Widespread Distribution of Trypodendron laeve in the Carpathian Mountains (Romania)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Field Research
2.2. Determining the Date of the Commencement of the Flight and the Number of Missed Flight Days
2.3. Studies of Collection Material
2.4. Data Processing
2.5. Maps
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Raffa, K.F.; Grégoire, J.-C.; Staffan Lindgren, B. Natural history and ecology of bark beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: San Diego, CA, USA, 2015; pp. 1–40. [Google Scholar]
- Postner, M. Scolytidae (=Ipidae), Borkenkäfer. In Die Forstschädlinge Europas. 2. Band; Schwenke, W., Ed.; Paul Parey: Berlin, Germany; Hamburg, Germany, 1974; pp. 334–482. [Google Scholar]
- Moeck, H.A. Ethanol as the primary attractant for the ambrosia beetle Trypodendron lineatum (Coleoptera: Scolytidae). Can. Entomol. 1970, 102, 985–995. [Google Scholar] [CrossRef]
- Bauer, J.; Vité, J. Host selection by Trypodendron lineatum. Naturwissenschaften 1975, 62, 539. [Google Scholar] [CrossRef]
- Nijholt, W.; Shonherr, J. Chemical response behavior of scolytids in West Germany and Western Canada. Can. For. Serv. Bi-Mon. Res. Note 1976, 32, 31–32. [Google Scholar]
- Kohnle, U. Untersuchungen über die Pheromonsysteme sekundärer Borkenkäfer (Col., Scolytidae). Z. Angew. Entomol. 1985, 100, 197–218. [Google Scholar] [CrossRef]
- Klimetzek, D.; Vité, J.; König, E. Über das Verhalten mitteleuropäischer Trypodendron-Arten gegenüber natürlichen und synthetischen Lockstoffen. Allg. For. Jagd-Zeit. 1981, 152, 64–70. [Google Scholar]
- Schurig, V.; Weber, R.; Klimetzek, D.; Kohnle, U.; Mori, K. Enantiomeric composition of ‘lineatin’ in three sympatric ambrosia beetles. Naturwissenschaften 1982, 69, 602–603. [Google Scholar] [CrossRef]
- Vité, J.; Bakke, A. Synergism between chemical and physical stimuli in host colonization by an ambrosia beetle. Naturwissenschaften 1979, 66, 528–529. [Google Scholar] [CrossRef]
- Shore, T.L.; Lindgren, B.S. Effect of ethanol and α-pinene on response of ambrosia beetle, Trypodendron lineatum, to lineatin-baited funnel and drainpipe traps. J. Chem. Ecol. 1996, 22, 2187. [Google Scholar] [CrossRef] [PubMed]
- Byers, J.A. Attraction of bark beetles, Tomicus piniperda, Hylurgops palliatus, and Trypodendron domesticum and other insects to short-chain alcohols and monoterpenes. J. Chem. Ecol. 1992, 18, 2385–2402. [Google Scholar] [CrossRef] [PubMed]
- Kühnholz, S.; Borden, J.H.; Uzunovic, A. Secondary ambrosia beetles in apparently healthy trees: Adaptations, potential causes and suggested research. Integr. Pest Manag. Rev. 2001, 6, 209–219. [Google Scholar] [CrossRef]
- Borden, J.H. The striped ambrosia beetle. In Dynamics of Forest Insect Populations: Patterns, Causes, Implications; Berryman, A.A., Ed.; Springer: Boston, MA, USA, 1988; pp. 579–596. [Google Scholar]
- Smith, S.M.; Hulcr, J. Scolytus and other economically important bark and ambrosia beetles. In Bark Beetles: Biology and Ecology of Native and Invasive Species; Vega, F.E., Hofstetter, R.W., Eds.; Elsevier: San Diego, CA, USA, 2015; pp. 495–531. [Google Scholar]
- Knížek, M. Scolytinae. In Catalogue of Palearctic Coleoptera; Löbl, I., Smetana, A., Eds.; Apollo Books: Stenstrup, Danmark, 2011; Volume 7, p. 373. [Google Scholar]
- Pfeffer, A. Zentral- und Westpaläarktische Borken- und Kernkäfer: (Coleoptera: Scolytidae, Platypodidae); Pro Entomologia, c/o Naturhistorisches Museum: Basel, Switzerland, 1995; p. 310. [Google Scholar]
- Lindgren, B.S. Trypodendron lineatum (Olivier) (Coleoptera: Scolytidae) breeding in bigleaf maple, Acer mocrophyllum. J. Entomol. Soc. B. C. 1986, 83, 44. [Google Scholar]
- Strand, A. Seven new species of coleoptera from Norway. Nor. Entomol. Tidskr. 1946, 7, 168–172. [Google Scholar]
- Holzschuh, C. Ein neuer, gefährlicher Nutzholzborkenkäfer in Österreich. Forstsch.-Aktuell Wien 1990, 3, 2. [Google Scholar]
- Martikainen, P. Flight period and ecology of Trypodendron proximum (Niijima) (Col., Scolytidae) in Finland. J. Appl. Entomol. 2000, 124, 57–62. [Google Scholar] [CrossRef]
- Kvamme, T. Trypodendron piceum Strand (Col., Scolytidae): Flight period and response to synthetic pheromones. Fauna Nor. Ser. B 1988, 35, 65–70. [Google Scholar]
- Eggers, H. Japanische Borkenkäfer II. Arb. morph. taxon. Ent. Berlin-Dahlem 1939, 6, 114–123. [Google Scholar]
- Balachowsky, A.S. Faune de France 50. Coléoptères, Scolytides; Paul Lechevalier: Paris, France, 1949; p. 320. [Google Scholar]
- Stark, V.N. Zhestkokrylye, Koroedy. Fauna SSSR; Akademia Nauk SSSR: Moskva, Russia; Leningrad, Russia, 1952; p. 463. [Google Scholar]
- Nunberg, M. Klucze do Oznaczania Owadów Polski, Cz. Xix Chrząszcze—Coleoptera, Zeszyt 99–100, Korniki—Scolytidae, Wyrynniki—Platypodidae; Panstwowe Wydawnictwo Naukowe: Warszawa, Poland, 1954; p. 106. [Google Scholar]
- Pfeffer, A. Kůrovci—Scolytoidea. FAUNA Čsr, Svazek 6; ČSAV: Praha, Czech Republic, 1955; p. 324. [Google Scholar]
- Grüne, S. Handbuch zur Bestimmung der Europäischen Borkenkäfer: Brief Illustrated Key to European Bark Beetles; M. & H. Schaper: Hannover, Germany, 1979; p. 182. [Google Scholar]
- Schedl, K.E. 91. Familie: Scolytidae (Borken-und Ambrosiakäfer). In Die Käfer Mitteleuropas; Freude, H., Harde, K.W., Lohse, G.A., Eds.; Spektrum Akademischer Verlag: Berlin/Heidelberg, Germany, 1981; Volume 10, pp. 34–99. [Google Scholar]
- Annila, E.; Bakke, A.; Bejer-Petersen, B.; Lekander, B. Flight period and brood emergence in Trypodendron lineatum (Oliv.) (Coleoptera, Scolytidae) in the Nordic countries. Commun. Inst. For. Fenn. 1972, 76, 1–28. [Google Scholar]
- Lindelöw, Å. Aktuellt om svenska barkborrar (Coleoptera; Curculionidae, Scolytinae). Entomol. Tidskr. 2010, 131, 97–104. [Google Scholar]
- Pfeffer, A. Taxonomischer Status einer Arten der Gattung Xyloterus Erichson (Coleoptera, Scolytidae). Acta Entomol. Bohemoslov. 1989, 86, 129–136. [Google Scholar]
- Pfeffer, A. 91. Familie: Scolytidae. In Die Käfer Mitteleuropas; Lohse, G.A., Lucht, W.H., Eds.; Springer Spektrum: Berlin/Heidelberg, Germany, 1994; Volume 14. [Google Scholar]
- Wood, S.L. Nomenclatural changes and new species of Scolytidae (Coleoptera), Part IV. Great Basin Nat. 1989, 49, 167–185. [Google Scholar]
- Mandelshtam, M.Y.; Popovichev, B.G. Annotated list of bark beetles (Coleoptera, Scolytidae) of Leningrad province. Ėntomol. Obozr. 2000, 79, 599–618. [Google Scholar]
- Holzschuh, C. Ergebnisse von Untersuchungen über die Einschleppung von Borkenkäfern an Holzlager- und Umschlagplätzen. Forstsch.-Aktuell Wien 1990, 5, 7–8. [Google Scholar]
- Holzschuh, C. Forstschädlinge, die in den letzten fünfzig Jahren in Österreich eingewandert sind oder eingeschleppt wurden. Stapfia Lienz 1995, 37, 129–141. [Google Scholar]
- Krehan, H.; Holzschuh, C. Trypodendron laeve—Vorkommen in Österreich. Forstsch.-Aktuell Wien 1999, 23/24, 6–8. [Google Scholar]
- Kenis, M. Insects—Insecta. In Invasive Alien Species in Switzerland—An Inventory of Alien Species and Their Threat to Biodiversity and Economy in Switzerland; Wittenberg, R., Kenis, M., Blick, T., Hänggi, A., Gassmann, A., Weber, E., Eds.; Federal Office for the Environment (FOEN): Bern, Switzerland, 2006; pp. 71–100. [Google Scholar]
- DAISE. Handbook of Alien Species in Europe; Springer: Berlin/Heidelberg, Germany, 2009; p. 399. [Google Scholar]
- Köhler, F.; Klausnitzer, B. Verzeichnis der Käfer Deutschlands. Ent. Nachr. Ber. (Dresden) Beiheft 1998, 4, 1–185. [Google Scholar]
- Zelený, J.; Doležal, P. Kůrovcoviti brouci (Scolytidae, Coleoptera) na smrku na Šumavě. Akt. Šumav. Výzkumu 2004, 2, 221–223. [Google Scholar]
- Zelený, J. Nejčastější kůrovcovití na smrku na Šumavě. Lesn. Práce 2000, 80, 258–259. [Google Scholar]
- Olenici, N.; Knížek, M.; Olenici, V.; Duduman, M.-L.; Biriş, I.-A. First report of three scolytid species (Coleoptera: Curculionidae, Scolytinae) in Romania. Ann. For. Res. 2014, 57, 87. [Google Scholar] [CrossRef]
- Sweeney, J.D.; Silk, P.; Grebennikov, V.; Mandelshtam, M. Efficacy of semiochemical-baited traps for detection of Scolytinae species (Coleoptera: Curculionidae) in the Russian Far East. Eur. J. Entomol. 2016, 113, 84–97. [Google Scholar] [CrossRef]
- Lekander, B.; Bejer-Petersen, B.; Kangas, E.; Bakke, A. The distribution of bark beetles in the Nordic countries. Acta Entomol. Fenn. 1977, 32, 1–37. [Google Scholar]
- Muona, J. Four species of beetles new to Finland. Not. Entomol. 1989, 69, 195–197. [Google Scholar]
- Muona, J. Tarkennuksia eräiden kuoriaislajien esiintymiseen Suomessa ja Venäjän Karjalassa (Coleoptera). Sahlbergia 1994, 1, 7–10. [Google Scholar]
- Martikainen, P.; Siitonen, J.; Kaila, L.; Punttila, P. Intensity of forest management and bark beetles in non-epidemic conditions: A comparison between Finnish and Russian Karelia. J. Appl. Entomol. 1996, 120, 257–264. [Google Scholar] [CrossRef]
- Martikainen, P.; Siitonen, J.; Kaila, L.; Punttila, P.; Rauh, J. Bark beetles (Coleoptera, Scolytidae) and associated beetle species in mature managed and old-growth boreal forests in southern Finland. For. Ecol. Manag. 1999, 116, 233–245. [Google Scholar] [CrossRef]
- Voolma, K.; Mandelshtam, M.J.; Shcherbakov, A.N.; Yakovlev, E.B.; Õunap, H.; Süda, I.; Popovichev, B.G.; Sharapa, T.V.; Galasjeva, T.V.; Khairetdinov, R.R. Distribution and spread of bark beetles (Coleoptera: Scolytidae) around the gulf of Finland: A comparative study with notes on rare species of Estonia, Finland and North-Western Russia. Entomol. Fenn. 2004, 15, 198–210. [Google Scholar]
- Öhrn, P.; Lindelöw, Å.; Långström, B. Flight activity of the ambrosia beetles Trypodendron laeve and Trypodendron lineatum in relation to temperature in southern Sweden. In Biotic Risks and Climate Change in Forests; Delb, H., Pontuali, S., Eds.; Berichte Freiburger Forstliche Forschung, Forest Research Institute of Baden–Württemberg: Freiburg, Germany, 2011; Volume 89, pp. 86–90. [Google Scholar]
- Lukášová, K.; Knížek, M.; Holuša, J.; Čejka, M.; Kacprzyk, M. Is the bark beetle Trypodendron laeve (Coleoptera: Curculionidae: Scolytinae) an alien pest in the Czech Republic and Poland? Pol. J. Ecol. 2012, 60, 789–795. [Google Scholar]
- Witkowski, R.; Załuska, M.T.; Buchholz, L.; Mazur, A. Nowe dane o występowaniu Trypodendron laeve Eggers, 1939 (Coleoptera, Curculionidae, Scolytinae) w Polsce. Acta Sci. Pol. Silv. Colendar. Rat. Ind. Lignar. 2015, 14, 81–86. [Google Scholar]
- Haylock, M.R.; Hofstra, N.; Klein Tank, A.M.G.; Klok, E.J.; Jones, P.D.; New, M. A European daily high-resolution gridded data set of surface temperature and precipitation for 1950–2006. J. Geophys. Res. Atmos. 2008, 113. [Google Scholar] [CrossRef]
- Micu, D.M.; Dumitrescu, A.; Cheval, S.; Birsan, M.-V. Climate of the Romanian Carpathians: Variability and Trends; Springer: London, UK, 2014; p. 213. [Google Scholar]
- Vasiliu, M.; Zaharia, D.; Ignat, C. Catalogul scolitidelor din colecţia “Ştefan Negru” a Muzeului Judeţean Suceava (Coleoptera, Scloytoidea). In Studii şi Comunicări, Ştiinţele Naturii; Muzeul Judeţean: Suceava, Romania, 1978; pp. 37–58. [Google Scholar]
- Negru, Ș. Les Scolytoides (Coleoptera, Scolytoidea) de la collection scientifique du Musée Brukenthal—Sibiu. Trav. Mus. d’Hist. Nat. Grigore Antipa Buchar. 1966, 6, 397–405. [Google Scholar]
- Borden, J.; Fockler, C. Emergence and orientation behavior of brood Trypodendron lineatum (Coleoptera: Scolytidae). J. Entomol. Soc. B. C. 1973, 70, 34–38. [Google Scholar]
- Zar, J.H. Biostatistical Analysis, 5th ed.; Pearson Prentice Hall: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Park, J.; Reid, M.L. Distribution of a bark beetle, Trypodendron lineatum, in a harvested landscape. For. Ecol. Manag. 2007, 242, 236–242. [Google Scholar] [CrossRef]
- Lukášová, K.; Holuša, J. Comparison of Trypodendron lineatum, T. domesticum and T. laeve (Coleoptera: Curculionidae) flight activity in Central Europe. J. For. Sci. 2014, 60, 382–387. [Google Scholar] [CrossRef]
- Bussler, H.; Schmidt, O. Remarks on the taxonomy, distribution and ecology of Trypodendron laeve Eggers, 1939 (Coleoptera: Scolytidae). Nachrichtenbl. Bayer. Entomol. 2008, 57, 62–65. [Google Scholar]
- Klimetzek, D.; Vité, J.P. Tierische Schädlinge. In Die Fichte; Schmidt-Vogt, H., Ed.; Krankheiten, Schäden, Fichtensterben; Paul Parey: Berlin, Germany, 1989; Band II/2; pp. 40–133. [Google Scholar]
- Salom, S.M.; McLean, J.A. Flight behavior of scolytid beetle in response to semiochemicals at different wind speeds. J. Chem. Ecol. 1991, 17, 647–661. [Google Scholar] [CrossRef] [PubMed]
- Salom, S.M.; McLean, J.A. Environmental influences on dispersal of Trypodendron lineatum (Coleoptera: Scolytidae). Environ. Entomol. 1991, 20, 565–576. [Google Scholar] [CrossRef]
- Petri, K. Siebenbürgens Käferfauna auf Grund ihrer Erforschung bis zum Jahre 1911; Drotleff: Hermannstadt, Romania, 1912; p. 376. [Google Scholar]
- Merkl, O. Data to the knowledge on the beetle fauna of Maramures, Romania (Coleoptera). Stud. Univ. Vasile Goldis Ser. Stiintele Vietii. 2008, 18, 243–311. [Google Scholar]
- Kocs, I. A Magyar Természettudományi Múzeum Bogárgyűjteményében Található, Székelyföldön gyűjtött ormányosalkatú bogarak fajlistája (Coleoptera: Curculionoidea). Acta Siculica 2010, 2010, 105–121. [Google Scholar]
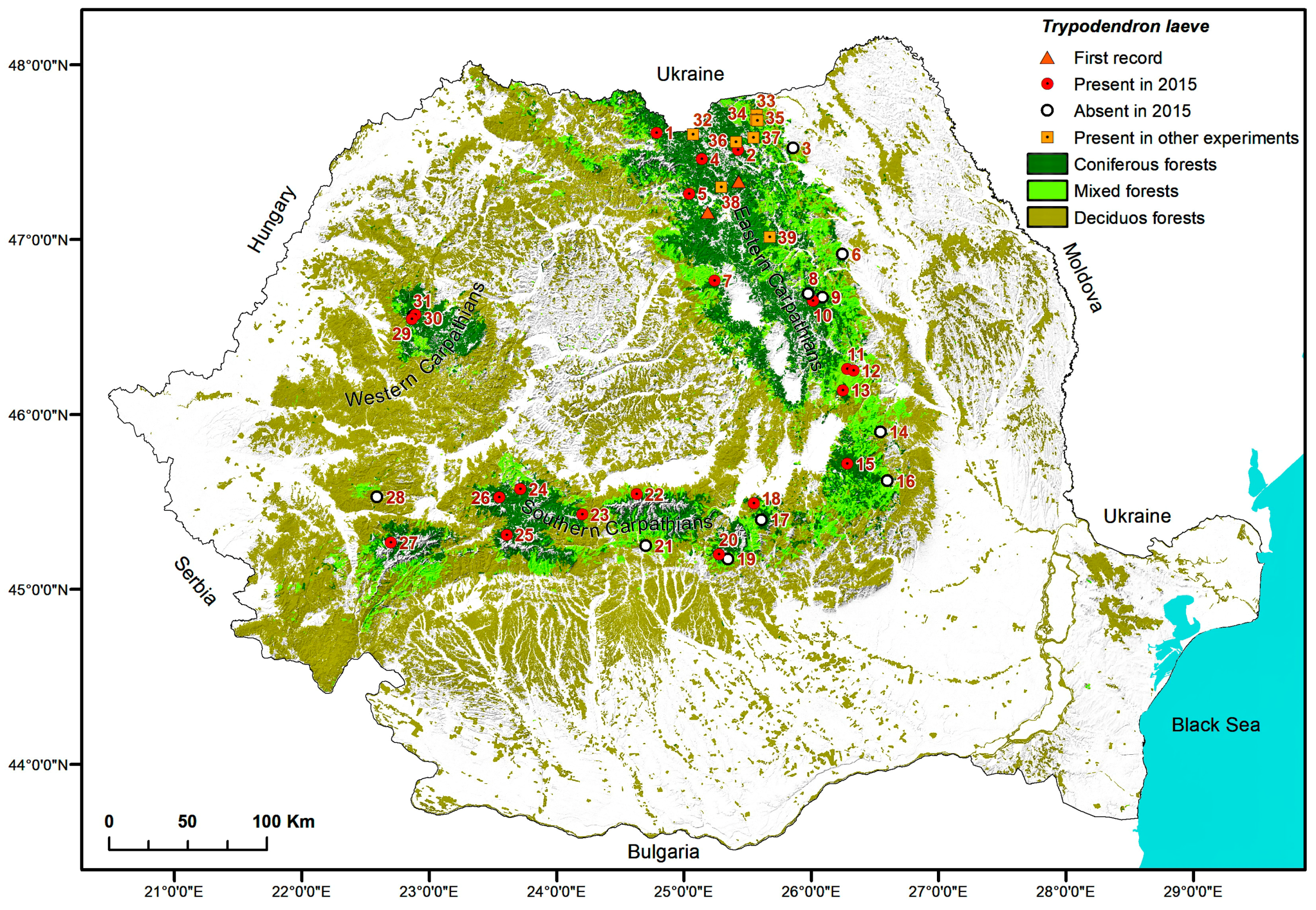
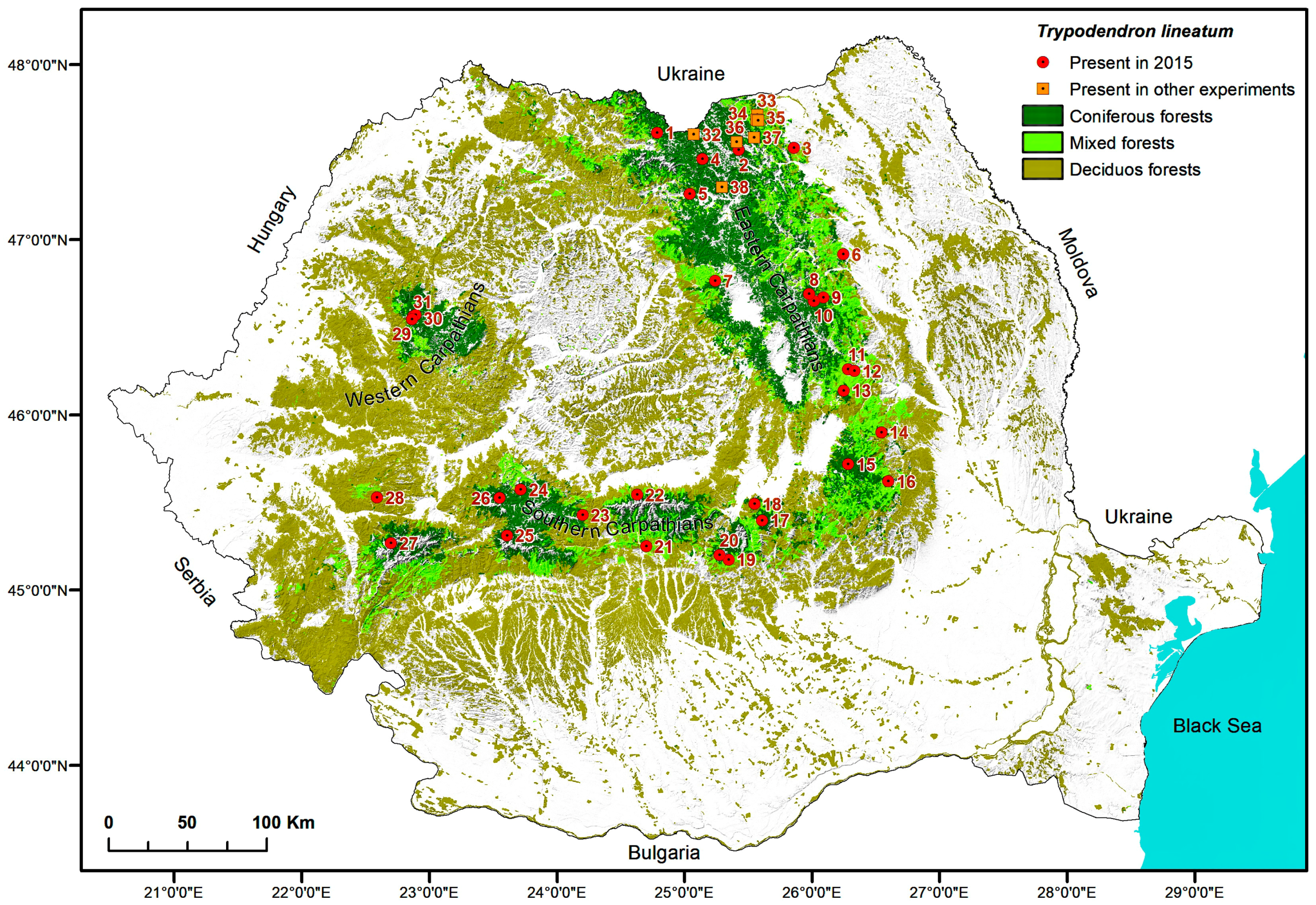
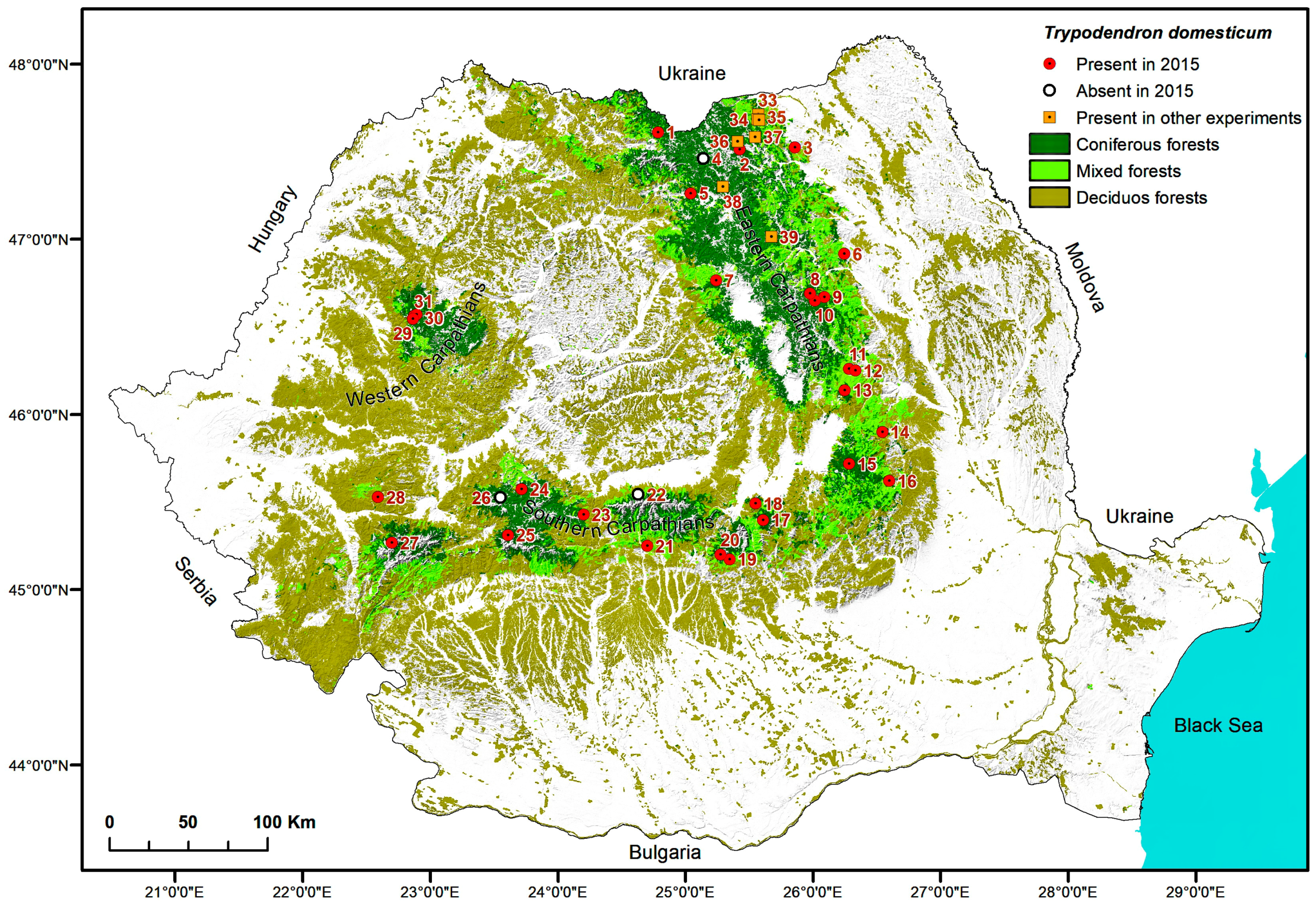
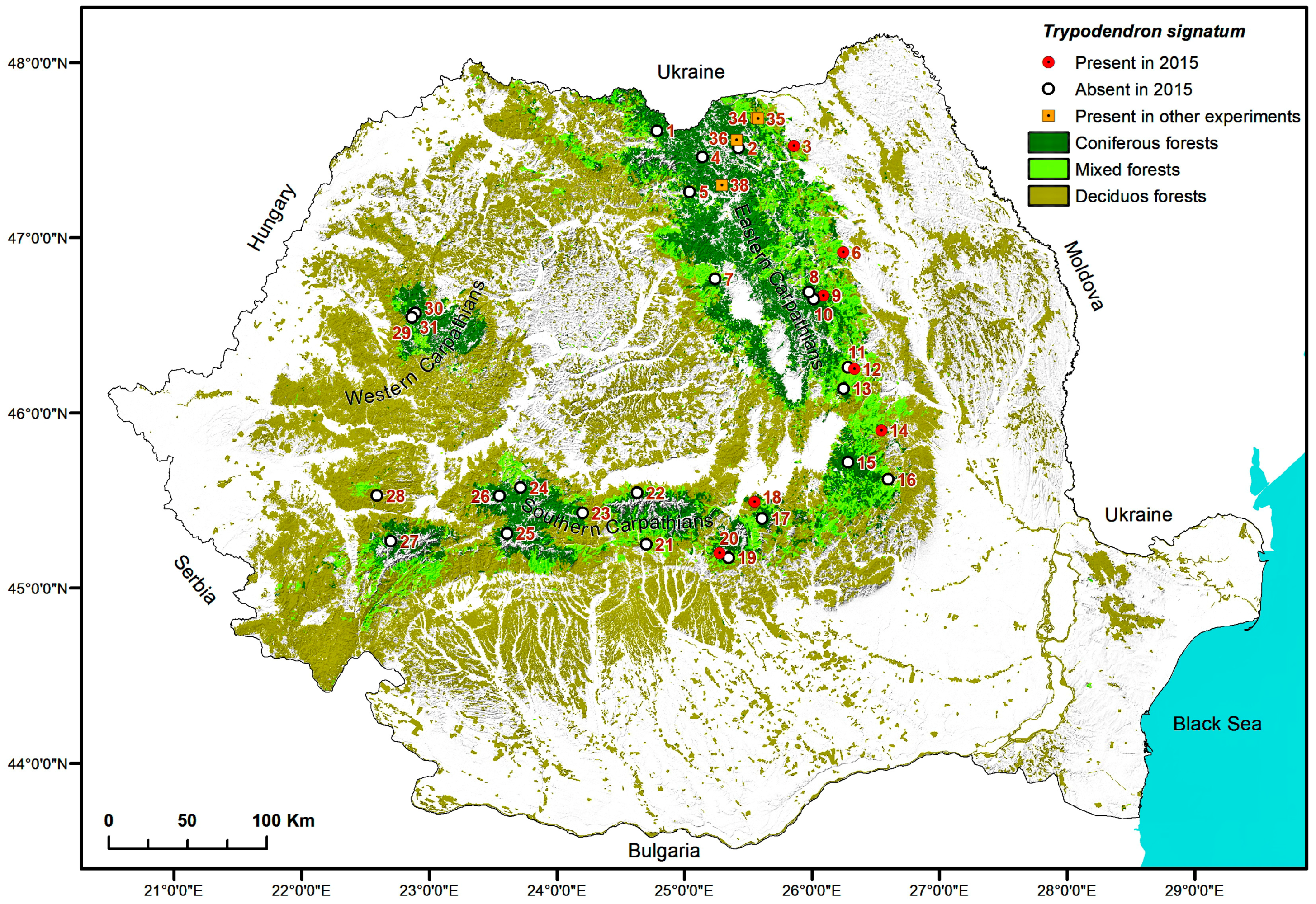
| No. | Location, County | Forest District, Production Unit, Compartment | Coordinates N/E | Elevation (m)/Aspect | Forest Composition (%) | Forest Age (Years) |
|---|---|---|---|---|---|---|
| Trap locations for detection of Trypodendon laeve in 2015 | ||||||
| 1. | Vișeul de Sus, Maramureș | Vișeu, VI Mira, 9C | 47.7227333 24.7672555 | 1520 NW | 100 Pa | 160 |
| 2. | Paltinu, Suceava | Vama, II Paltinu, 87A | 47.6236667 25.4564722 | 1180–1250 E | 100 Pa | 80 |
| 3. | Cacica, Suceava | Solca, II Cacica, 9B | 47.6322889 25.9207638 | 460 E | 40 Aa 40 Pa 20 Fs | 120 |
| 4. | Cârlibaba, Suceava | Cârlibaba, VI Cârlibaba, 129A | 47.5716028 25.1482083 | 1100 NW | 100 Pa | 80 |
| 5. | Lunca Ilvei, Bistrița-Năsăud | Lunca Ilvei, I Lunca Ilvei, 150 | 47.3729028 25.0417055 | 1070 SW | 60 Pa 30 Fs 10 Aa | 120 |
| 6. | Almaș, Neamț | Gârcina, III Almaș, 28B | 47.0176806 26.3233083 | 650 NE | 40 Fs 20 Aa 20 Pa 20 Ap | 55 |
| 7. | Stânceni, Mureș | Lunca Bradului, Trupul Gudea, 132B | 46.864583 25.245783 | 1144 W | 80 Pa 10 Aa 10 Fs | 115 |
| 8. | Brateș_3, Neamț | Tarcău, VII Ața, 54A | 46.7954444 26.0342027 | 983 NW | 60 Pa 30 Aa 10 Fs | 150 |
| 9. | Brateș_1, Neamț | Tarcău, V Bolovăniș, 179A | 46.7748000 26.1507416 | 580 NE | 60 Pa 30 Aa 10 Dt | 80 |
| 10. | Brateș_2, Neamț | Tarcău, VI Brateș, 179A | 46.7554583 26.0735944 | 818 NW | 70 Pa 30 Aa | 110 |
| 11. | Dărmănești, Bacău | Dărmănești, III Dărmănești, 79B | 46.3614333 26.3454722 | 1020 E | 50 Fs 30 Pa 20 Dt | 100 |
| 12. | Sălătruc, Bacău | Lignum, UBII Lapos, 101, 102 | 46.351275 26.398533 | 820 E | 50 Fs 30 Aa 20 Pa | 100 |
| 13. | Poiana Uzul, Bacău | Dărmănești, III Bărzăuța, 54A | 46.2393833 26.3076777 | 1160 N | 90 Pa 10 Dr | 100 |
| 14. | Soveja, Vrancea | Soveja, II Soveja, 64C | 45.9973083 26.6106000 | 810 SE | 80 Pa 20 Aa | 75 |
| 15. | Covasna, Covasna | Comandău, IV Obârșia Bâscii, 99B,C | 45.8171028 26.3339861 | 1340 N | 100 Pa | 30–130 |
| 16. | Brădăcești, Vrancea | Zăbala-Nereju, I Bârsești, 124 | 45.7158333 26.6588888 | 1060 | 60 Fs 40 Pa | 110 |
| 17. | Predeal, Brașov | RPLP Kronstadt, III Postăvaru, 133A | 45.5023472 25.6298277 | 1345 N | 100 Pa | 110 |
| 18. | Poiana Brașov, Brașov | RPLP Kronstadt, VI Tâmpa, 38A | 45.5970556 25.5668472 | 1096 NW | 40 Pa 30 Aa 30 Ld | 110 |
| 19. | Moroeni, Dâmbovița | A.O.S. Carpathia, III Raciu, 56C | 45.2785944 25.3500000 | 1200 NE | 100 Pa | 80 |
| 20. | Bădeni, Argeș | A.O.S. Carpathia, VII Bădeanca, 100A | 45.3062000 25.2803972 | 1420 SW | 90 Pa 10 Fs | 90 |
| 21. | Căpățâneni, Argeș | Vidraru, VI Limpedea, 42B, 43B | 45.3556778 24.6877555 | 1520 S/SW | 100 Pa | 125 |
| 22. | Cârțișoara, Sibiu | Arpaș, V Bâlea, 14H | 45.6500000 24.6119444 | 1503 W | 100 Pa | 100 |
| 23. | Paltin, Sibiu | Sibiu, I Dealul Paltinului, 34G | 45.5313889 24.1694444 | 1494 NE | 100 Pa | 120 |
| 24. | Bistra, Sibiu | Miercurea Sibiului, III Miercurea Sibiului, 164B | 45.6716667 23.6638888 | 1490 N | 100 Pa | 100 |
| 25. | Jieț, Hunedoara | Petroșani, V Jieț, 62A | 45.4090278 23.5594361 | 1205 SE | 60 Fs 40 Pa | 150 |
| 26. | Cugir, Alba | Cugir, Valea Bosorog-Parva, 130A,B | 45.6228889 23.4930194 | 1400 E/SE | 100 Pa | 95 |
| 27. | Poiana Mărului, Caraș-Severin | Oțelu Roșu, VI Obârșia Bistrei Mărului, 66A/66B/69B | 45.3493333 22.6219722 | 1215 NW/NE/N | 90 Pa 10 Aa/100 Pa/100 Pa | 90/130/130 |
| 28. | Rusca Montană, Caraș-Severin | Rusca Montană, V Rusca Montană, 138A, 139 | 45.6071111 22.4974722 | 802 N/N | 70 Pa 30 Fs 60 Pa40Fs | 110 110 |
| 29. | Padiş_2, Bihor | Beliș, II Ponor, 69A | 46.6338028 22.7409138 | 1135 N | 100 Pa | 115 |
| 30. | Doda Pilii, Cluj | Beliș, II Ponor, 143A | 46.657875 22.7680111 | 1280 SE | 100 Pa | 160 |
| 31. | Padiş_1, Bihor | Beliș, II Ponor, 128A | 46.650975 22.7502583 | 1440 NE | 70 Pa 30 Fs | 110 |
| Trap location for other studies with synthetic pheromone of Trypodendron lineatum | ||||||
| 32. | Bobeica, Suceava | Cârlibaba, VII Buhăiescu, 49I | 47.7132194 25.0763333 | 1200 S | 100 Pa | 115 |
| 33. | Putna_1, Suceava | Putna, II Putnișoara, 105A | 47.827604 25.607495 | 650 SE | 70 Pa 10 Aa 20 Fs | 110 |
| 34. | Putna_2, Suceava | Putna, II Putnișoara, 121A | 47.799167 25.603056 | 750 E | 40 Pa 20 Aa 30 Fs 10 Ap | 90 |
| 35. | Putna_3, Suceava | Putna, II Putnișoara, 131A | 47.792221 25.611334 | 850 V | 40 Pa 20 Aa 40 Fs | 45 |
| 36. | Demacușa, Suceava | Tomnatic, I Demacușa, 50G | 47.6701575 25.4389286 | 890–1000 NE | 60 Pa 20 Aa 20 Fs | 80 |
| 37. | Ciumârna, Suceava | Vama, III Dragoșa, 344A | 47.6943417 25.5870722 | 800 SE | 60 Pa 20 Aa 20 Fs | 110 |
| 38. | Iacobeni, Suceava | Iacobeni, U.P. VI Botoş—Orata, 5A | 47.4114722 25.3118611 | 835–1115 W | 100 Pa | 100 |
| 39. | Borca, Neamț | Borca, II Borca, 73A | 47.1216389 25.7174388 | 1010 N | 100 Pa | 40 |
| Site No. | Location | No. of Traps | Monitored Period | Start of Flight | No. Flight Days Lost | Tmax.aver | Total Number of Trypodendron Beetles per Study Site | Ratio T. laeve/T. lineatum | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| laeve | lineatum | domesticum | signatum | ||||||||
| 1. | Vișeul de Sus | 3 | 21.04–19.05.15 | 26.04.15 | 0 | 43 | 1832 | 1 | 0 | 1/42.6 | |
| 2. | Paltinu | 3 | 20.03–16.05.15 | 12.04.15 | 0 | 56 | 1270 | 314 | 0 | 1/22.5 | |
| 3. | Cacica | 3 | 03.04–18.05.15 | 24.03.15 | 3 | 15.9 | 0 | 63 | 9 | 12 | |
| 4. | Cârlibaba | 3 | 07.04–25.05.15 | 26.03.15 | 1 | 13.9 | 217 | 4464 | 0 | 0 | 1/19.6 |
| 5. | Lunca Ilvei | 3 | 26.03–14.05.15 | 26.03.15 | 0 | 3 | 60 | 118 | 0 | 1/17.0 | |
| 6. | Almaș | 3 | 04.04–07.05.15 | 25.03.15 | 2 | 14.9 | 0 | 474 | 18 | 3 | |
| 7. | Stânceni | 3 | 15.04–29.05.15 | 26.03.15 | 4 | 14.4 | 46 | 4527 | 13 | 0 | 1/89.8 |
| 8. | Brateș_3 | 1 | 08.04–20.05.15 | 26.03.15 | 1 | 14.6 | 0 | 5 | 6 | 0 | |
| 9. | Brateș_1 | 1 | 01.04–12.05.15 | 24.03.15 | 4 | 15.1 | 0 | 12 | 3 | 2 | |
| 10. | Brateș_2 | 1 | 01.04–12.05.15 | 25.03.15 | 2 | 14.9 | 1 | 130 | 4 | 0 | 1/22.0 |
| 11. | Dărmănești | 1 | 30.03–11.05.15 | 26.03.15 | 1 | 13.6 | 6 | 51 | 14 | 0 | 1/8.5 |
| 12. | Sălătruc | 2 | 30.03–11.05.15 | 25.03.15 | 2 | 14.4 | 9 | 181 | 45 | 17 | 1/19.1 |
| 13. | Poiana Uzul | 3 | 02.04–07.05.15 | 11.04.15 | 0 | 61 | 204 | 41 | 0 | 1/3.3 | |
| 14. | Soveja | 3 | 28.04–02.06.15 | 26.03.15 | 13 | 17.4 | 0 | 180 | 9 | 29 | |
| 15. | Covasna | 3 | 28.04–26.05.15 | 11.04.15 | 9 | 14.2 | 11 | 3609 | 10 | 0 | 1/298.3 |
| 16. | Brădăcești | 3 | 26.04–31.05.15 | 11.04.15 | 8 | 15.9 | 0 | 71 | 6 | 0 | |
| 17. | Predeal | 3 | 23.05–04.07.15 | 11.04.15 | 19 | 15.5 | 0 | 4285 | 10 | 0 | |
| 18. | Poiana Brașov | 3 | 30.03–11.05.15 | 26.03.15 | 1 | 13.3 | 178 | 1387 | 17 | 3 | 1/7.8 |
| 19. | Moroeni | 3 | 02.04–21.05.15 | 11.04.15 | 0 | 0 | 69 | 5 | 0 | ||
| 20. | Bădeni | 3 | 02.04–23.05.15 | 11.04.15 | 0 | 67 | 722 | 348 | 56 | 1/3.2 | |
| 21. | Căpățâneni | 3 | 03.05–14.06.15 | 16.04.15 | 5 | 13.8 | 0 | 165 | 5 | 0 | |
| 22. | Cârțișoara | 3 | 02.04–14.05.15 | 16.04.15 | 0 | 68 | 517 | 0 | 0 | 1/7.4 | |
| 23. | Paltin | 3 | 02.04–14.05.15 | 16.04.15 | 0 | 6 | 606 | 18 | 0 | 1/101.0 | |
| 24. | Bistra | 3 | 01.05–05.06.15 | 11.04.15 | 6 | 13.9 | 2 | 365 | 1 | 0 | 1/151.5 |
| 25. | Jieț | 3 | 20.04–25.05.15 | 26.03.15 | 6 | 15.7 | 83 | 818 | 67 | 0 | 1/7.7 |
| 26. | Cugir | 3 | 23.04–08.06.15 | 11.04.15 | 4 | 14.8 | 1 | 769 | 0 | 0 | 1/671.0 |
| 27. | Poiana Mărului | 3 | 14.04–19.05.15 | 26.03.15 | 4 | 15.9 | 1 | 88 | 12 | 0 | 1/68.0 |
| 28. | Rusca Montană | 3 | 14.04–19.05.15 | 25.03.15 | 7 | 17.1 | 0 | 64 | 2 | 0 | |
| 29. | Padiș_2 | 1 | 07.05–10.06.15 | 25.03.15 | 16 | 16.5 | 3 | 262 | 19 | 0 | 1/31.3 |
| 30. | Doda Pilii | 1 | 07.05–10.06.15 | 26.03.15 | 13 | 16.1 | 1 | 364 | 12 | 0 | 1/364.0 |
| 31. | Padiș_1 | 1 | 07.05–10.06.15 | 26.03.15 | 10 | 15.6 | 0 | 427 | 22 | 0 | |
| Total | 78 | 863 | 28,041 | 1149 | 122 | ||||||
| Site No. | Location | No. of Traps | Monitored Period | Total Number of Trypodendron Beetles per Study Site | |||
|---|---|---|---|---|---|---|---|
| laeve | lineatum | domesticum | signatum | ||||
| 32. | Bobeica | 3 | 19.04–16.09.14 | 1 | 37,291 | 0 | 0 |
| 33. | Putna_1 | 3 | 13.03–24.08.17 | 5 | 353 | 19 | 0 |
| 34. | Putna_2 | 3 | 13.03–24.08.17 | 41 | 686 | 42 | 4 |
| 35. | Putna_3 | 3 | 13.03–24.08.17 | 237 | 4125 | 47 | 5 |
| 36. | Demacușa | 20 | 12.04–15.06.16 | 1 | 19,366 | 524 | 1 |
| 37. | Ciumârna | 20 | 06.05–09.06.15 | 1 | 14,095 | 57 | 0 |
| 38. | Iacobeni | 20 | 13.04–16.06.16 | 3 | 31,906 | 47 | 3 |
| 39. | Borca | - | 23.03.17 | 1 | - | 2 | - |
| Total | 72 | 290 | 107,822 | 738 | 13 | ||
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olenici, N.; Duduman, M.-L.; Isaia, G.; Knížek, M.; Vasian, I. Widespread Distribution of Trypodendron laeve in the Carpathian Mountains (Romania). Forests 2018, 9, 286. https://doi.org/10.3390/f9060286
Olenici N, Duduman M-L, Isaia G, Knížek M, Vasian I. Widespread Distribution of Trypodendron laeve in the Carpathian Mountains (Romania). Forests. 2018; 9(6):286. https://doi.org/10.3390/f9060286
Chicago/Turabian StyleOlenici, Nicolai, Mihai-Leonard Duduman, Gabriela Isaia, Miloš Knížek, and Iuliana Vasian. 2018. "Widespread Distribution of Trypodendron laeve in the Carpathian Mountains (Romania)" Forests 9, no. 6: 286. https://doi.org/10.3390/f9060286
APA StyleOlenici, N., Duduman, M.-L., Isaia, G., Knížek, M., & Vasian, I. (2018). Widespread Distribution of Trypodendron laeve in the Carpathian Mountains (Romania). Forests, 9(6), 286. https://doi.org/10.3390/f9060286






