Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Sites and Field Sampling
2.2. Data Analysis
3. Results
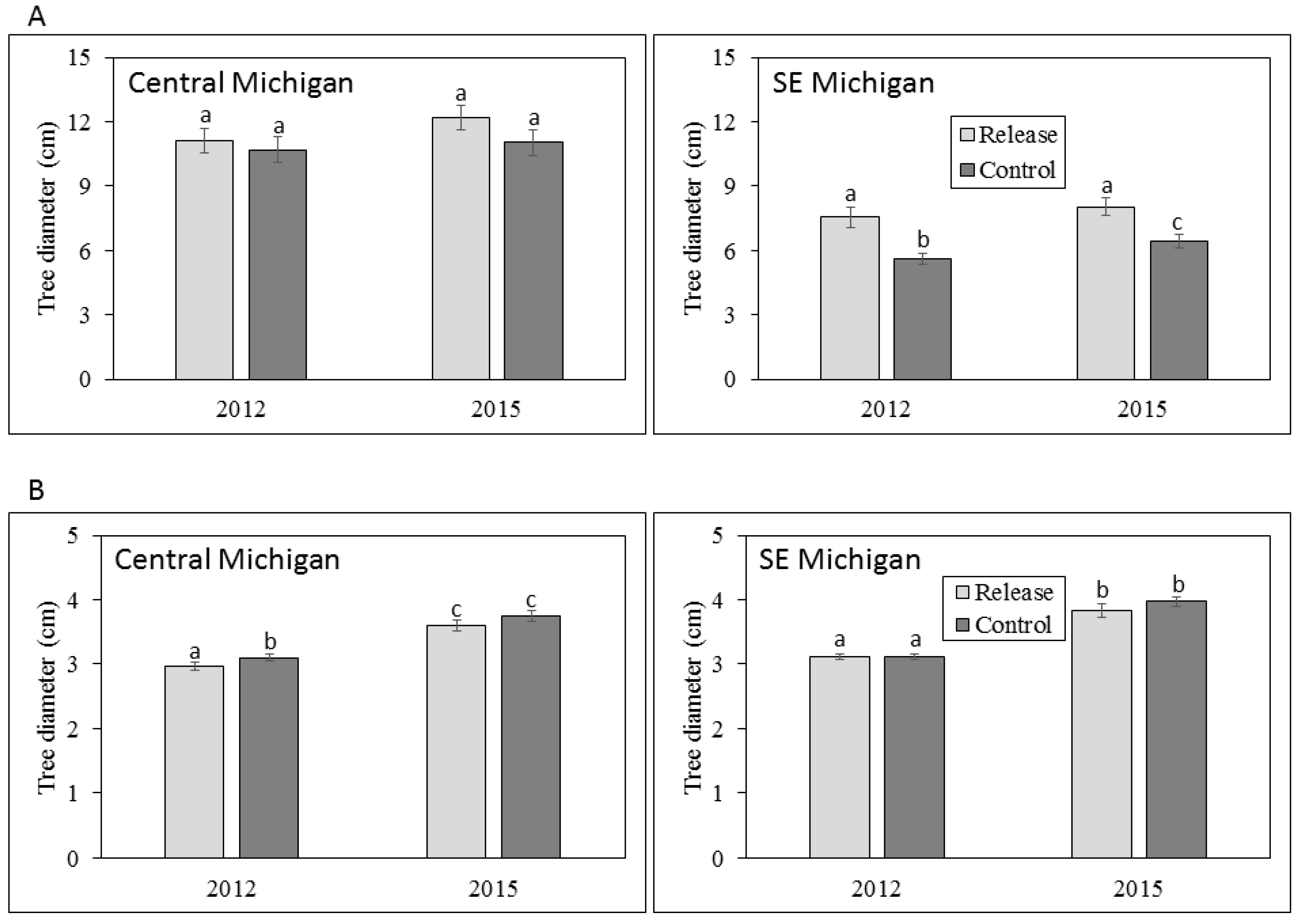
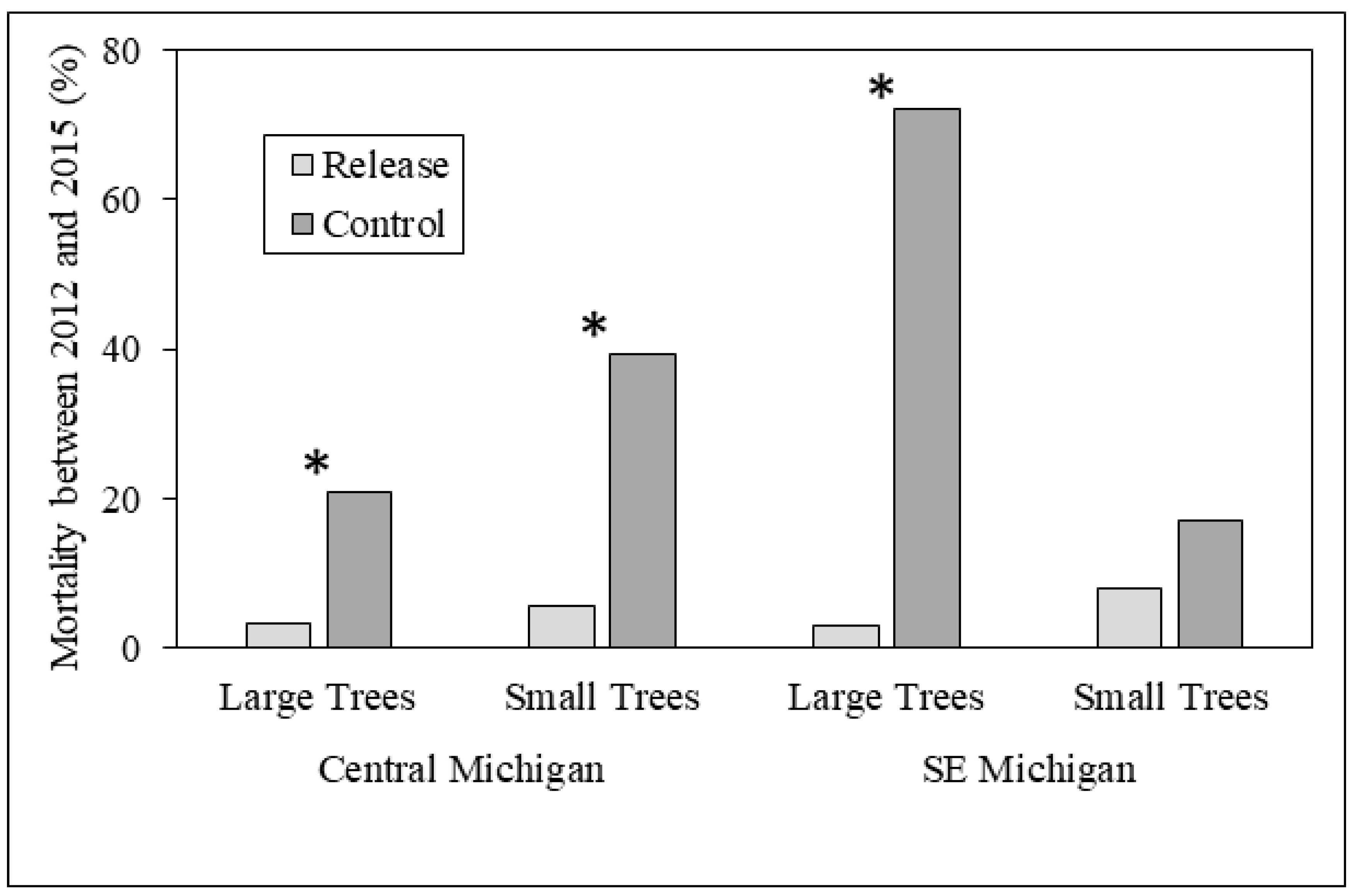
3.1. Ash Tree Diameters and Mortality
3.2. Ash Tree Health
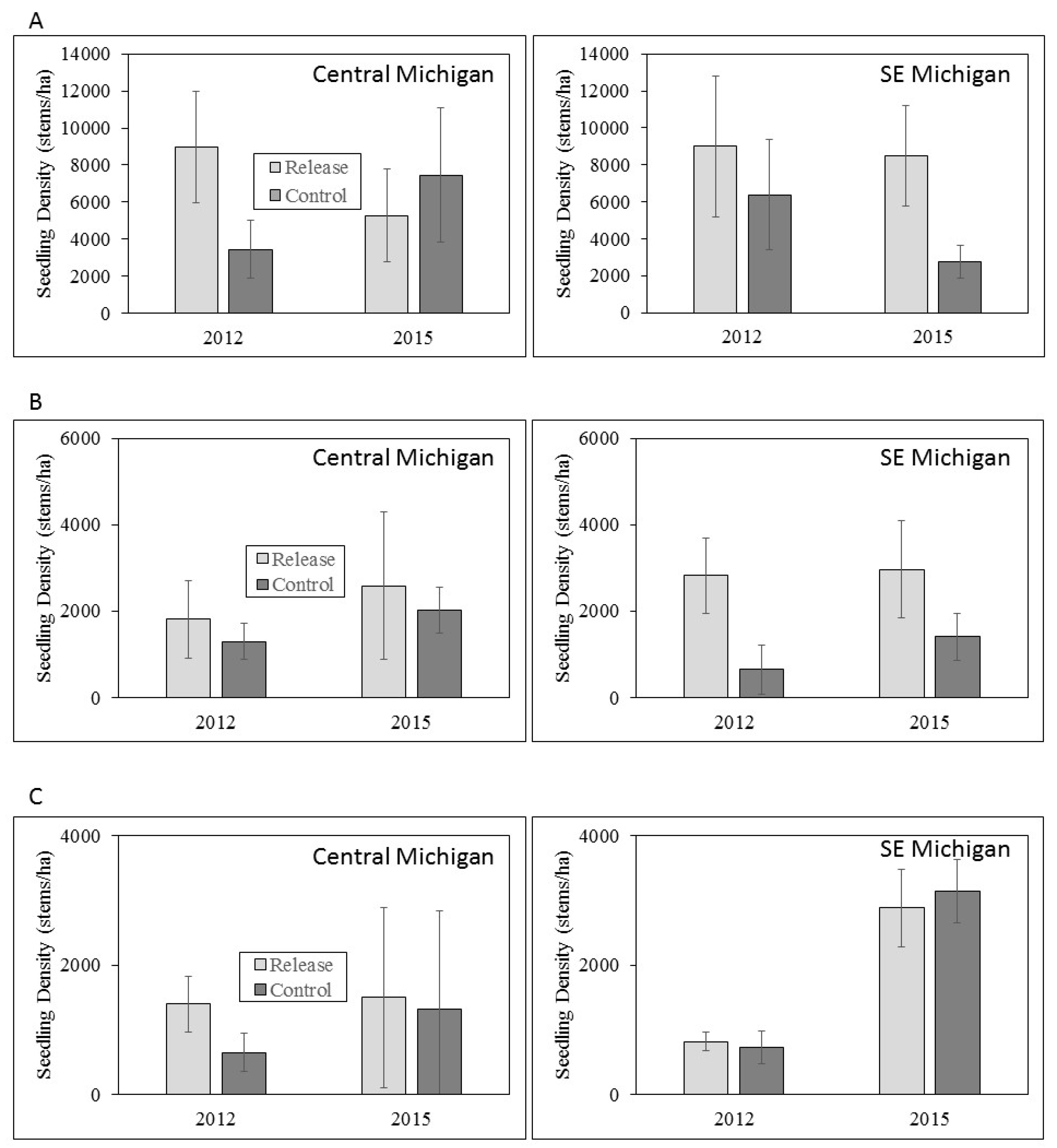
3.3. Ash Regeneration
4. Discussion
4.1. Ash Diameter and Health
4.2. Ash Regeneration
4.3. Future Research
5. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
Appendix A
| Site Name * | Release-Plot Codes ** | Treatment *** | Ash Relative Dominance (%) | Dominant Overstory Tree Species |
|---|---|---|---|---|
| Central Michigan | ||||
| Central Park | CPNMLT | Release | 3.7 | American elm, boxelder |
| Control | 8.3 | American elm | ||
| Legg Park | LPRFLT | Release | 2.3 | Silver maple, American elm, hackberry |
| Control | 1.0 | Silver maple, sycamore, American elm | ||
| Burchfield | BFPKLT | Release | 3.8 | Silver maple, American elm |
| Control | 3.8 | Silver maple, American elm | ||
| Gratiot-Saginaw | GSW | Release | 2.1 | Red maple, red oak |
| Control | 1.9 | Red maple, red oak | ||
| Rose Lake | RL | Release | 1.7 | Red maple, basswood, American elm |
| Control | 1.1 | Red maple, black cherry, American elm | ||
| Southeastern Michigan | ||||
| Lake Erie | LKEMP | Release | 0.1 | American elm, silver maple |
| Oakwoods | Control | 0.1 | Red maple, American elm, shagbark hickory | |
| Pinckney | PNKSL | Release | 0.1 | Red oak, red maple |
| Control | 0.1 | Red oak, red maple | ||
| Willow North | WMPKN | Release | 0.1 | American elm |
| Willow South | WMPKS | Release | 4.0 | Eastern cottonwood |
| Lower Huron | Control | 0.1 | Silver maple, American elm | |
Appendix B
| Site Codes | Year | O. agrili Released | T. planipennisi Released | ||||
|---|---|---|---|---|---|---|---|
| Month(s) | Total N (Females) | No. of Releases | Month(s) | Total N (Females) | No. of Releases | ||
| Central Michigan * | |||||||
| CPNMLT | 2007 | August | 700 | 6 | September–October | 870 | 3 |
| 2008 | June–August | 330 | 4 | June | 150 | 1 | |
| 2009 | June | 300 | 1 | June–October | 3000 | 9 | |
| LPRFLT | 2008 | July | 200 | 2 | September–October | 200 | 5 |
| 2009 | June | 300 | 1 | May–September | 3250 | 10 | |
| BFPKLT | 2008 | July–August | 200 | 2 | July | 110 | 1 |
| 2009 | June | 300 | 1 | May–September | 3200 | 9 | |
| GSW | 2009 | August | 375 | 1 | August–September | 700 | 2 |
| 2010 | June–July | 1110 | 2 | June–September | 3290 | 6 | |
| RL | 2010 | July | 1160 | 2 | June–September | 3880 | 7 |
| SE Michigan ** | |||||||
| LKEMP | 2011 | June | 240 | 1 | June | 1740 | 3 |
| PNKSL | 2011 | July–Aug | 300 | 3 | July | 710 | 2 |
| WMPKN | 2011 | June–July | 180 | 2 | June–July | 1830 | 2 |
| WMPKS | 2011 | June–July | 160 | 2 | June–July | 400 | 2 |
References
- Mattson, W.; Vanhanen, H.; Veteli, T.; Sivonen, S.; Niemelä, P. Few immigrant phytophagous insects on woody plants in Europe: Legacy of the European crucible? Biol. Invasions 2007, 9, 957–974. [Google Scholar] [CrossRef]
- Liebhold, A.M.; MacDonald, W.L.; Bergdahl, D.; Mastro, V.C. Invasion by exotic forest pests: A threat to forest ecosystems. For. Sci. Monogr. 1995, 41, 1–49. [Google Scholar] [CrossRef]
- Simberloff, D. Nonindigneous species: A global threat to biodiversity and stability. In Nature and Human Society: The Quest for a Sustainable World; Raven, P., Williams, T., Eds.; National Academy Press: Washington, DC, USA, 2000; pp. 325–336. [Google Scholar]
- Aukema, J.E.; McCullough, D.G.; Von Holle, B.; Liebhold, A.M.; Britton, K.; Frankel, S.J. Historical accumulation of non-indigenous forest pests in the continental United States. BioScience 2010, 60, 886–897. [Google Scholar] [CrossRef]
- Lovett, G.M.; Weiss, M.; Liebhold, A.M.; Holmes, T.; Leung, B.; Lambert, K.F.; Orwig, D.A.; Campbell, F.T.; Rosenthal, J.; McCullough, D.G.; et al. Nonnative forest insects and pathogens in the United States: Impacts and policy options. Ecol. Soc. Am. 2016, 26, 1437–1455. [Google Scholar] [CrossRef] [PubMed]
- Kenis, M.; Hurley, B.P.; Hajek, A.E.; Cock, M.J.W. Classical biological control of insect pests of trees: Facts and figures. Biol. Invasions 2017, 19, 3401–3417. [Google Scholar] [CrossRef]
- Sadof, C.S.; Hughes, G.P.; Witte, A.R.; Peterson, D.J.; Ginzel, M.D. Tools for staging and managing emerald ash borer in the urban forest. Arboric. Urban For. 2017, 43, 15–26. [Google Scholar]
- Liebhold, A.M.; McCullough, D.G.; Blackburn, L.M.; Frankel, S.J.; Von Holle, B.; Aukema, J.E. A highly aggregated geographical distribution of forest pest invasions in the USA. Divers. Distrib. 2013, 19, 1208–1216. [Google Scholar] [CrossRef]
- Levine, J.M.; D’Antonio, C.M. Forecasting biological invasions with increasing international trade. Conserv. Biol. 2003, 17, 322–326. [Google Scholar] [CrossRef]
- Rebek, E.J.; Herms, D.A.; Smitley, D.R. Interspecific variation in resistance to emerald ash borer (Coleoptera: Buprestidae) among North American and Asian ash (Fraxinus spp.). Environ. Entomol. 2008, 37, 242–246. [Google Scholar] [CrossRef]
- Tanis, S.R.; McCullough, D.G. Differential persistence of blue ash (Fraxinus quadrangulata) and white ash (Fraxinus americana) following emerald ash borer (Agrilus planipennis) invasion. Can. J. For. Res. 2012, 42, 1542–1550. [Google Scholar] [CrossRef]
- Spei, B.A.; Kashian, D.M. Potential for persistence of blue ash in the presence of emerald ash borer in southeastern Michigan. For. Ecol. Manag. 2017, 392, 137–143. [Google Scholar] [CrossRef]
- Cappaert, D.; McCullough, D.G.; Poland, T.M.; Siegert, N.W. Emerald ash borer in North America: A research and regulatory challenge. Am. Entomol. 2005, 51, 152–165. [Google Scholar] [CrossRef]
- USDA–APHIS. Initial County EAB Detection Map. 2018. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/MultiState.pdf (accessed on 15 May 2018).
- Marshall, J.M.; Smith, E.L.; Mech, R. Estimates of Agrilus planipennis infestation rates and potential survival of ash. Am. Midland Nat. 2013, 169, 179–193. [Google Scholar] [CrossRef]
- Nowak, D.; Crane, D.; Stevens, J.; Walton, J. Potential Damage from Emerald Ash Borer; United States Department of Agriculture, Forest Service, Northern Research Station: Syracuse, NY, USA, 2003; pp. 1–5. Available online: https://www.nrs.fs.fed.us/disturbance/invasive_species/eab/local-resources/downloads/EAB_potential.pdf (accessed on 20 March 2018).
- Herms, D.A.; McCullough, D.G. Emerald ash borer invasion of North America: History, biology, ecology, impacts, and management. Ann. Rev. Entomol. 2014, 59, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Wagner, D.L.; Todd, K.J. New ecological assessment for the emerald ash borer: A cautionary tale about unvetted host-plant literature. Am. Entomol. 2016, 62, 26–35. [Google Scholar] [CrossRef]
- Jennings, D.E.; Duan, J.J.; Bean, D.; Kimberly, A.R.; Williams, G.L.; Bells, S.K.; Shurtleff, A.S.; Shrewsbury, P.M. Effects of the emerald ash borer invasion on the community composition of arthropods associated with ash tree boles in Maryland, U.S.A. Agric. For. Entomol. 2017, 19, 122–129. [Google Scholar] [CrossRef]
- Taylor, P.B.; Duan, J.J.; Fuester, R.W.; Hoddle, M.; Van Driesche, R.G. Parasitoid Guilds of Agrilus Woodborers Coleoptera: Buprestidae: Their Diversity and Potential for Use in Biological Control. Psyche 2012, 2012, 813929. [Google Scholar]
- Liu, H.; Bauer, L.S.; Gao, R.; Zhao, T.; Petrice, T.R.; Haack, R.A. Exploratory survey for the emerald ash borer, Agrilus planipennis (Coleoptera: Buprestidae), and its natural enemies in China. Great Lakes Entomol. 2003, 36, 191–204. [Google Scholar]
- Liu, H.; Bauer, L.S.; Miller, D.L.; Zhao, T.; Gao, R.; Song, L.; Luan, Q.; Jin, R.; Gao, C. Seasonal abundance of Agrilus planipennis (Coleoptera: Buprestidae) and its natural enemies Oobius agrili (Hymenoptera: Encyrtidae) and Tetrastichus planipennisi (Hymenoptera: Eulophidae) in China. Biol. Control 2007, 42, 61–71. [Google Scholar] [CrossRef]
- Bauer, L.S.; Duan, J.J.; Gould, J.R. Emerald ash borer Agrilus planipennis Fairmaire Coleoptera: Buprestidae. In The Use of Classical Biological Control to Preserve Forests in North America; FHTET-2013-2; Van Driesche, R., Reardon, R., Eds.; United States Department of Agriculture, Forest Service, Forest Health and Technology Enterprise Team: Morgantown, WV, USA, 2014; pp. 189–209. Available online: https://www.nrs.fs.fed.us/pubs/48051 (accessed on 20 March 2018).
- Van Driesche, R.; Hoddle, M.; Center, T. Control of Pests and Weeds by Natural Enemies; Blackwell Publishing: Malden, MA, USA, 2008. [Google Scholar]
- Bauer, L.S.; Duan, J.J.; Gould, J.R.; Van Driesche, R.G. Progress in the classical biological control of Agrilus planipennis Fairmaire (Coleoptera: Buprestidae) in North America. Can. Entomol. 2015, 147, 300–317. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Van Driesche, R.G.; Gould, J.G. Progress and challenges of protecting North American ash trees from the emerald ash borer using biological control. Forests 2018, 9, 142. [Google Scholar] [CrossRef]
- Wang, X.Y.; Cao, L.M.; Yang, Z.Q.; Duan, J.J.; Gould, J.R.; Bauer, L.S. Natural enemies of emerald ash borer (Coleoptera: Buprestidae) in northeast China, with notes on two species of parasitic Coleoptera. Can. Entomol. 2016, 148, 329–342. [Google Scholar] [CrossRef]
- Federal Register. Availability of an environmental assessment for the proposed release of three parasitoids for the biological control of the emerald ash borer Agrilus planipennis in the Continental United States. Fed. Regist. 2007, 72, 28947–28948. Available online: http://www.regulations.gov/#!documentDetail;D= APHIS-2007-0060-0043 (accessed on 20 March 2018).
- Federal Register. Availability of an environmental assessment for field release of the parasitoid Spathius galinae for the biological control of the emerald ash borer (Agrilus planipennis) in the contiguous United States. Fed. Regist. 2015, 80, 7827–7828. Available online: https://www.regulations.gov/docket?D=APHIS-2014-0094 (accessed on 20 March 2018).
- USDA–APHIS/ARS/FS. USDA Animal Plant Health Inspection Service/Agricultural Research Service/Forest Service. Emerald Ash Borer Biological Control Release and Recovery Guidelines. 2017. Available online: https://www.aphis.usda.gov/plant_health/plant_pest_info/emerald_ash_b/downloads/EAB-FieldRelease-Guidelines.pdf (accessed on 20 March 2018).
- MapBioControl.org. Agent Release Tracking and Data Management for Federal, State, and Researchers Releasing Biocontrol Agents for Management of the Emerald Ash Borer. 2018. Available online: http://www.mapbiocontrol.org/ (accessed on 20 March 2018).
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Lelito, J.P.; Van Driesche, R.G. Establishment and abundance of Tetrastichus planipennisi (Hymenoptera: Eulophidae) in Michigan: Potential for success in classical biocontrol of the invasive emerald ash borer (Coleoptera: Buprestidae). J. Econ. Entomol. 2013, 106, 1145–1154. [Google Scholar] [CrossRef] [PubMed]
- Duan, J.J.; Bauer, L.S.; Abell, K.J.; Ulyshen, M.D.; Van Driesche, R.G. Population dynamics of an invasive forest insect and associated natural enemies in the aftermath of invasion: Implications for biological control. J. Appl. Ecol. 2015, 52, 1246–1254. [Google Scholar] [CrossRef]
- Abell, K.J.; Bauer, L.S.; Duan, J.J.; Van Driesche, R.G. Long-term monitoring of the introduced emerald ash borer (Coleoptera: Buprestidae) egg parasitoid, Oobius agrili (Hymenoptera: Encyrtidae), in Michigan, USA and evaluation of a newly developed monitoring technique. Biol. Control. 2014, 79, 36–42. [Google Scholar] [CrossRef]
- Abell, K.J.; Duan, J.J.; Bauer, L.S.; Lelito, J.P.; Van Driesche, R.G. The effect of bark thickness on the effectiveness of Tetrastichus planipennisi (Hymen: Eulophidae) and Atanycolus spp. (Hymen: Braconidae) two parasitoids of emerald ash borer (Coleop: Buprestidae). Biol. Control. 2012, 63, 320–325. [Google Scholar] [CrossRef]
- Duan, J.J.; Bauer, L.S.; Van Driesche, R.G. Emerald ash borer biocontrol in ash saplings: The potential for early stage recovery of North American ash trees. For. Ecol. Manag. 2017, 394, 64–72. [Google Scholar] [CrossRef]
- Margulies, E.; Bauer, L.; Ibanez, I. Buying time: Preliminary assessment of biocontrol in the recovery of native forest vegetation in the aftermath of the invasive emerald ash borer. Forests 2017, 8, 369. [Google Scholar] [CrossRef]
- Knight, K.S.; Brown, J.P.; Long, R.P. Factors affecting the survival of ash (Fraxinus spp.) trees infested by emerald ash borer (Agrilus planipennis). Biol. Invasions 2013, 15, 371–383. [Google Scholar] [CrossRef]
- Vercken, E.; Vincent, F.; Mailleret, L.; Ris, N.; Tabone, E.; Fauvergue, X. Time-lag in extinction dynamics in experimental populations: Evidence for a genetic Allee effect? J. Anim. Ecol. 2013, 82, 621–631. [Google Scholar] [CrossRef] [PubMed]
- Vercken, E.; Fauvergue, X.; Ris, N.; Crochard, D.; Mailleret, L. Temporal autocorrelation in host density increases establishment success of parasitoids in an experimental system. Ecol. Evol. 2015, 5, 2684–2693. [Google Scholar] [CrossRef] [PubMed]
- Abell, K.J.; Bauer, L.S.; Miller, D.L.; Duan, J.J.; Van Driesche, R.G. Monitoring the establishment and flight phenology of parasitoids of emerald ash borer (Coleoptera: Buprestidae) in Michigan by using sentinel eggs and larvae. Fla. Entomol. 2016, 99, 667–672. [Google Scholar] [CrossRef]
- Kashian, D.M.; Witter, J.A. Assessing the potential for ash canopy tree replacement via current regeneration following emerald ash borer-caused mortality on southeastern Michigan landscapes. For. Ecol. Manag. 2011, 261, 480–488. [Google Scholar] [CrossRef]
- Klooster, W.S.; Herms, D.A.; Knight, K.S.; Herms, C.P.; McCullough, D.G.; Smith, A.S.; Gandhi, K.J.K.; Cardina, J. Ash (Fraxinus spp.) mortality, regeneration, and seed bank dynamics in mixed hardwood forests following invasion by emerald ash borer (Agrilus planipennis). Biol. Invasions 2014, 16, 859–873. [Google Scholar] [CrossRef]
- Siegert, N.W.; McCullough, D.G.; Liebhold, A.M.; Telewski, F.W. Dendrochronological reconstruction of the epicentre and early spread of emerald ash borer in North America. Divers. Distrib. 2014, 20, 847–858. [Google Scholar] [CrossRef]
- Smith, A. Effects of community structure on forest susceptibility and response to the emerald ash borer invasion of the Huron River Watershed in southeastern Michigan. Master’s Thesis, Ohio State University, Columbus, OH, USA, 2006. [Google Scholar]
- IBM Corp. IBM SPSS Statistics for Windows, version 23.0; Released; IBM Corp.: Armonk, NY, USA, 2015. [Google Scholar]
- Kashian, D.M. Sprouting and seed production may promote persistence of green ash in the presence of the emerald ash borer. Ecosphere 2016, 7, 1–15. [Google Scholar] [CrossRef]
- Murphy, T.C.; Van Dreische, R.G.; Gould, J.R.; Elkinton, J. Can Spathius galinae attack emerald ash borer larvae feeding in large ash trees? Biol. Control 2017, 114, 8–14. [Google Scholar] [CrossRef]
- Hausman, C.E. The Ecological Impacts of the Emerald Ash Borer (Agrilus plannipennis): Identification of Conservation and Forest Management Strategies. Ph.D. Thesis, Kent State University, Kent, OH, USA, 2010; p. 174. [Google Scholar]
- Costanza, K.K.L.; Livingston, W.H.; Kashian, D.M.; Slesak, R.A.; Tardif, J.C.; Dech, J.P.; Diamond, A.K.; Daigle, J.J.; Ranco, D.J.; Siegert, N.W.; et al. The precarious state of a cultural keystone species: Biological and tribal assessments of the role and future of black ash. J. For. 2017, 115, 435–446. [Google Scholar] [CrossRef]
- Palik, B.J.; Ostry, M.E.; Venette, R.C.; Abdela, E. Tree regeneration in black ash (Fraxinus nigra) stands exhibiting dieback in northern Minnesota. For. Ecol. Manag. 2012, 269, 26–30. [Google Scholar] [CrossRef]
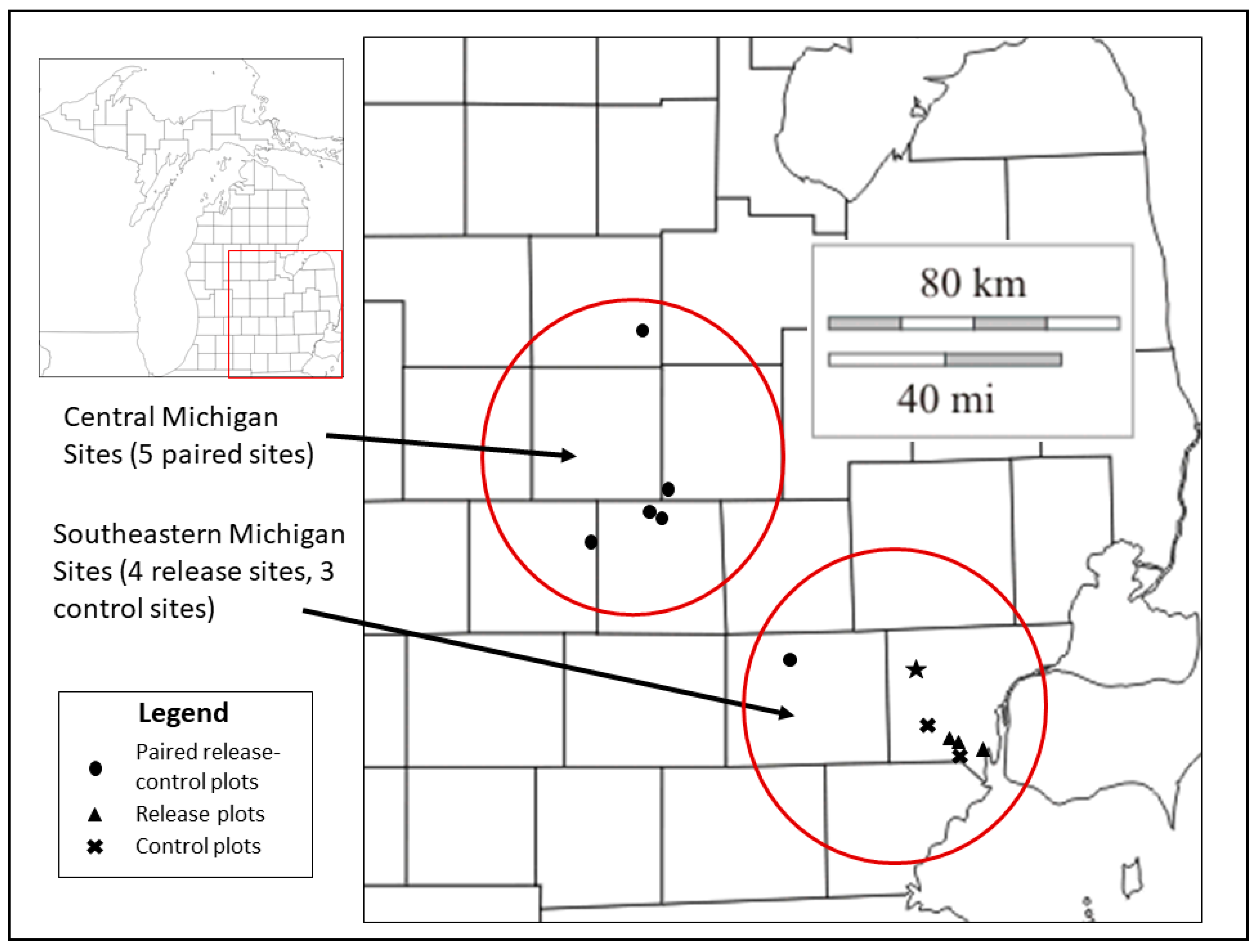
| Site | Location | Treatment | Year Parasitoid Releases Began |
|---|---|---|---|
| Central Michigan | |||
| Central Park | Okemos, MI | Paired | 2007 |
| Legg Park | Okemos, MI | Paired | 2008 |
| Burchfield Park | Holt, MI | Paired | 2008 |
| Gratiot-Saginaw West | Ashley, MI | Paired | 2009 |
| Rose Lake | Lansing, MI | Paired | 2010 |
| Southeastern Michigan | |||
| Lake Erie Metropark * | Brownstown, MI | Release | 2011 |
| Oakwoods Metropark * | Flat Rock, MI | Control | |
| Pinckney Recreation Area | Pinckney, MI | Paired | 2011 |
| Willow Metropark North ** | New Boston, MI | Release | 2011 |
| Willow Metropark South ** | New Boston, MI | Release | 2011 |
| Lower Huron Metropark ** | Belleville, MI | Control | |
| Central Michigan | Release | Control |
| Large Trees 2012 | 2.60 (0.11) a | 2.34 (0.14) a |
| Large Trees 2015 | 3.49 (0.15) b | 3.27 (0.21) b |
| Small Trees 2012 | 1.48 (0.08) 1 | 1.42 (0.07) 1 |
| Small Trees 2015 | 2.30 (0.12) 2 | 2.14 (0.12) 2 |
| Southeastern Michigan | Release | Control |
| Large Trees 2012 | 1.83 (0.08) a | 2.05 (0.16) a |
| Large Trees 2015 | 3.46 (0.15) b | 3.53 (0.15) b |
| Small Trees 2012 | 1.80 (0.09) 1 | 1.85 (0.12) 1 |
| Small Trees 2015 | 3.20 (0.14) 2 | 2.68 (0.16) 3 |
| Central Michigan | Release | Control |
| Large Trees 2012 | 86.4 a | 72.8 b |
| Large Trees 2015 | 97.5 c | 94.9 c |
| Small Trees 2012 | 48.8 1 | 37.6 1 |
| Small Trees 2015 | 74.6 2 | 89.5 3 |
| Southeastern Michigan | Release | Control |
| Large Trees 2012 | 85.0 a | 70.7 b |
| Large Trees 2015 | 90.7 a | 98.1 a |
| Small Trees 2012 | 61.0 1 | 50.7 1 |
| Small Trees 2015 | 84.3 2 | 85.5 2 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kashian, D.M.; Bauer, L.S.; Spei, B.A.; Duan, J.J.; Gould, J.R. Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan. Forests 2018, 9, 296. https://doi.org/10.3390/f9060296
Kashian DM, Bauer LS, Spei BA, Duan JJ, Gould JR. Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan. Forests. 2018; 9(6):296. https://doi.org/10.3390/f9060296
Chicago/Turabian StyleKashian, Daniel M., Leah S. Bauer, Benjamin A. Spei, Jian J. Duan, and Juli R. Gould. 2018. "Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan" Forests 9, no. 6: 296. https://doi.org/10.3390/f9060296
APA StyleKashian, D. M., Bauer, L. S., Spei, B. A., Duan, J. J., & Gould, J. R. (2018). Potential Impacts of Emerald Ash Borer Biocontrol on Ash Health and Recovery in Southern Michigan. Forests, 9(6), 296. https://doi.org/10.3390/f9060296




