Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China
Abstract
1. Introduction
2. Materials and Methods
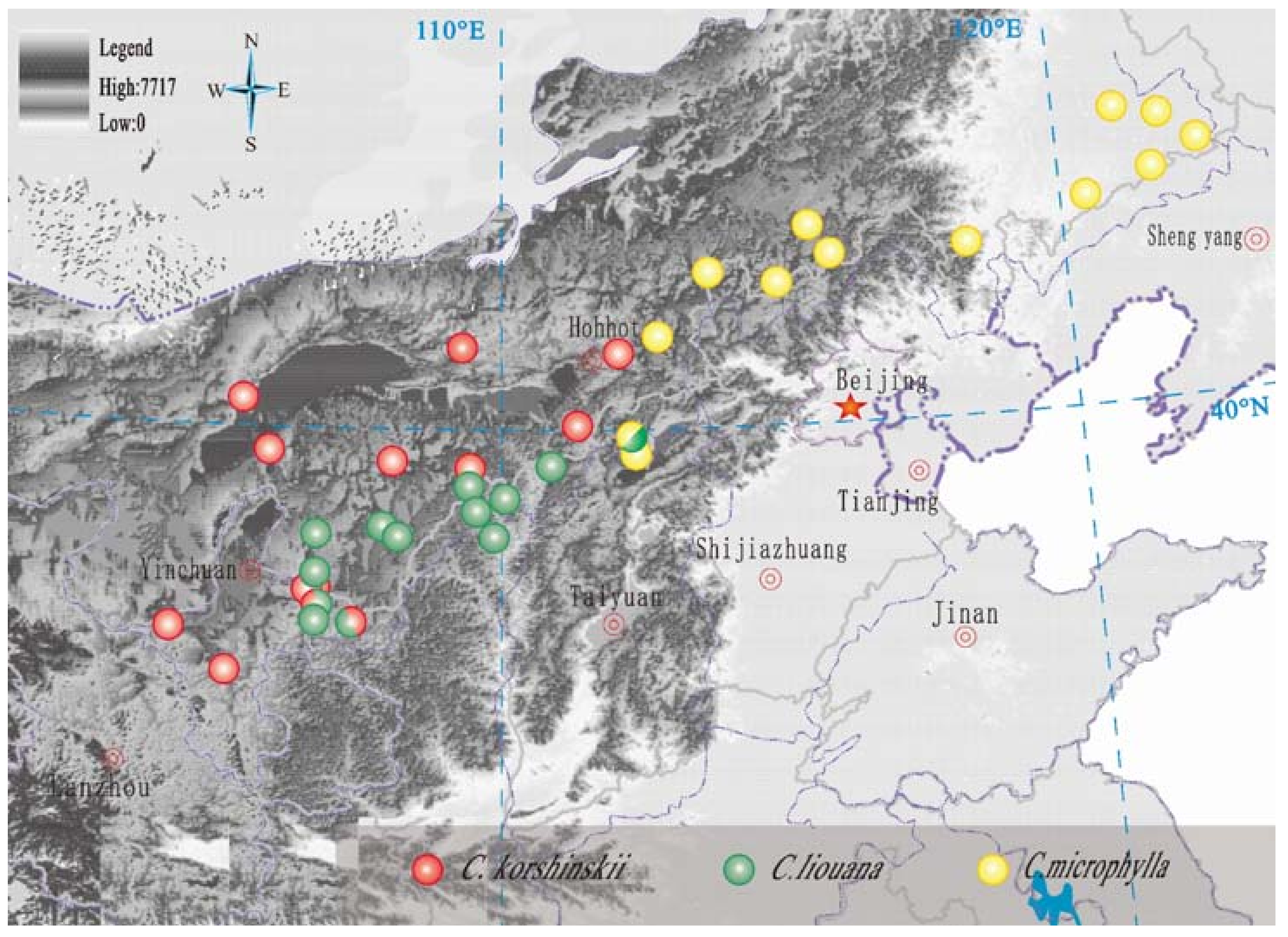
2.1. Study Area
2.2. Sampling and Measurements
2.3. Meteorological Data
2.4. Data Analysis
3. Results
3.1. Variation across the Sites in Climate and Leaf Nutrient Composition of Three Caragana Species
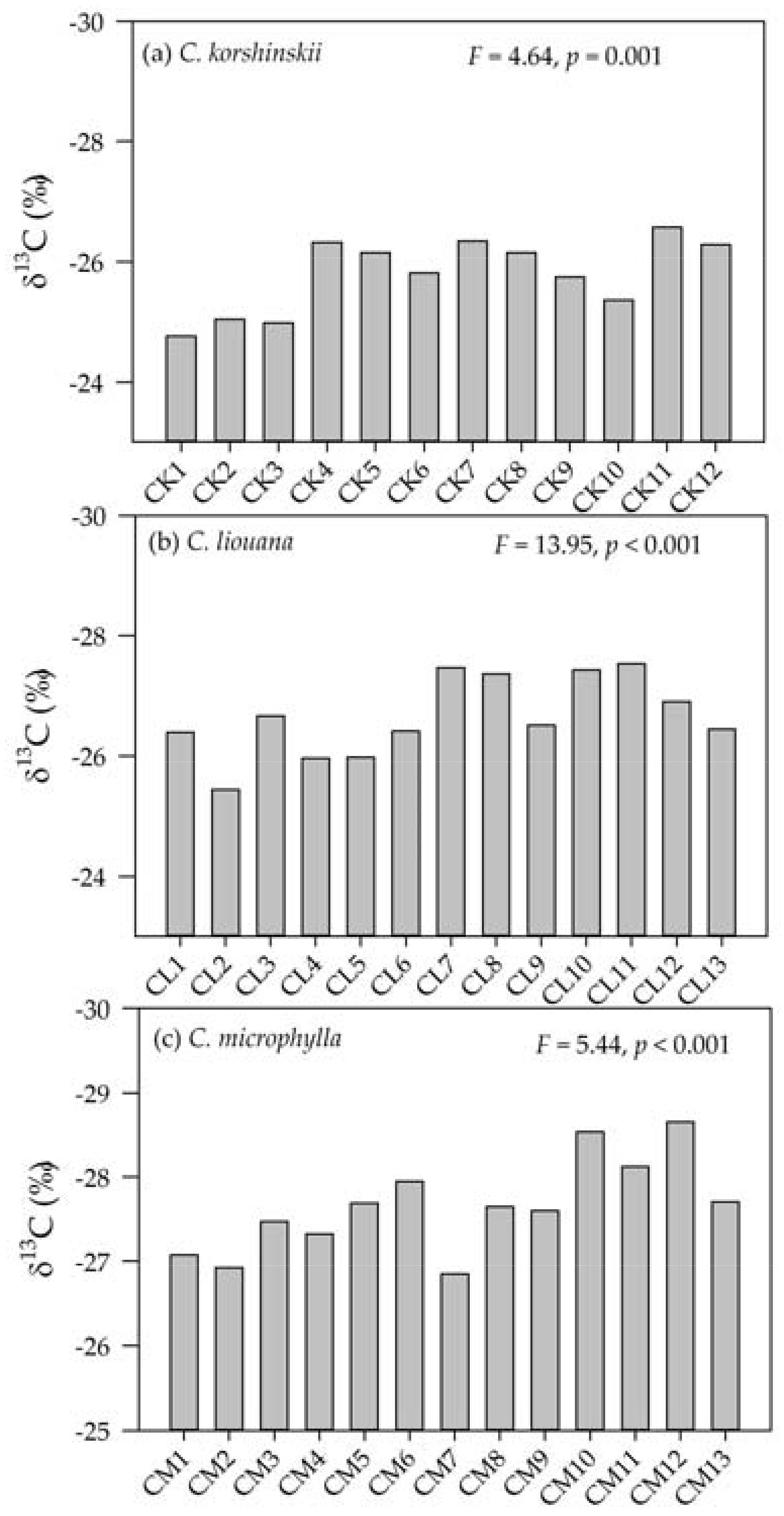
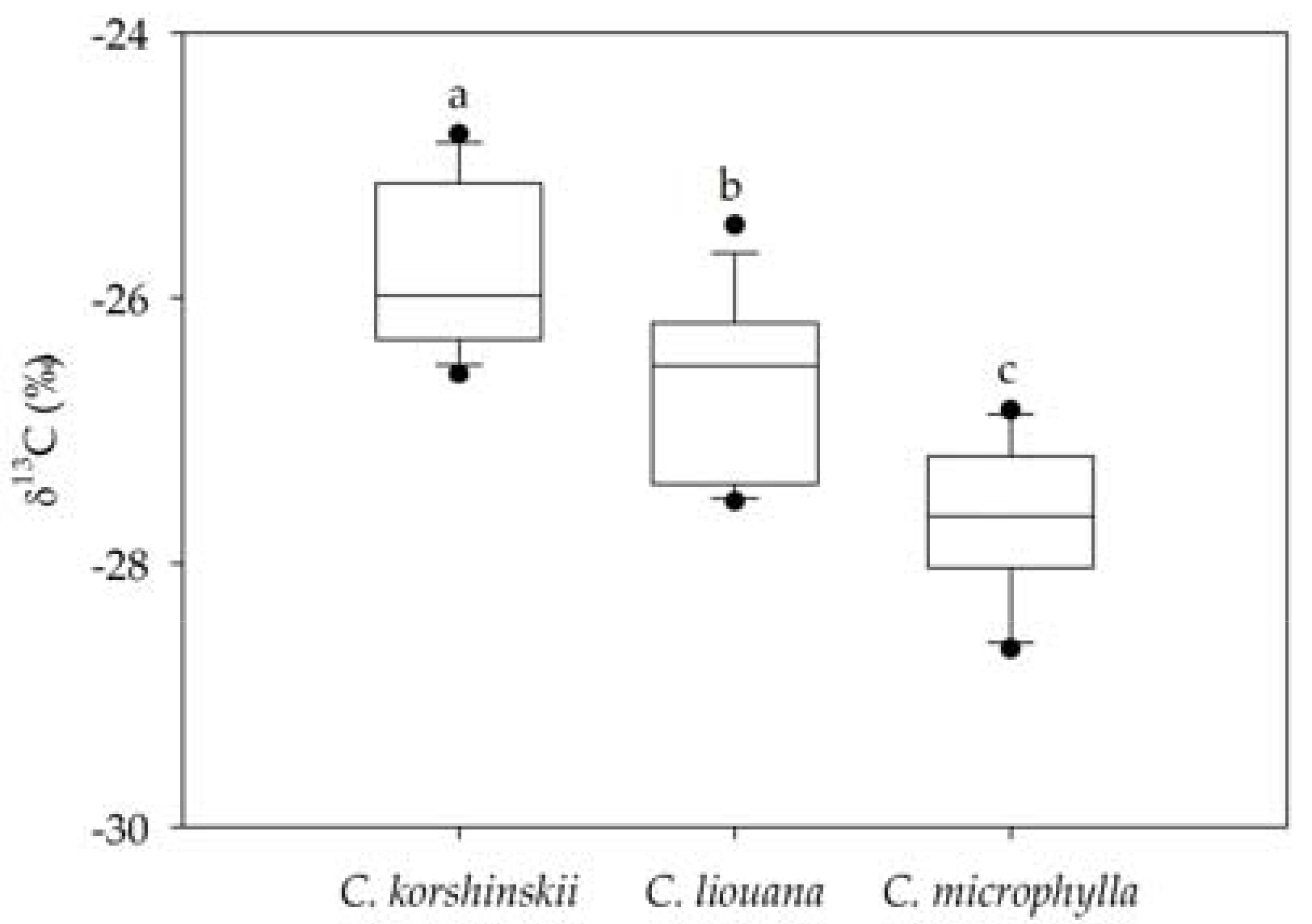
3.2. Variation in Leaf δ13C within and Among Species
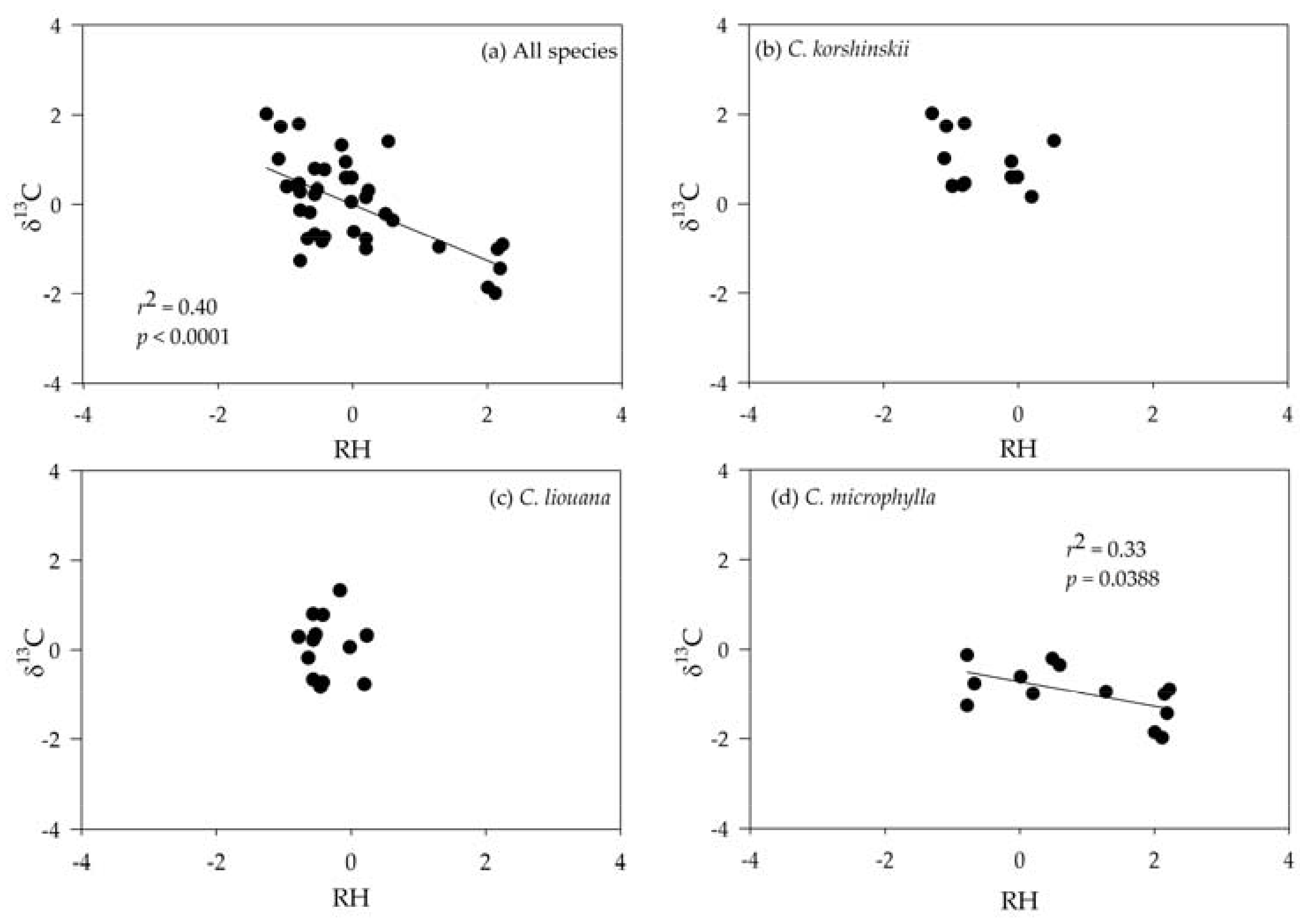
3.3. Correlating Leaf δ13C with Climatic Variables
3.4. Correlating Leaf δ13C with Altitude
3.5. Correlating Leaf δ13C with Leaf Nutrient Concentrations
3.6. Relationship between Leaf δ13C and Climatic Variables, Altitude, and Leaf Nutrients
4. Discussion
4.1. Variation in Leaf δ13C of Three Caragana Species along the Transect
4.2. Difference in WUE between the Three Caragana Species: Implications for Their Geographical Distribution
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate extremes: Observations, modeling, and impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef] [PubMed]
- Dawson, T.E.; Mambelli, S.; Plamboeck, A.H.; Templer, P.H.; Tu, K.P. Stable isotopes in plant ecology. Annu. Rev. Ecol. Syst. 2002, 33, 507–559. [Google Scholar] [CrossRef]
- Knapp, A.K.; Beier, C.; Briske, D.D.; Classen, A.T.; Luo, Y.Q.; Reichstein, M.; Smith, M.D.; Bell, J.E.; Fay, P.A.; Heisler, J.L.; et al. Consequences of more extreme precipitation regimes for terrestrial ecosystems. BioScience 2008, 58, 811–821. [Google Scholar] [CrossRef]
- Ma, J.Y.; Sun, W.; Sun, H.L.; Wang, S.M. Stable carbon isotope characteristics of desert plants in the Junggar Basin, China. Ecol. Res. 2012, 27, 115–124. [Google Scholar] [CrossRef]
- Swap, R.J.; Aranibar, J.N.; Dowty, PR.; Gilhooly, W.P., III; Macko, S.A. Natural abundance of 13C and 15N in C3 and C4 vegetation of southern Africa: Patterns and implications. Glob. Chang. Biol. 2004, 10, 350–358. [Google Scholar] [CrossRef]
- Li, Z.; Yang, L.; Lu, W.; Guo, W.; Gong, X.; Xu, J.; Yu, D. Spatial patterns of leaf carbon, nitrogen stoichiometry and stable carbon isotope composition of Ranunculus natans C.A. Mey. (Ranunculaceae) in the arid zone of northwest China. Ecol. Eng. 2015, 77, 9–17. [Google Scholar] [CrossRef]
- Liu, X.; Su, Q.; Li, C.; Zhang, Y.; Wang, Q. Responses of carbon isotope ratios of C3 herbs to humidity index in northern China. Turk. J. Earth Sci. 2014, 23, 100–111. [Google Scholar] [CrossRef]
- Peri, P.L.; Ladd, B.; Pepper, D.A.; Bonser, S.P.; Laffan, S.W.; Amelung, W. Carbon (δ13C) and nitrogen (δ15N) stable isotope composition in plant and soil in Southern Patagonia’s native forests. Glob. Chang. Biol. 2012, 18, 311–321. [Google Scholar] [CrossRef]
- Wang, C.; Liu, D.; Luo, W.; Fang, Y.; Wang, X.; Lü, X.; Jiang, Y.; Han, X.G.; Bai, E. Variations in leaf carbon isotope composition along an arid and semi-arid grassland transect in Northern China. J. Plant Ecol. 2016, 112, 576–585. [Google Scholar] [CrossRef]
- Chen, L.T.; Flynn, D.F.; Zhang, X.W.; Gao, X.L.; Lin, L.; Luo, J.; Zhao, C.M. Divergent patterns of foliar δ13C and δ15N in Quercus aquifolioides with an altitudinal transect on the Tibetan Plateau: An integrated study based on multiple key leaf functional traits. J. Plant Ecol. 2015, 8, 303–312. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Hubick, K.; Condon, A.G.; Richards, R.A. Carbon isotope fractionation and plant water-use efficiency. In Stable Isotopes in Ecological Research; Rundel, P.W., Ehleringer, J.R., Nagy, K.A., Eds.; Springer: New York, NY, USA, 1989; pp. 21–40. [Google Scholar]
- Warren, C.R.; McGrath, J.F.; Adams, M.A. Water availability and carbon isotope discrimination in conifers. Oecologia 2001, 127, 476–486. [Google Scholar] [CrossRef] [PubMed]
- Fleck, I.; Peña-Rojas, K.; Aranda, X. Mesophyll conductance to CO2 and leaf morphological characteristics under drought stress during Quercus ilex L. resprouting. Ann. For. Sci. 2010, 67, 308. [Google Scholar] [CrossRef]
- Körner, C.H.; Farquhar, G.D.; Wong, S.C. Carbon isotope discrimination by plants follows latitudinal and altitudinal trends. Oecologia 1991, 88, 30–40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Shangguan, Z. Spatial patterns of foliar stable carbon isotope compositions of C3 plant species in the Loess Plateau of China. Ecol. Res. 2007, 22, 342–353. [Google Scholar] [CrossRef]
- Diefendorf, A.F.; Mueller, K.E.; Wing, S.L.; Koch, P.L.; Freeman, K.H.; Hayes, J.M. Global patterns in leaf 13C discrimination and implications for studies of past and future climate. Proc. Natl. Acad. Sci. USA 2010, 107, 5738–5743. [Google Scholar] [CrossRef] [PubMed]
- Chamaillard, S.; Fichot, R.; Vincentbarbaroux, C.; Bastien, C.; Depierreux, C.; Dreyer, E.; Villar, M.; Brignolas, F. Variations in bulk leaf carbon isotope discrimination, growth and related leaf traits among three Populus nigra L. populations. Tree Physiol. 2011, 31, 1076–1087. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.C.; Fan, J.W.; Zhang, W.Y.; Harris, W.; Zhong, H.P.; Hu, Z.M.; Song, L.L. Factors influencing altitudinal patterns of C3 plant foliar carbon isotope composition of grasslands on the Qinghai-Tibet Plateau, China. Alp. Bot. 2011, 121, 79–90. [Google Scholar] [CrossRef]
- Wang, N.; Xu, S.S.; Jia, X.; Gao, J.; Zhang, W.P.; Qiu, Y.P.; Wang, G.X. Variations in foliar stable carbon isotopes among functional groups and along environmental gradients in China—A meta-analysis. Plant Biol. 2013, 15, 144–151. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.K.; Liu, W.Q.; Liu, G.X.; Chen, T.; Zhang, W.; Wu, X.K.; Zhang, G.S.; Zhang, Y.H.; Li, L.; Zhang, B.G.; et al. Temporal and spatial variations in the stable carbon isotope composition and carbon and nitrogen contents in current-season twigs of Tamarix chinensis Lour. and their relationships to environmental factors in the Laizhou Bay wetland in China. Ecol. Eng. 2016, 90, 417–426. [Google Scholar] [CrossRef]
- Aguilar-Romero, R.; Pineda-Garcia, F.; Paz, H.; González-Rodríguez, A.; Oyama, K. Differentiation in the water-use strategies among oak species from central Mexico. Tree Physiol. 2017, 37, 915–925. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.X.; Zang, R.G.; Li, C.Y. Physiological and morphological variations of Picea asperata populations originating from different altitudes in the mountains of southwest China. For. Ecol. Manag. 2006, 221, 285–290. [Google Scholar] [CrossRef]
- Prentice, I.C.; Meng, T.T.; Wang, H.; Harrison, S.P.; Ni, J.; Wang, G.H. Evidence of a universal scaling relationship for leaf CO2 drawdown along an aridity gradient. New Phytol. 2011, 190, 169–180. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Wu, B.; Yang, W.; Luo, T. Low rainfall induced shift in leaf trait relationship within species along a semi-arid sandy land transect in northern China. Plant Biol. 2011, 13, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.M.; Chen, L.T.; Ma, F.; Yao, B.Q.; Liu, J.Q. Altitudinal differences in the leaf fitness of juvenile and mature alpine spruce trees (Picea crassifolia). Tree Physiol. 2008, 28, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.W.; Marshall, J.D. Population differences in water use efficiency of well-watered and water-stressed western larch seedlings. Can. J. Forest Res. 1994, 24, 92–99. [Google Scholar] [CrossRef]
- Friend, A.D.; Woodward, F.I.; Switsuer, V.R. Field measurements of photosynthesis stomatal conductance, leaf nitrogen and δ13C along altitudinal gradients in Scotland. Funct. Ecol. 1989, 3, 117–122. [Google Scholar] [CrossRef]
- Hultine, D.R.; Marshall, J.D. Altitude trends in conifer leaf morphology and stable carbon isotope composition. Oecologia 2000, 123, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sparks, J.; Ehleringer, J. Leaf carbon isotope discrimination and nitrogen content for riparian trees along elevational transects. Oecologia 1997, 109, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Hamerlynck, E.P.; Huxman, T.E.; McAuliffe, J.R.; Smith, S.D. Carbon isotope discrimination and foliar nutrient status of Larrea tridentata (creosote bush) in contrasting Mojave desert soils. Oecologia 2004, 138, 210–215. [Google Scholar] [CrossRef] [PubMed]
- Springer, C.J.; Delucia, E.H.; Thomas, R.B. Relationships between net photosynthesis and foliar nitrogen concentrations in a loblolly pine forest ecosystem grown in elevated atmospheric carbon dioxide. Tree Physiol. 2005, 25, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Domingues, T.F.; Meir, P.; Feldpausch, T.R.; Saiz, G.; Veenendaal, E.M.; Schrodt, F.; Bird, M.; Djagbletey, G.; Hien, F.; Compaore, H. Co-limitation of photosynthetic capacity by nitrogen and phosphorus in West Africa woodlands. Plant Cell Environ. 2010, 33, 959–980. [Google Scholar] [CrossRef] [PubMed]
- Walia, A.; Guy, R.D.; White, B. Carbon isotope discrimination in western hemlock and its relationship to mineral nutrition and growth. Tree Physiol. 2010, 30, 728–740. [Google Scholar] [CrossRef] [PubMed]
- Walker, A.P.; Beckerman, A.P.; Gu, L.; Kattge, J.; Cernusak, L.A.; Domingues, T.F.; Scales, J.C.; Wohlfahrt, G.; Wullschleger, S.D.; Woodward, F.I. The relationship of leaf photosynthetic traits-Vcmax and Jmax-to leaf nitrogen, leaf phosphorus, and specific leaf area: A meta-analysis and modeling study. Ecol. Evol. 2014, 4, 3218–3235. [Google Scholar] [CrossRef] [PubMed]
- Cordell, S.; Goldstein, G.; Meinzer, F.C.; Handley, L.L. Allocation of nitrogen and carbon in leaves of Metrosiderospolymorpha regulates carboxylation capacity and δ13C along an altitudinal gradient. Funct. Ecol. 1999, 13, 811–818. [Google Scholar] [CrossRef]
- Kloeppel, B.D.; Gower, S.T.; Treichel, I.W.; Kharuk, S. Foliar carbon isotope discrimination in Larix species and sympatric evergreen conifers: A global comparision. Oecologia 1998, 114, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Damesin, C.; Rambal, S.; Joffre, R. Between-tree variations leaf δ13C of Quercus pubescens and Quercus ilex among Mediterranean habitats with different water availability. Oecologia 1997, 111, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Warren, C.R.; Adams, M.A. Phosphorus affects growth and partitioning of nitrogen to Rubisco in Pinus pinaster. Tree Physiol. 2002, 22, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Chen, F.; Xia, G.; Su, H.; Duan, Z.; Wang, G. Correlations between leaf δ13C and physiological parameters of desert plant Reaumuria soongorica. Chin. J. Appl. Ecol. 2008, 19, 1166–1171. [Google Scholar]
- Li, S.J.; Zhang, Y.F.; Chen, T. Relationships between foliar stable carbon isotope composition and environmental factors and leaf element contents of Pinus tabulaeformis in northwestern China. Chin. J. Plant Ecol. 2011, 35, 596–604. [Google Scholar] [CrossRef]
- Zhou, Y.C.; Fan, J.W.; Harris, W.; Zhong, H.P.; Zhang, W.Y.; Cheng, X.L. Relationships between C3 plant foliar carbon isotope composition and element contents of grassland species at high altitudes on the Qinghai-Tibet Plateau, China. PLoS ONE 2013, 8, e60794. [Google Scholar] [CrossRef] [PubMed]
- Merah, O. Carbon isotope discrimination and mineral composition of three organs in durum wheat genotypes grown under Mediterranean conditions. C. R. Acad. Sci. Ser. III 2001, 324, 355–363. [Google Scholar] [CrossRef]
- Chen, S.P.; Bai, Y.F.; Lin, G.H.; Han, X.G. Variations in life-form composition and foliar carbon isotope discrimination among eight plant communities under different soil moisture conditions in the Xilin River Basin, Inner Mongolia, China. Ecol. Res. 2005, 20, 167–176. [Google Scholar] [CrossRef]
- Van de Water, P.K.; Leavitt, S.W.; Betancourt, J.L. Leaf δ13C variability with elevation, slope aspect, and precipitation in the southwest United States. Oecologia 2002, 132, 332–343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L.; Fritsch, P.W. Evolutionary response of Caragana (Fabaceae) to Qinghai–Tibetan Plateau uplift and Asian interior aridification. Plant Syst. Evol. 2010, 288, 191–199. [Google Scholar] [CrossRef]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L.; Wu, J.B.; Xu, J.S. Physiological adaptations of four dominant Caragana species in the desert region of the Inner Mongolia Plateau. J. Arid Environ. 2008, 72, 247–254. [Google Scholar] [CrossRef]
- Meng, Q.X.; Niu, Y.; Niu, X.W.; Roubin, R.H.; Hanrahan, J.R. Ethnobotany, phytochemistry and pharmacology of the genus Caragana used in traditional Chinese medicine. J. Ethnopharmacol. 2009, 124, 350–368. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.F.; Li, Y.M.; Hu, H.; Liu, X.; Qi, L. Chemical constituents from Caragana tangutica. Biochem. Syst. Ecol. 2013, 51, 288–290. [Google Scholar] [CrossRef]
- Na, X.F.; Xu, T.T.; Li, M.; Ma, F.; Kardol, P. Bacterial diversity in the rhizosphere of two phylogenetically closely related plant species across environmental gradients. J. Soils Sediments 2016, 17, 1–11. [Google Scholar] [CrossRef]
- Na, X.F.; Li, X.R.; Zhang, Z.Y.; Li, M.; Kardol, P.; Xu, T.T.; Wang, M.; Cao, X.N.; Ma, F. Bacterial community dynamics in the rhizosphere of a long-lived, leguminous shrub across a 40-year age sequence. J. Soils Sediments 2017, 1, 1–9. [Google Scholar] [CrossRef]
- Ma, F.; Na, X.F.; Xu, T.T. Drought responses of three closely related Caragana species: Implication for their vicarious distribution. Ecol. Evol. 2016, 6, 2763–2773. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.C.; Gao, Y.B.; Guo, H.Y.; Wang, J.L. Photosynthesis, transpiration, and water use efficiency of Caragana microphylla, C. intermedia, and C. korshinskii. Photosynthetica 2004, 43, 65–70. [Google Scholar] [CrossRef]
- O’Leary, M.H. Carbon isotopes in photosynthesis (fractionation techniques may reveal new aspects of carbon dynamics in plants). BioScience 1988, 38, 328–336. [Google Scholar]
- Ehleringer, J.R.; Cooper, T.A. Correlations between carbon isotope ratio and microhabit in desert plants. Oecologia 1988, 76, 562–566. [Google Scholar] [CrossRef] [PubMed]
- Rundel, P.W.; Esler, K.J.; Cowling, R.M. Ecological and phylogenetic patterns of carbon isotope discrimination in the winter-rain-fall flora of the Richterveld, South Africa. Plant Ecol. 1999, 142, 133–148. [Google Scholar] [CrossRef]
- Vitoria, A.P.; Vieira, T.D.O.; Camargo, P.D.B.; Santiago, L.S. Using leaf δ13C and photosynthetic parameters to understand acclimation to irradiance and leaf age effects during tropical forest regeneration. Forest Ecol. Manag. 2016, 379, 50–60. [Google Scholar] [CrossRef]
- Stewart, G.R.; Turnbull, M.H.; Schmidt, S.; Erskine, P.D. 13C natural abundance in plant communities along a rainfall gradient: A biological integrator of water availability. Aust. J. Plant Physiol. 1995, 22, 51–55. [Google Scholar] [CrossRef]
- Leffler, A.J.; Enquist, B.J. Carbon isotope composition of tree leaves from Guanacaste, Costa Rica: Comparison across tropical forests and tree life history. J. Trop. Ecol. 2002, 18, 151–159. [Google Scholar] [CrossRef]
- Troughton, J.H.; Card, K.A. Temperature effects on the carbon isotope ratio of C3, C4 and crassulacean-acid-metabolism (CAM) plants. Planta 1975, 123, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Welker, J.M.; Wookey, P.A.; Parsons, A.N.; Press, M.C.; Callaghan, T.V.; Lee, J.A. Leaf carbon isotope discrimination and vegetative responses of Dryas octopetala to temperature and water manipulations in a High Arctic polar semidesert, Svalbard. Oecologia 1993, 95, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yang, F.; Xiao, X.; Zhang, S.; Korpelainen, H.; Li, C. Sex-specific responses of Populus cathayana to drought and elevated temperatures. Plant Cell Environ. 2008, 31, 850–860. [Google Scholar] [CrossRef] [PubMed]
- Morecroft, M.D.; Woodward, F. Experiments on the causes of altitudinal differences in the leaf nutrient contents, size and δ13C of Alchemilla alpina. New Phytol. 1996, 134, 471–479. [Google Scholar] [CrossRef]
- Tsialtas, J.T.; Handley, L.L.; Kassioumi, M.T.; Veresoglou, D.S.; Gagianas, A.A. Interspecific variation in potential water-use efficiency and its relation to plant species abundance in a water-limited grassland. Funct. Ecol. 2001, 15, 605–614. [Google Scholar] [CrossRef]
- Cordell, S.; Goldstein, G.; Mueller-Dombois, D.; Webb, D.; Vitousek, P.M. Physiological and morphological variation in Metrosideros polymorpha, a dominant Hawaiian tree species, along an altitudinal gradient: The role of phenotypic plasticity. Oecologia 1998, 113, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Z.; Wang, G.A.; Zhang, R.N.; Li, L. A negative relationship between foliar carbon isotope composition and mass-based nitrogen concentration on the eastern slope of Mount Gongga, China. PLoS ONE 2016, 11, E0166958. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.C.; Cheng, X.L.; Fan, J.W.; Harris, W. Relationships between foliar carbon isotope composition and elements of C3 species in grasslands of Inner Mongolia, China. Plant Ecol. 2016, 217, 883–897. [Google Scholar] [CrossRef]
- Cernusak, L.A.; Winter, K.; Aranda, J.; Turner, B.L.; Marshall, J.D. Transpiration efficiency of a tropical pioneer tree (Ficus insipida) in relation to soil fertility. J. Exp. Bot. 2007, 58, 3549–3566. [Google Scholar] [CrossRef] [PubMed]
- Cramer, M.D.; Hawkins, H.J.; Verboom, G.A. The importance of nutritional regulation of plant water flux. Oecologia 2009, 161, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Q.; Li, J.Y.; Wang, J.H.; Wang, N.; Sun, K.; Jia, Z.R.; Dong, L.; Pan, X. Relationship on leaf δ13C and nutrients in leaf and soil of different Nitraria tangutorum populations in Tsaidam Basin. Acta Bot. Boreali-Occident. Sin. 2013, 33, 2301–2308. [Google Scholar]
- Li, J.; Wang, G.; Liu, X.; Han, J.; Liu, M.; Liu, X. Variations in carbon isotope ratios of C3 plants and distribution of C4 plants along an altitudinal transect on the eastern slope of mount Gongga. Sci. China Ser. D 2009, 52, 1714–1723. [Google Scholar] [CrossRef]
- Zhu, Y.; Siegwolf, R.T.W.; Durka, W.; Körner, C. Phylogenetically balanced evidence for structural and carbon isotope responses in plants along elevational gradients. Oecologia 2010, 162, 853–863. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.L. Studies on geographical distribution pattern of the subgenus Pogonophace (Fabaceae: Astragalus) in China using GIS technique. Acta Bot. Sin. 2000, 42, 849–854. [Google Scholar]
- Zhu, H.; Ma, Y.X.; Yan, L.C.; Hu, H. The relationship between geography and climate in the generic-level patterns of Chinese seed plants. Acta Phytotaxon. Sin. 2007, 45, 134–166. [Google Scholar]
- Thompson, J.; Charpentier, A.; Bouguet, G.; Charmassona, F.; Rosetb, S.; Buatoisa, B.; Vernetd, P.; Gouyone, P.H. Evolution of a genetic polymorphism with climate change in a Mediterranean landscape. Proc. Natl. Acad. Sci. USA 2013, 110, 2893–2897. [Google Scholar] [CrossRef] [PubMed]
- Pyke, C.R.; Condit, R.; Aguilar, S.; Lao, S. Floristic composition across a climatic gradient in a neotropical lowland forest. J. Veg. Sci. 2001, 12, 553–566. [Google Scholar] [CrossRef]

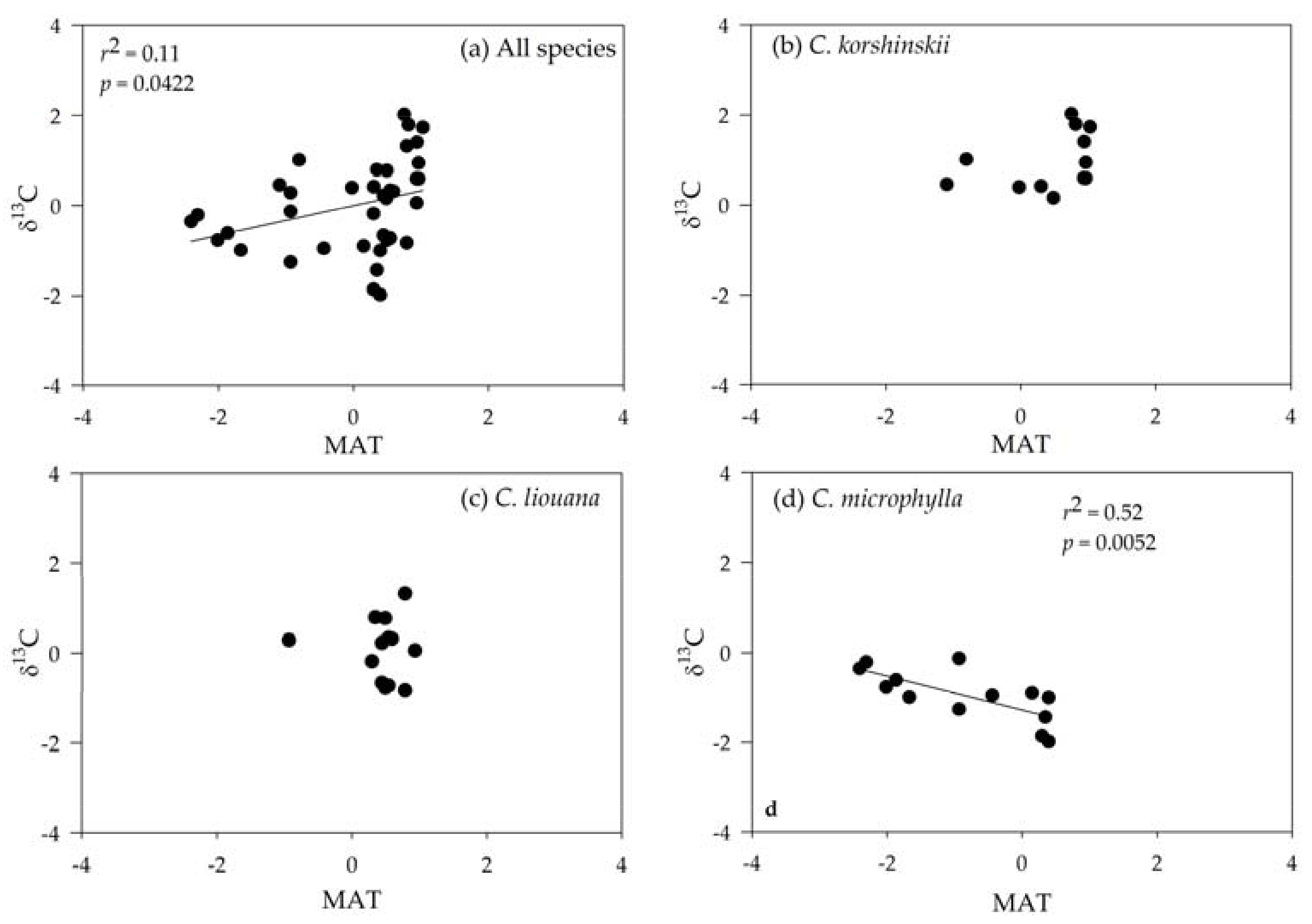
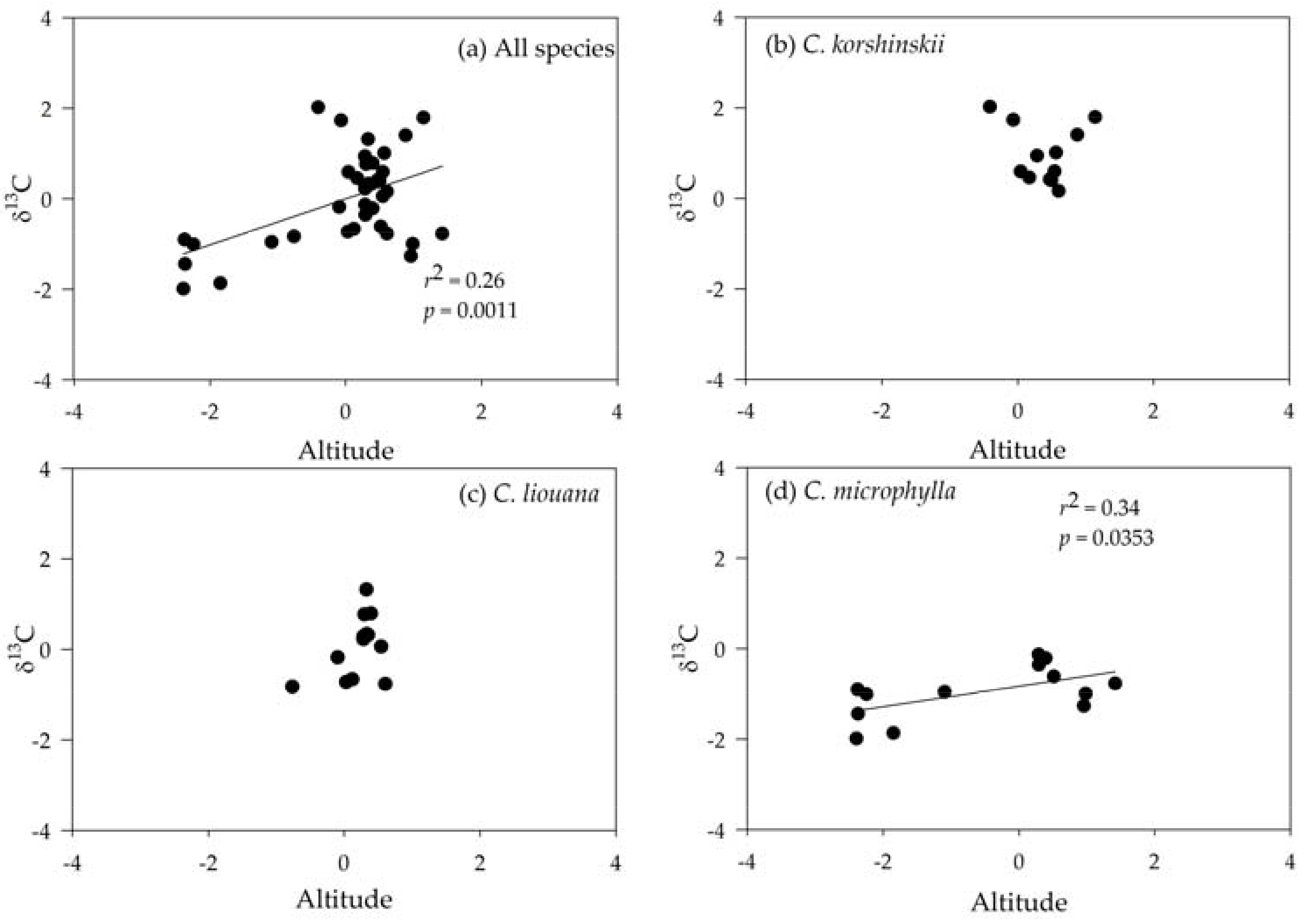
| Variables | C. korshinskii | C. liouana | C. microphylla |
|---|---|---|---|
| Mean ± SD | Mean ± SD | Mean ± SD | |
| Altitude (m) | 1368.8 ± 173.1 a | 1293.2 ± 142.9 a | 971.9 ± 612.7 b |
| MAP (mm) | 232.9 ± 81.6 b | 330.2 ± 69.1 a | 372.9 ± 40.0 a |
| MAT (°C) | 7.2 ± 1.5 b | 7.2 ± 0.9 b | 4.6 ± 2.2 a |
| RH (%) | 55.5 ± 0.6 b | 56.0 ± 0.9 b | 59.3 ± 3.3 a |
| Leaf N (mg∙g−1) | 32.62 ± 4.59 b | 38.32 ± 1.89 a | 39.76 ± 2.6 a |
| Leaf P (mg∙g−1) | 1.38 ± 0.23 b | 1.70 ± 0.17 a | 1.71 ± 0.24 a |
| Species | MAP | MAT | RH | N | P |
|---|---|---|---|---|---|
| All species | −0.274 | −0.193 | −0.780 *** | −0.099 | −0.267 |
| C. korshinskii | 0.239 | 0.048 | 0.291 | 0.344 | 0.388 |
| C. liouana | −0.510 | −0.090 | 0.400 | −0.017 | 0.294 |
| C. microphylla | 0.020 | −0.890 *** | −0.947 *** | 0.400 | −0.344 |
| Nutrient Variables | Species | Statistic | ||
|---|---|---|---|---|
| Slope | r2 | p-Value | ||
| Leaf N | All species | −0.11 | 0.26 | 0.0010 |
| C. korshinskii | −0.10 | 0.03 | 0.6026 | |
| C. liouana | −0.25 | 0.03 | 0.6061 | |
| C. microphylla | 0.46 | 0.23 | 0.0975 | |
| Leaf P | All species | −0.44 | 0.20 | 0.0051 |
| C. korshinskii | −0.24 | 0.12 | 0.2760 | |
| C. liouana | 0.14 | 0.02 | 0.6625 | |
| C. microphylla | −0.03 | 0.002 | 0.8936 | |
| Species | a (Constant) | bx1 (MAP) | Standardized Coefficients | cx2 (MAT) | Standardized Coefficients | dx3 (RH) | Standardized Coefficients | ex4 (N) | Standardized Coefficients | F | p Regression | r2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All species | −16.37 | −0.01 | −0.63 p < 0.001 | −0.14 | −0.41 p < 0.001 | 49.78 | <0.001 | 0.74 | ||||
| C. korshinskii | −26.22 | −0.01 | −1.15 p < 0.001 | 0.08 | 0.56 p = 0.013 | 21.08 | <0.001 | 0.82 | ||||
| C. liouana | −21.00 | −0.01 | −0.91 p = 0.003 | −0.39 | −0.55 p = 0.045 | 7.29 | 0.011 | 0.59 | ||||
| C. microphylla | −26.84 | −0.18 | −0.72 p = 0.005 | 12.03 | 0.005 | 0.52 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, F.; Liang, W.; Zhou, Z.; Xiao, G.; Liu, J.; He, J.; Jiao, B.; Xu, T. Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China. Forests 2018, 9, 297. https://doi.org/10.3390/f9060297
Ma F, Liang W, Zhou Z, Xiao G, Liu J, He J, Jiao B, Xu T. Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China. Forests. 2018; 9(6):297. https://doi.org/10.3390/f9060297
Chicago/Turabian StyleMa, Fei, Wenyu Liang, Zhaona Zhou, Guoju Xiao, Jili Liu, Jun He, Bingzhong Jiao, and Tingting Xu. 2018. "Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China" Forests 9, no. 6: 297. https://doi.org/10.3390/f9060297
APA StyleMa, F., Liang, W., Zhou, Z., Xiao, G., Liu, J., He, J., Jiao, B., & Xu, T. (2018). Spatial Variation in Leaf Stable Carbon Isotope Composition of Three Caragana Species in Northern China. Forests, 9(6), 297. https://doi.org/10.3390/f9060297





