]
- Bréda, N.; Huc, R.; Granier, A.; Dreyer, E. Temperate forest trees and stands under severe drought: A review of ecophysiological responses, adaptation processes and long-term consequences. Ann. For. Sci. 2006, 63, 625–644. [Google Scholar] [CrossRef]
- Kogan, F.; Guo, W. 2006–2015 mega-drought in the western USA and its monitoring from space data. Geomat. Nat. Hazards Risk 2015, 6, 651–668. [Google Scholar] [CrossRef]
- Lindner, M.; Maroschek, M.; Netherer, S.; Kremer, A.; Barbati, A.; Garcia-Gonzalo, J.; Seidl, R.; Delzon, S.; Corona, P.; Kolström, M. Climate change impacts, adaptive capacity, and vulnerability of european forest ecosystems. For. Ecol. Manag. 2010, 259, 698–709. [Google Scholar] [CrossRef]
- Swidrak, I.; Schuster, R.; Oberhuber, W. Comparing growth phenology of co-occurring deciduous and evergreen conifers exposed to drought. Flora-Morphol. Distrib. Funct. Ecol. Plants 2013, 208, 609–617. [Google Scholar] [CrossRef]
- Weber, P.; Bugmann, H.; Rigling, A. Radial growth responses to drought of pinus sylvestris and quercus pubescens in an inner-alpine dry valley. J. Veg. Sci. 2007, 18, 777–792. [Google Scholar] [CrossRef]
- Klos, R.J.; Wang, G.G.; Bauerle, W.L.; Rieck, J.R. Drought impact on forest growth and mortality in the southeast USA: An analysis using forest health and monitoring data. Ecol. Appl. 2009, 19, 699–708. [Google Scholar] [CrossRef] [PubMed]
- Coops, N.C.; Waring, R.H. Estimating the vulnerability of fifteen tree species under changing climate in northwest north america. Ecol. Model. 2011, 222, 2119–2129. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Species-specific drought effects on flower and fruit production in a mediterranean holm oak forest. Forestry 2007, 80, 351–357. [Google Scholar] [CrossRef]
- Arend, M.; Kuster, T.; Günthardt-Goerg, M.S.; Dobbertin, M. Provenance-specific growth responses to drought and air warming in three european oak species (Quercus robur, Q. Petraea and Q. Pubescens). Tree Physiol. 2011, 31, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Montserrat-Marti, G.; Camarero, J.J.; Palacio, S.; Pérez-Rontomé, C.; Milla, R.; Albuixech, J.; Maestro, M. Summer-drought constrains the phenology and growth of two coexisting mediterranean oaks with contrasting leaf habit: Implications for their persistence and reproduction. Trees 2009, 23, 787–799. [Google Scholar] [CrossRef]
- Kuster, T.M.; Dobbertin, M.; Günthardt-Goerg, M.S.; Schaub, M.; Arend, M. A phenological timetable of oak growth under experimental drought and air warming. PLoS ONE 2014, 9, e89724. [Google Scholar] [CrossRef] [PubMed]
- Maseyk, K.; Grünzweig, J.M.; Rotenberg, E.; Yakir, D. Respiration acclimation contributes to high carbon-use efficiency in a seasonally dry pine forest. Glob. Chang. Biol. 2008, 14, 1553–1567. [Google Scholar] [CrossRef]
- Arend, M.; Sever, K.; Pflug, E.; Gessler, A.; Schaub, M. Seasonal photosynthetic response of european beech to severe summer drought: Limitation, recovery and post-drought stimulation. Agric. For. Meteorol. 2016, 220, 83–89. [Google Scholar] [CrossRef]
- Peters, M.P.; Iverson, L.R.; Matthews, S.N. Long-term droughtiness and drought tolerance of eastern us forests over five decades. For. Ecol. Manag. 2015, 345, 56–64. [Google Scholar] [CrossRef]
- Wells, N.; Goddard, S.; Hayes, M.J. A self-calibrating palmer drought severity index. J. Clim. 2004, 17, 2335–2351. [Google Scholar] [CrossRef]
- Rohli, R.V.; Bushra, N.; Lam, N.S.; Zou, L.; Mihunov, V.; Reams, M.A.; Argote, J.E. Drought indices as drought predictors in the south-central USA. Nat. Hazards 2016, 83, 1567–1582. [Google Scholar] [CrossRef]
- Dai, A. Increasing drought under global warming in observations and models. Nat. Clim. Chang. 2013, 3, 52. [Google Scholar] [CrossRef]
- Melaas, E.K.; Sulla-Menashe, D.; Gray, J.M.; Black, T.A.; Morin, T.H.; Richardson, A.D.; Friedl, M.A. Multisite analysis of land surface phenology in north american temperate and boreal deciduous forests from landsat. Remote Sens. Environ. 2016, 186, 452–464. [Google Scholar] [CrossRef]
- Goward, S.N.; Dye, D.; Kerber, A.; Kalb, V. Comparison of north and south american biomes from avhrr observations. Geocarto Int. 1987, 2, 27–39. [Google Scholar] [CrossRef]
- Beck, P.S.; Juday, G.P.; Alix, C.; Barber, V.A.; Winslow, S.E.; Sousa, E.E.; Heiser, P.; Herriges, J.D.; Goetz, S.J. Changes in forest productivity across alaska consistent with biome shift. Ecol. Lett. 2011, 14, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nigh, T.A.; Schroeder, W.A. Atlas of Missouri Ecoregions; Missouri Department of Conservation: Jefferson City, MO, USA, 2002.
- McNab, W.H.; Avers, P.E. Ecological Subregions of the United States, Section Descriptions; Food and Agriculture Organization of the United Nations: Rome, Italy, 1994. [Google Scholar]
- Jones, P.; Harris, I. CRU TS3.25: Climatic Research Unit (CRU) Time-Series (TS) Version 3.25 of High-Resolution Gridded Data of Month-by-month Variation in Climate (Jan. 1901–Dec. 2016). Available online: http://data.ceda.ac.uk/badc/cru/data/cru_ts/cru_ts_3.25/ (accessed on 21 September 2017).
- Schrier, G.; Barichivich, J.; Briffa, K.; Jones, P. A scpdsi-based global data set of dry and wet spells for 1901–2009. J. Geophys. Res. Atmos. 2013, 118, 4025–4048. [Google Scholar] [CrossRef]
- Homer, C.; Dewitz, J.; Yang, L.; Jin, S.; Danielson, P.; Xian, G.; Megown, K. Completion of the 2011 National Land Cover Database for the conterminous United States–representing a decade of land cover change information. Photogramm. Eng. Remote Sens. 2015, 81, 345–354. [Google Scholar]
- Didan, K. MOD13Q1 MODIS/Terra Vegetation Indices 16-Day L3 Global 250m SIN Grid. Available online: https://lpdaac.usgs.gov/node/844 (accessed on 30 September 2016).
- Bjornsson, H.; Venegas, S. A Manual for EOF and SVD Analyses of Climatic Data; CCGCR Report; McGill University: Montreal, QC, Canada, 1997; Volume 97, pp. 112–134. [Google Scholar]
- Liu, X.; Wang, S.; Zhou, Y.; Wang, F.; Li, W.; Liu, W. Regionalization and spatiotemporal variation of drought in china based on standardized precipitation evapotranspiration index (1961–2013). Adv. Meteorol. 2015, 2015, 950262. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Eklundh, L.; Hellström, M.; Bärring, L.; Jönsson, P. Annual changes in modis vegetation indices of swedish coniferous forests in relation to snow dynamics and tree phenology. Remote Sens. Environ. 2010, 114, 2719–2730. [Google Scholar] [CrossRef]
- Jönsson, A.M.; Schroeder, L.M.; Lagergren, F.; Anderbrant, O.; Smith, B. Guess the impact of ips typographus—An ecosystem modelling approach for simulating spruce bark beetle outbreaks. Agric. For. Meteorol. 2012, 166, 188–200. [Google Scholar] [CrossRef]
- Ivits, E.; Cherlet, M.; Sommer, S.; Mehl, W. Addressing the complexity in non-linear evolution of vegetation phenological change with time-series of remote sensing images. Ecol. Indic. 2013, 26, 49–60. [Google Scholar] [CrossRef]
- Husson, F.; Lê, S.; Pagès, J. Exploratory Multivariate Analysis by Example Using R; Chapman and Hall/CRC: Boca Raton, FL, USA, 2017. [Google Scholar]
- Wang, C.; He, H.S.; Kabrick, J.M. A remote sensing-assisted risk rating study to predict oak decline and recovery in the missouri ozark highlands, USA. GIScience Remote Sens. 2008, 45, 406–425. [Google Scholar] [CrossRef]
- Seco, R.; Karl, T.; Guenther, A.; Hosman, K.P.; Pallardy, S.G.; Gu, L.; Geron, C.; Harley, P.; Kim, S. Ecosystem-scale volatile organic compound fluxes during an extreme drought in a broadleaf temperate forest of the missouri ozarks (central USA). Glob. Chang. Biol. 2015, 21, 3657–3674. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Pallardy, S.G.; Meyers, T.P.; GU, L.H.; Hanson, P.J.; Wullschleger, S.D.; Heuer, M.; Hosman, K.P.; Riggs, J.S.; Sluss, D.W. Environmental controls on water use efficiency during severe drought in an ozark forest in missouri, USA. Glob. Chang. Biol. 2010, 16, 2252–2271. [Google Scholar] [CrossRef]
- Fan, Z.; Fan, X.; Crosby, M.K.; Moser, W.K.; He, H.; Spetich, M.A.; Shifley, S.R. Spatio-temporal trends of oak decline and mortality under periodic regional drought in the ozark highlands of arkansas and missouri. Forests 2012, 3, 614–631. [Google Scholar] [CrossRef]
- Dunn, A.L.; Barford, C.C.; Wofsy, S.C.; Goulden, M.L.; Daube, B.C. A long-term record of carbon exchange in a boreal black spruce forest: Means, responses to interannual variability, and decadal trends. Glob. Chang. Biol. 2007, 13, 577–590. [Google Scholar] [CrossRef]
- LeBlanc, D.C. Interactive effects of acidic deposition, drought, and insect attack on oak populations in the midwestern united states. Can. J. For. Res. 1998, 28, 1184–1197. [Google Scholar] [CrossRef]
- Ogaya, R.; Peñuelas, J. Tree growth, mortality, and above-ground biomass accumulation in a holm oak forest under a five-year experimental field drought. Plant Ecol. 2007, 189, 291–299. [Google Scholar] [CrossRef]
- Hinckley, T.; Dougherty, P.; Lassoie, J.; Roberts, J.; Teskey, R. A severe drought: Impact on tree growth, phenology, net photosynthetic rate and water relations. Am. Midl. Nat. 1979, 307–316. [Google Scholar] [CrossRef]
- Seneviratne, S.I. Climate science: Historical drought trends revisited. Nature 2012, 491, 338–339. [Google Scholar] [CrossRef] [PubMed]
- Buitenwerf, R.; Rose, L.; Higgins, S.I. Three decades of multi-dimensional change in global leaf phenology. Nat. Clim. Chang. 2015, 5, 364–368. [Google Scholar] [CrossRef]
- Aguadé, D.; Poyatos, R.; Rosas, T.; Martínez-Vilalta, J. Comparative drought responses of quercus ilex l. and pinus sylvestris l. In a montane forest undergoing a vegetation shift. Forests 2015, 6, 2505–2529. [Google Scholar] [CrossRef]
- Pretzsch, H.; Schütze, G.; Uhl, E. Resistance of european tree species to drought stress in mixed versus pure forests: Evidence of stress release by inter-specific facilitation. Plant Biol. 2013, 15, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Morin, X.; Fahse, L.; Scherer-Lorenzen, M.; Bugmann, H. Tree species richness promotes productivity in temperate forests through strong complementarity between species. Ecol. Lett. 2011, 14, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Hommel, R.; Siegwolf, R.; Zavadlav, S.; Arend, M.; Schaub, M.; Galiano, L.; Haeni, M.; Kayler, Z.E.; Gessler, A. Impact of interspecific competition and drought on the allocation of new assimilates in trees. Plant Biol. 2016, 18, 785–796. [Google Scholar] [CrossRef] [PubMed]
- Ruehr, N.K.; Offermann, C.A.; Gessler, A.; Winkler, J.B.; Ferrio, J.P.; Buchmann, N.; Barnard, R.L. Drought effects on allocation of recent carbon: From beech leaves to soil CO2 efflux. New Phytol. 2009, 184, 950–961. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, S.; Friedl, M.A.; Tan, B.; Zhang, X.; Verma, M. Land surface phenology from modis: Characterization of the collection 5 global land cover dynamics product. Remote Sens. Environ. 2010, 114, 1805–1816. [Google Scholar] [CrossRef]
- Lewińska, K.E.; Ivits, E.; Schardt, M.; Zebisch, M. Alpine forest drought monitoring in south tyrol: PCA based synergy between scPDSI data and modis derived NDVI and NDII7 time series. Remote Sens. 2016, 8, 639. [Google Scholar] [CrossRef]


| Value in NLCD | Forest Types | Descriptions |
|---|---|---|
| 41 | Oak forest | These areas are dominated by oak species. |
| >42 | >Pine forest | >These areas are dominated by pine species. |
| 43 | Mixed oak-pine forest | These areas are co-dominated by oak and pine species. |
| 11, 12, 21–24, 31, 51, 52, 71–74, 81, 82, 90, 95 | Others | Water, urban or built-up land, agriculture, barren, tundra, wetland, rangeland, perennial snow or ice. |
| Drought Zone | # of Samples in Oak Forest | # of Samples in Pine Forest | # of Samples in Mixed Forests | Total |
|---|---|---|---|---|
| DZ1 | 474 | 55 | 83 | 612 |
| DZ2 | 458 | 65 | 73 | 596 |
| DZ3 | 445 | 71 | 81 | 597 |
| DZ4 | 521 | 21 | 11 | 553 |
| Drought Zone (Avg. scPDSI for 2000–2015) | Type of Forest | Phenology Parameters | Drought Year (avg. scPDSI for the Drought Event) | ||||
|---|---|---|---|---|---|---|---|
| Minor drought zone (1.05) | 2000 (−0.92) | 2001 (−0.79) | 2007 (−0.48) | 2012 (−0.52) | |||
| Oak forest | SL (days) | 190.9 d | 197.2 c | 208.9 a | 201.5 b | ||
| NDVIlin | 117,012.1 c | 119,454.3 b | 123,368.5 a | 119,560.8 b | |||
| Pine forest | SL (days) | 190.0 b | 196.3 b | 210.1 a | 201.9 a | ||
| NDVIlin | 117,133.0 b | 119,557.0 a | 124,128.0 a | 119,103.0 a | |||
| Mixed forest | SL (days) | 187.8 c | 194.8 c | 208.5 a | 199.9 b | ||
| NDVIlin | 116,292.8 b | 118,645.2 b | 123,500.6 a | 119,462.3 b | |||
| Cyclic drought zone (0.60) | 2000 (−1.20) | 2001 (−0.77) | 2005 (−0.29) | 2012 (−1.01) | |||
| Oak forest | SL (days) | 195.4 c | 201.1 b | 203.6 a | 200.2 b | ||
| NDVIlin | 122,278.9 b | 124,853.4 a | 124,742.4 a | 117,687.1 c | |||
| Pine forest | SL (days) | 194.3 b | 199.9 a | 204.0 a | 200.0 a | ||
| NDVIlin | 121,145.0 ab | 123,408.0 a | 125,588.0 a | 118,018.0 c | |||
| Mixed forest | SL (days) | 195.0 c | 198.3 b | 204.8 a | 201.0 b | ||
| NDVIlin | 121,145.3 a | 123,408.3 a | 125,588.0 a | 118,017.9 b | |||
| Moderate drought zone (0.95) | 2000 (−1.08) | 2001 (−0.12) | 2005 (−0.22) | 2006 (−0.55) | 2007 (−0.43) | ||
| Oak forest | SL (days) | 207.7 c | 208.6 b | 202.4 d | 215.3a | 212.4 b | |
| NDVIlin | 122,209.1 b | 122,786.7 b | 120,134.1 c | 124,507.9 a | 124,243.3 a | ||
| Pine forest | SL (days) | 206.0 b | 207.9 b | 203.9 c | 216.2 a | 212.2 a | |
| NDVIlin | 122,420.0 a | 123,654.0 a | 121,759.0 a | 125,948.0 a | 125,226.0 a | ||
| Mixed forest | SL (days) | 208.2 b | 209.7 b | 204.0 c | 214.3 a | 214.0 a | |
| NDVIlin | 123,573 a | 124,072 a | 122,588 a | 125,401 a | 125,918 a | ||
| Sporadic severe drought zone (−0.12) | 2003 (−0.83) | 2005 (−0.72) | 2006 (−2.80) | 2007 (−3.11) | 2013 (−1.2) | ||
| Oak forest | SL (days) | 187.1 d | 195.1 c | 199.6 b | 200.8 b | 209.3 a | |
| NDVIlin | 117,956.3 c | 125,627.6 b | 127,971.8 a | 126,930.9 a | 126,513.8 a | ||
| Pine forest | SL (days) | 184.2 c | 188.3 b | 190.7 b | 197.6 b | 218.9 a | |
| NDVIlin | 123,085.0 c | 127,783.0 b | 129,003.0 b | 131,911.0 b | 135,909.0 a | ||
| Mixed forest | SL (days) | 183.9 a | 192.0 a | 198.4 a | 199.4 a | 203.8 a | |
| NDVIlin | 114,653 a | 124,281 a | 124,906 a | 123,075 a | 123,527 a | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Duan, S.; He, H.S.; Spetich, M. Effects of Growing-Season Drought on Phenology and Productivity in the West Region of Central Hardwood Forests, USA. Forests 2018, 9, 377. https://doi.org/10.3390/f9070377
Duan S, He HS, Spetich M. Effects of Growing-Season Drought on Phenology and Productivity in the West Region of Central Hardwood Forests, USA. Forests. 2018; 9(7):377. https://doi.org/10.3390/f9070377
Chicago/Turabian StyleDuan, Shengwu, Hong S. He, and Marty Spetich. 2018. "Effects of Growing-Season Drought on Phenology and Productivity in the West Region of Central Hardwood Forests, USA" Forests 9, no. 7: 377. https://doi.org/10.3390/f9070377
APA StyleDuan, S., He, H. S., & Spetich, M. (2018). Effects of Growing-Season Drought on Phenology and Productivity in the West Region of Central Hardwood Forests, USA. Forests, 9(7), 377. https://doi.org/10.3390/f9070377




